Abstract
Overused vegetable oils, which are considered to be a waste and available in huge quantities after frying processes, were directly ethoxylated using a conventional cheap catalyst in order to obtain an economically valuable ethoxylated product to replace the imported intermediate derivatives and at the same time the environment will be rid of one of its pollutants. Therefore, this work was devoted to exploring its application as a fatliquoring agent in the leather industry. Overused sunflower and olein oils were directly ethoxylated using ethylene oxide gas in the presence of 3% KOH catalyst at 180 °C for 20 h. The prepared products were applied as nonionic fatliquors. The fatliquoring process is the operation in which a fatty matter is introduced into the leather fibers. The results obtained showed that the prepared ethoxylated overused oils were effective fatliquors with high HLB values giving stable oil in water emulsion as well as high stability against acid, alkali and different metallic salts. The fatliquored leather had improved mechanical properties such as tensile strength and elongation at break. In addition, a significant enhancement of the texture of the treated leather by the two prepared fatliquors as indicated from the scanning electron microscope images was observed. Also the results indicated that ethoxylated overused sunflower oil gave better results than those of ethoxylated overused olein oil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, ethoxylation has been considered to be an effective process by which the fatty materials yield good nonionic surface active agents and at the same time is a non-polluting method. Ethylene oxide-based surfactants are compounds that contain a poly(ethylene oxide) chain as a hydrophile and act as an intermediate material in several industries. They comprise a number of major surfactant classes, including alcohol ethoxylates; alcohol ether sulfates; alkyl phenol ethoxylates; ethoxylated nitrogen compounds, such as alkylamine ethoxylates; and ethylene oxide/propylene oxide block copolymers [1]. Ethoxylated fatty acid esters are well known as ether-ester-type nonionic surfactants with multiple uses in applications like emulsifiers, dispersants or oil phase adjusters in cosmetics or in industrial products. Also ethoxylated methyl laureates have been studied as wetting agents [2]. A few publications reported that ethoxylated vegetable oils or fats were used in emulsion fatliquoring of various tanned leather (chrome- or glutaraldehyde-tanned) to produce highly elastic and ultra soft leather [3, 4].
The leather industry utilizes several processes for converting animal hides or skins into leather which have a great effect on the whole characters of the leather produced [5, 6]. The preliminary processes of de-hairing and bating remove most of the natural fats from the skin. Therefore, as water is removed during the drying stage from chrome tanned leather, cohesion of the fibers take place resulting in hard intractable leather which is quite difficult to re-hydrate [7]. This means that, chrome tanned leather when it dries out, will become bony, hard and thus will be unsuitable for use for most purposes, in addition, its color becomes darker and less appealing. Therefore, incorporation of fatliquor into leather reduces the damaging effect of air oxidation and controls the differential shrinkage of the grain versus the corium of the leather during the drying process.
Fatliquor helps to prevent the loosening of the leather grain and is intended to lubricate the tanned leather fibers to obtain leather of full and soft handle, abrasion resistance, flexibility, pliability and stretching as well as improving its mechanical properties [8]. Therefore, introducing a lubricant into the leather keeps the fibers apart during drying and reduces frictional forces within the fiber weaves thus allowing the fibers to move laterally over each other. Also, it imparts to the leather grains specific properties which make it suitable for its most effective utilization [9]. Therefore, one of the most important industrial processes which render the penetration of fat molecules is the formation of surface active compounds. Nowadays, the world is directed in all fields to replace synthetic based materials with natural ones. In the field of surface active agents which are usually based mainly on expensive petrochemical precursors, the trend is that they be prepared from natural sources to be used as natural intermediate derivatives in several industries in order to reduce environmental pollution and health hazards combined with synthetic materials.
In Egypt, the annual consumption of edible oils and fats is about 850,000 tons/year. Eighty percent of this consumption is used in frying. After frying, 300,000 tons of overused oils remain as a waste available in huge quantities.
In this work, overused vegetable oils (sunflower and olein) were used directly to prepare ethoxylated nonionic surfactants using a conventional cheap and available catalyst which means a low cost of preparation and the produced derivatives will have an economic retrieval with good ability to replace the imported intermediate derivatives and at the same time the environment will dispose of one of its pollutants. The emulsion stability of the prepared nonionic fatliquors was evaluated as well as their application in leather fatliquoring. Also, the investigation of the resulting fatliquored chrome tanned leather was taken into consideration.
