Abstract
Jojoba oil is of immense importance for industrial applications. There are a lot of published articles concerning its various uses in cosmetics, detergents, surfactants and lubricants. Therefore, this work was devoted to exploring its application for further use in the leather industry as a fat-liquoring agent. The fat-liquoring process is one important step in leather manufacturing, with the intention of obtaining leather of full, soft handle, flexibility, and pliability as well as improving its mechanical properties. The study involved preparation of jojoba fat-liquor via a sulfitation process. An improvement of the sulfitation process based on combined SO3 content was achieved under phase transfer catalysis (PTC). Two differently prepared types of phase transfer catalyst of phosphonium and ammonium types were investigated, namely, benzyl tri-phenyl phosphonium chloride (BTPP) and tri-ethyl benzyl ammonium chloride (TEBA). The fat-liquored leather led to an improvement in its mechanical properties such as tensile strength and elongation at break. In addition, a significant enhancement of the texture of the treated leather by jojoba fat-liquor as indicated in the scanning electron microscope (SEM) images was observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the tanning industry, various chemical and mechanical treatment processes are applied to produce finished leather. The process of tanning hides to leather has the primary purpose of preserving the fibre structure from any damage due to bacterial attack. Chrome-tanned leather in its wet condition (wet blue) is relatively more flexible than vegetable tanned leather. As the water is removed during the drying process, cohesion of the fibres take place resulting to hard intractable leather which is quite difficult to rehydrate [1]. In addition, when chrome tanned leather when dries out, it becomes bony, hard and thus will be rendered unsuitable for use in most purposes. Besides, its colour turns darker and becomes less appealing.
The fat-liquoring process is one important step in leather manufacturing by introducing a lubricant into the leather. The fat-liquor keeps the fibres apart during drying and reduces frictional forces within the fibre weaves thus allowing the fibres to move laterally over each other. Also, the incorporation of fatty matter into leather reduces the damaging effect of air oxidation [2, 3].
In a previous work, the authors studied the sulfation and sulfonation of fatty materials [4]. Oleum, sulfuric acid, SO3 gas and recently chlorosulfonic acid as well as sodium bisulfite have been proposed as sulfiting agents [5, 6].
Originally, jojoba oil was used in fried foods and bakery products, but these uses showed some potential and yet had some definite limitations [7]. Currently, Jojoba oil is commonly used in industrial applications e.g. cosmetics, detergent, surfactant and lubricants [8–10].
This work was devoted to study the sulfitation of jojoba oil with sodium bisulfite under phase transfer catalyses (PTC) in comparison with sulfuric acid as a sulfating agent, as well as its utilisation in fat-liquoring of leather. Evaluation of the resulting fat-liquored chrome tanned leather was taken into consideration.
Materials and Methods
Materials
-
Representative samples of developed jojoba seeds were collected from 2009 production in Ismailiya, Egypt.
-
Sodium bisulfite and sulfuric acid (98.5%) were pure chemical grades.
-
Benzyl tri-phenyl phosphonium chloride (BTPP) and tri-ethyl benzyl ammonium chloride (TEBA) were prepared as described below.
-
Local commercial full grain chrome tanned leather was used for the present investigation and obtained from the Radio Tannery, Cairo, Egypt.
Methods
Preparation of TEBA
TEBA was prepared by refluxing 5 g (6.9 ml, 49.4 mmol) of triethylamine and 6.3 g (5.7 ml, 49.8 mmol) of benzyl chloride in 25 ml of acetonitrile for 1 h. The mixture was then left to cool to room temperature and diluted with diethyl ether. After complete precipitation, the product was separated on a suction filter, and recrystallised with dry ethyl acetate /ethanol mixture (1:1) to give pure TEBA as white crystalline and strong hygroscopic solid. The yield was 95% (m. p., 189 °C).
Preparation of BTPP