Materials and Methods
Materials
-
Refined, bleached and deodorized sunflower and olein oils were purchased from the local market and Egypt—Gulf Company for oil processing, respectively.
-
Overused sunflower and olein oils were obtained by heating 1 kg of fresh oil in a domestic aluminium fryer at 180 °C for 6 h a day for 10 days up to a total of 60 h of heating time. This heating process was carried out to obtain the overused individual oils with a simulated characteristic fatty acid composition like those obtained after frying processes in homes, restaurants and fried food factories but which might be unknown mixed oils.
-
An ethylene oxide gas cylinder was purchased from the Etico Gas Company (EL-Sharqia for gases, 10th of Ramadan Industrial City).
-
Potassium carbonate catalyst and all solvents and chemicals used were of a highly pure grade.
-
Local bovine full grain chrome tanned leather was used for the present investigation and obtained from Radio Tannery, Cairo, Egypt.
Methods
Fatty Acid Compositions
Overused oil methyl esters were prepared according to Ludde et al. [10]. The identification of the components of the fatty acid methyl esters was done using gas liquid chromatography on a Hewlett Packard Model 6890 chromatograph under the following conditions:
-
Separation was done on an INNO wax (polyethylene glycol) Model No. 19095 N-123, 240 °C maximum, capillary column 30.0 m × 530 μm × 1.0 μm, nominal flow 15 ml/min. with average velocity 89 cm/s and pressure 8.2 psi.
-
Column temperature was 240 °C with temperature programming: initial temperature 100–240 °C maximum with 10 °C rising for each minute and then hold at 240 °C for 10 min.
-
Injection temperature 280 °C, back inlet, with split ratio 8:1, split flow 120 ml/min., gas saver 20 ml/min.
-
Carrier gas was nitrogen with flow rate 15 ml/min.
-
Flame ionization detector temperature 280 °C.
-
Hydrogen flow rate 30 ml/min.
-
Air flow rate 300 ml/min.
Reaction with Ethylene Oxide
The reaction of the oil with ethylene oxide was carried out in the closed system shown in Fig. 1 following essentially the procedure of Wrigley et al. [11]. Overused sunflower or olein oil (5 g) and 3% potassium hydroxide (KOH) catalyst were mixed in the round-bottom flask of the system. The reaction mixture was stirred using a magnetic stirrer and heated under a nitrogen atmosphere to the desired reaction temperature (180 °C). The gentle flow of nitrogen was then halted and the nitrogen was replaced by ethylene oxide which was thereafter kept in harmony with the rate of the reaction.
After the required period of time (20 h) the flow of ethylene oxide was stopped and replaced by nitrogen to cool the reaction mixture.
Evaluation of the Ethoxylated Overused Oils (Fatliquors)
The prepared ethoxylated overused oils were evaluated by the following analysis:
-
a.
Chemical characteristics determination
Iodine value (IV), acid value (AV) and saponification value (SV) were carried out according to AOCS Official Methods Cd 3d-63, Cc 18-80 and Tl 1a-64 [12] respectively.
-
b.
FT-IR analysis
The change in the functional groups of overused sunflower and olein oils before and after ethoxylation was studied using FT-IR analysis; it was performed using Mattson 5000 FTIR, USA spectrophotometer with resolution 4 cm−1.
-
c.
Molecular weight determination by gel permeation chromatography (GPC)
The average molecular weights of overused sunflower and olein oils before and after ethoxylation were determined using a gel permeation chromatograph (GPC) coupled with an RI detector. Samples were dissolved in tetrahydrofuran and the GPC instrument used in the measurements was a modified HPLC, Waters 600 System Controller, 717 plus Autosampler. Columns: Phenomenex Phenogel 10 μm 500 A, 250 × 8 mm Phenomenex Phenogel 5 μm 50 A, 300 × 7.8 mm. Detection: Waters model 2410 Refractive index, ATTN = 16×. Eluent: dimethylformamide DMF (100% by vol). Flow rate: 0.7 ml/min. Temperature: 50 °C. Injection volume: 25 μl.
-
d.
Quantitative determination of ethoxylated products
The ethoxylated oils were quantitatively determined through:
-
1.
The number of moles of ethylene oxide (n) introduced into the overused oil was estimated depending on the difference in determined molecular weight of the sample before and after ethoxylation.
-
2.
The degree of ethoxylation was calculated by dividing the molecular weight of the group’s number of ethylene oxide added by the total molecular weight of the ethoxylated sample multiplied by 100, Eq. 1
where
- n :
-
Number of ethylene oxide moles,
- 44:
-
Molecular weight of ethylene oxide,
- R :
-
Molecular weight of the hydrophobe (fatty acid fraction).
-
e.
Hydrophile-lipophile balance (HLB)
The hydrophile-lipophile balance of nonionic fatliquors was calculated based on Eq. 2 [13].
where
- EO%:
-
is the percent of introduced ethylene oxide.
-
f.
Stability of ethoxylated overused oils (Fatliquors)
The stability of ethoxylated overused oils (fatliquors) emulsion was carried out through:
-
1.
Stability to acid
The following qualitative method [14] provides an indication of acid stability over an acid concentration range from 0.1 to 10.0%. Fatliquor solution (100 ml, 1.0%) was boiled under reflux. The appearance of the solution was recorded. 1 ml of sulfuric acid (10%) was added to the fatliquored solution and boiled for 15 min. The appearance of the solution was recorded so that, turbidity and/or separation of an oil indicated lack of stability. The process was repeated with 1, 3, 5, 7 and 10% acidity and stability was recorded as “stable” “partially decomposed” or “decomposed” at these concentrations indicated.
-
2.
Stability to alkali
The following method provides a qualitative test used to determine the “salting out” of a product as well as its chemical stability [14]. The fatliquor (1 g) was dissolved in 74 ml of water, 25 g of NaOH was added and the mixture was stirred to dissolve the NaOH and the appearance of the system was recorded. The mixture was then boiled under reflux for 15 min and the appearance was again recorded. The contents of the flask were cooled and the solution was decanted through a filter paper. The insoluble material was dissolved in 25 ml distilled water and titrated with dilute acid to a faintly acid end point of methyl orange. The mixture was finally boiled and allowed to cool to room temperature. If oil has been separated under this condition, the material is considered as being unstable. If the insoluble material has completely dissolved in acidified solution and showed no separation, the product is considered as being “stable”.
-
3.
Stability towards metallic ions
Solutions of different metallic salts (1.0%) were used to determine the stability of the fatliquors. The solution of the metallic salt (1%) was added dropwise to the solution of the fatliquor (10 ml, 1%) until the solution becomes turbid or precipitation occurred. The volume of the metallic salt used was recorded. The flask containing the mixture was heated to boiling until the solution became clear, additional metallic salt solution (up to a maximum of 10 ml) was used to attain the end point.
Fatliquoring Process
The leather samples were worked up in a wet-finishing process as follow: the leather pieces were first washed with water for about 15 min and water drained off. Then the neutralization process was carried out using 1% sodium formate and running the drum at ≈10 rpm for 15 min at 30 °C. Thereafter, 0.5% sodium bicarbonate was added and the drum was run for an additional 10 min at the same speed. The leather pieces had turned a greenish blue color with bromo cresol green throughout the whole thickness (pH 5.0–5.3). The neutralized leather pieces were washed and dyed with 5% acid dye for 30 min. Then, the fat-emulsion was added to the dyeing bath at room temperature. After the complete addition of the fat liquor, the drum was run at ≈10 rpm for 40 min at 30 °C. The leather pieces were washed with water for about 10 min, removed from the drum, sammed and left to dry in air through hanging up at room temperature. The dried leather pieces were used for the various physical properties investigations.
-
All percentages and chemical additives doses were calculated on the basis of leather weight (w/w).
Mechanical Measurements
Dumbbell-shaped specimens 50 mm in length and 4 mm (neck width) were used for measurements of mechanical properties (tensile strength and elongation at break). The measured data are the average of four transverse and longitudinal measurements for each sample. These tests were carried out using an Instron Machine (model 1195) [15]. The cross-head speed was controlled at 50 mm/min and the tests were done at room temperature (25 °C).
Scanning Electron Microscope
Experimental and control specimens were prepared as circular samples (10 mm) and then subjected to sputter coating of gold ions to produce a conducting medium (sputter coater-Edwards-Model S -150 A, Eng). A Jeol scanning microscope (Japan) JSM-T20 was used for the microscopic study.
Results and Discussion
Fatty Acid Composition
The fatty acids content of both overused sunflower and olein oils can be divided into two main groups as illustrated in Table 1.
It can be seen from Table 1 that, the saturated fatty acids contents of overused sunflower oil and overused olein oil were (23.94 and 51.58%) and unsaturated fatty acids were (76.06 and 48.41%). Also, it was noted that, the ratio of total saturated fatty acids of overused olein oil is almost equal to the total unsaturated fatty acids (1:0.94), while this ratio for overused sunflower oil was almost different by about three times (1:3.18). Oleic acid constitutes more than 93% and 41% of the unsaturated fatty acids of overused olein oil and overused sunflower oil, respectively, while linoleic acid of overused sunflower oil had much higher value (44.4%) compared with overused olein oil (3.08%). On the other hand, palmitic acid showed more than 49% and 10% of the total fatty acids, respectively.
Evaluation of the Ethoxylated Overused Oils (Fatliquors)
-
a.
Chemical Characteristics of the ethoxylated products
The main chemical characteristics of overused sunflower and olein oils before and after ethoxylation are shown in Table 2. It can seen from Table 2 that all the parameters (iodine, acid and saponification values) were found to decrease after the ethoxylation reaction which indicates consumption of free fatty acids and the unsaturation moiety during ethoxylation and that the ethoxylation reaction had proceeded successfully. When vegetable oils are subjected to high temperatures during heating or frying for long periods of time their characteristics are found to differ than those of fresh oils due to thermal oxidation. Oxidation results in the formation of different products like aldehydes, ketones, oxidized fatty acids and other oxidation products, which is confirmed by FT-IR spectra (Figs. 2, 3).
This difference appeared clearly during the ethoxylation reaction as it was found to proceed successfully using a conventional, cheap and available catalyst such as KOH, while it failed with fresh vegetable oils (triglycerides) using the same catalyst as mentioned in some of the literature due to the lack of active hydrogen [6, 16, 17] which indicates that the mechanism of ethoxylation of overused oils differs from than that of fresh oils.
-
b.
FTIR analysis
The spectrum of oil before frying showed characteristic absorption bands associated with common oil. Figures 2 and 3 show FT-IR spectra of overused sunflower and olein oils before and after ethoxylation with the FT-IR in the range of 400–500 cm−1. The stretching and bending absorption peaks at 3,004 and 723 cm−1 were given by olefinic (cis=CH). The strong absorption peaks at around at 2,900–2,850 cm−1 were assigned to CH3 and CH2 asymmetric stretching vibration. Also, the spectra showed stretching absorption bands at 1,652 and 1,467 cm−1 which corresponding to non-conjugated (cis C=C) bond and C–H scissoring, respectively. While the absorption band at ~1,726 cm−1 corresponds to the carbonyl C=O of ester group, [18, 19].
The major changes that happened in the FTIR chart after ethoxylation reaction are:
-
1.
Appearance of absorption bands at ~960 and 850 cm−1 which corresponding to the C–O–C group. These two bands indicate the presence of the ethoxylated product.
-
2.
Disappearance of the absorption band at ~3,005 cm−1, which represents the unsaturation moiety in the overused oils.
-
3.
Spectra before ethoxylation (Figs. 2, 3) show two stretching absorption bands at positions ~2,925 and ~2,855 cm−1 (C–H stretching) in which became one sharp band after ethoxylation this may be due to the increase in the number of CH–2 groups.
-
4.
Also, the spectra show broadening and increase in the intensity of the characteristic absorption band at ~3,470 cm−1 for O–H stretching vibration intermolecular hydrogen bonded single bridge compounds.
The previously mentioned changes are reported for both ethoxylated overused sunflower and olein oils. Generally, these characteristic bands were detectable in both ethoxylated overused oils which prove that the reaction proceeded using the conventional catalyst. These results are in a good accordance with a reference chart of Fiveash Data Management [20] and the previous results reported by Ovalles et al. [21].
-
c.
Molecular weight and quantitative determination of ethoxylated products
The number and weight-average molecular weights (M ns, M ws) of the both overused oils before and after ethoxylation are shown in Table 3.
The molecular weight highly increased after ethoxylation by about three times resulting in a higher molecular weight of polymerization, due to the introduction of a high number of ethylene oxide molecules into the overused oil as illustrated in Tables 3 and 4. Also, it seems that the average molecular weight of the two prepared fatliquored is relatively similar.
The number of ethylene oxide moles introduced into the overused oils, ethylene oxide percent (EO %) and hydrophile-lipophile balance (HLB) were calculated based on the difference in molecular weight of the overused oils before and after ethoxylation. It can be noted that both overused oils gave good results as the introduced number of ethylene oxide groups and EO% were found to be high (Table 4).
The stability and hydrophile–lipophile balance (HLB) concept of fatliquor emulsion are the most important factors in the evaluation of fatliquor. If the fatliquor is unstable, it cannot give a proper fatliquoring effect, due to the separation of fat from the emulsion before its fixing to the leather fiber. So that, it is necessary to evaluate the stabilization of prepared non-ionic fatliquors emulsions through:
-
d.
HLB concept
Hydrophile–lipophile balance (HLB) is one of the most important properties of surfactants. The HLB is an expression of the relative simultaneous attraction of a surfactant for water and/or for oil (or for the two phases), this mean that the HLB of a surfactant determines the emulsion type that tends to be formed [22]. Emulsions of water in oil “W/O” type indicate that the emulsion is in continuous liquid phase consists of oil and the dispersed particles consists of water, and vice versa in the case of oil-in-water type (O/W) emulsions [22].
Akoh and Nwosu [23] found that a low HLB value of 3–6 will promote or stabilize W/O emulsions, while intermediate values (8–13) will stabilize O/W emulsions, and high values such as (15–18) will act as a solubilizier. In general, the relationship between the water-solubility of emulsifiers and their HLB value is shown in Table 5, [13].
The prepared ethoxylated overused sunflower oil has a HLB value of 13.31 and ethoxylated overused olein oil has a HLB value of 12.8 (Table 4) i.e., they form an “O/W” emulsion type, and are simply dispersible in water. This means that a fineness of emulsion is formed since ethoxylated portions as well as a non-ethoxylated portion of the ester, which is present in the fatliquor, are emulsifiable. So that the prepared ethoxylated overused oils can form a stable emulsion and transfer from the aqueous bath to the leather and penetrate in it, which means that they can be used as good fatliquors.
-
e.
Stability of ethoxylated overused oils (fatliquors)
The emulsion of fat liquor which will be applied in leather fatliquoring must have a degree of stability against acids and alkalis (different pH), hardness of water (Ca and Mg salts) and different metallic salts (Cr, Al, Zn and Fe). Thus, the fat separated from the emulsions gets deposited inside the leather and fixed to the fibers. Separation of fats from the emulsion before it is fixed to the leather fiber is not desired which cannot give a proper fatliquoring effect [24]. Therefore, the stability of fatliquor in its water emulsion is an important factor in leather fatliquoring.
-
1.
Acid stability
The pH and hardness of water used in fatliquoring operations contribute to the instability of emulsion and eventually the emulsion breaks. If the fatliquor emulsion is unstable it leads to oily patches in leather and fats spew. The acid-stable emulsion is used also among other purposes for acid-dyeing operation. Concerning the acid stability, it can generally be observed that the prepared fatliquor (ethoxylated overused sunflower and olein oils) are stable up to 7% (olein) and 10% (sunflower) (about pH 2), Table 6. After that concentration, the fatliquor showed turbidity (partially decomposed). It can generally be concluded that the prepared fatliquors are stable at different concentrations of acid, so they can be used even in beam-house operation also.
-
2.
Alkali stability
The stability to alkali determines the physical “salting out” of a product as well as its chemical stability. This means that fatliquoring agents are considered as stable to alkali when over sufficiently high caustic alkali concentration is reached. It is observed that the fatliquors under investigation are highly stable towards alkaline medium (Table 6).
-
3.
Metallic ion stability
The application of fatliquor can be greatly influenced by its stability against metallic salts. The stability towards metallic salts was expressed as the volume of metallic ion solution required to cause turbidity in the fat-liquor solution. The specific metallic ions were chosen in this test for the following reasons:
-
1.
Calcium and magnesium: because they are responsible for water hardness, since one of the factors of unfavorable surfactant properties is the formation of insoluble sticky lime and magnesia soaps.
-
2.
Aluminum and iron salts: because they readily form insoluble salts. Also if the prepared fatliquors are stable to them, they will be useful for alumina and iron tanning.
-
3.
Zinc salts: since they are somewhat amphoteric in nature and are present in certain processing baths.
-
4.
Basic chromium sulfate: the most and important common tanning agent all over the world.
It can be observed from Table 6 that the prepared fatliquors had a satisfactory stability towards the chosen metal ions. The results obtained were expressed as the volume of metallic ion solutions required to make the fatliquor solution turbid.
Also, it was observed that, the prepared fatliquor based on overused sunflower oil is more stable than that of overused olein oil.
Mechanical Characters of Fatliquored Leather
The fatliquoring process was carried out on neutralized leather using 6%/100 g leather. Mechanical properties include the measurement of the tensile strength and elongation at break, have been given in the greatest consideration on the evaluation of fatliquored leather, because, it gives an indication of fiber lubricity. The mechanical properties were evaluated according to the standard Egyptian physical testing method of leather (ES-123) [25] (Figs. 4, 5).
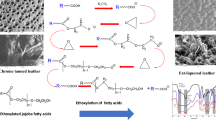
It is clearly seen from Figs. 4 and 5 that, the mechanical properties (tensile strength and elongation at break) of fatliquored leather are improved over those of the unfatliquored chrome tanned one. Also, it can be seen from Figs. 4 and 5 that the mechanical properties of leather treated with sunflower fatliquor was relatively better than that of olein oil fatliquor. The enhancement of mechanical properties is attributed to good lubrication of the fibers, as confirmed by scanning electron micrograph Figs. 6 and 7.
Scanning Electron Microscopy (SEM)
The Scanning Electron Micrograph is one of the important tools for the evaluation of fatliquored leather because SEM looks deeply into the hide fiber structure, and will show the effect of fat liquor on the fiber and grain surface. From microscopical analysis using SEM (Figs. 6 and 7) the treated leathers by the prepared fatliquors have smooth fibers and a soft grain, and a modified handle because the prepared fatliquors protect the surface of the fibers with a thin film of lubricant resulting in a soft leather. Scanning electron micrographs of the cross-section (×1,000) of the leather fibers before (Fig. 7a) and after fatliquoring (Fig. 7b, c) showed a significant lubrication of the fiber bundles and a surface grain of fine and loose texture.
Also, the grain surface (×50) of the treated leather exhibits no cracking (firm grain) and no fat-spew (Fig. 6a–c). This refers to the low percentage of free fatty acid in the prepared fatliquors, where, high free acid tends to cause spewing and leads to the production of narsh and cracky leather. In addition, a vivid shade of the fatliquored leather was obtained, i.e., the treated leather has a uniform shade. This is due to the prepared fatliquor has a lower iodine value.
The iodine value has a great effect on the color shade, where a higher iodine value indicates that the fatliquor will give yellow eventually when applied to white leathers due to light (UV light) and temperature may breakdown neutral fat into free fatty acids [26], resulting in fat-spews as well as possible fogging, also, polymerization and oxidation may take place, resulting in yellowing and the production of bad odors. The yellowing of fatliquors especially interferes with light shades.
Conclusions
The results of this work reveal that:
-
1.
Overused vegetable oils can be ethoxylated using a conventional catalyst giving a valuable surfactant with a high number of introduced ethylene oxide moles.
-
2.
Ethoxylating overused sunflower oil gave better results than ethoxylating overused olein oil, but still both gave good surfactants.
-
3.
The prepared ethoxylated fatliquors have superior properties of better penetration into the leather (due to its HLB).
-
4.
The aqueous emulsions have a remarkable stability to the salts and acids encountered in the leather manufacturing process.
-
5.
The mechanical properties (tensile strength and elongation at break) and texture of leather were markedly improved by the introduction of fatliquors.
-
6.
It has been demonstrated to a great extent that the prepared ethoxylated fatliquors based on overused vegetable oils are highly suitable for fatliquoring of chrome tanned leather.
-
7.
In conclusion, it should be pointed out that the Egyptian tanners can easily reach the figure required in the local specifications for such types of leather.
References
Cox MF (1994) Ethylene oxide-derived surfactants. In: Arno Cahn(ed) Proceedings of the 3rd world conference on detergents: global perspectives, pp 141–146
Schwartz AM, Perry JW (1949) Surface active agents their chemistry and technology. Interscience publishers, New York
Shin HS (2001) Manufacturing method of leather fat liquor by esterification and oxidation of fatty acid and monomer. Repub. Korean Kongkae Taeho Kongbo KR 2001 94,667 (Cl. D06N3/00), November 2001
Samsonova AM, Khlystova LG, Zurabyan KM (1993) Use of ethoxylated fats for fatliquoring of various types of leather. Kozh Obuvn Prom-st 8:20–21
Makdisi RichardS (1991) Tannery wastes definition, risk assessment and clean up options, Berkeley, California. J Hazard Mater 29:79–96
Alexander K, Donahue V (1990) Cleaner technologies in the tanning industry. EPA environmental challenge of the 1990s. International conference on pollution prevention: clean technologies and clean products pp 19–31, 10–13 June
Burgess D (1993) General aspects of fatliquoring: an introduction to the application and chemistry of fat liquoring. J Soc Leather Technol Chem 78:39–43
Heidemann E (1993) Fundamentals of leather manufacturing, Chap 15. In: Eduard Roether KG (ed), Darmstadt, ISBN: 3-7929-0206-0
Alexander KTW, Convington AD, Stosic RG (1993) The production of soft leather. Part 2. Drying and stress softening. J Am Leather Chem Assoc 88:254–269
Ludde FE, Barvord RA, Reimenschnider RW (1960) Direct conversion of lipid components to their fatty acid methyl esters. J Am Oil Chem Soc 73:447–451
Wrigley AN, Smith FD, Stirton AJ (1957) Synthetic detergents from animal fats. VIII. The ethenoxylation of fatty acids and alcohols. J Am Oil Chem Soc 34:39–43
AOCS (1996) Official Method Cd 3d-63, Cc 18-80 and Tl la-64. Official methods and recommended practices of the American Oil Chemists’ Society, AOCS, Champaign
Fujimoto T (1985) New introduction to surface active agents. Part 3. Chapter 3. Sanyo Chemical Industries Ltd., Japan, pp 187–218
Harris JC (1946) ASTM, Bulletin
Stevenson WTK, Sefton MV (1988) The equilibrium water content of some thermoplastic hydroxyalkyl methacrylate polymers. J Appl Polym Sci 36(7):1541–1553
Cox MF, Weerasooriya U (1997) Methyl ester ethoxylates. J Am Oil Chem Soc 74(7):847–859
Hama I, Okamoto T, Nakamura T (1995) Preparation and properties of ethoxylated fatty methyl ester nonionics. J Am Oil Chem Soc 72:781–784
Guillen MD, Cabo N (1997) Infrared spectroscopy in the study of edible oils and fats. J Sci Food Agric 75:1–11
Che Man YB, Setiowaty G (1999) Multivariate calibration of Fourier transform infrared spectra in determining iodine value of palm oil products. Food Chem 67:193–198
Fiveash Data Management (1997) FDM FTIR spectra library of surfactants
Ovalles C, Bolivar R, Cotte E, Aular W, Carrasquel J, Lujano E (2001) Novel ethoxylated surfactants from low-value refinery feed stocks Fuel 80:575–582
Griffin MC (1965) Emulsions in: encyclopedia of chemical technology, vol 8. Interscience publishers, New York pp 117–154
Akoh CC, Nwosu CV (1992) Emulsification properties of polyesters and sucrose ester blends II: alkyl glycoside polyesters. J Am Oil Chem Soc 69(1):14–19
Puntener A (1996) The influence of fatliquors on the light fastness of dyed leather. J Am Leather Chem Assoc 91(5):126–135
Egyptian Standard Specifications (1986) Physical methods of leather, E.S 123
Selvarangan R, Vijayalakshmi K, Raghunatha RAOD (1975) Leather Sci 22:265–276
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nashy, ES.H.A., Abo-ELwafa, G.A. Highly Stable Nonionic Fatliquors Based on Ethoxylated Overused Vegetable Oils. J Am Oil Chem Soc 88, 1611–1620 (2011). https://doi.org/10.1007/s11746-011-1809-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-011-1809-9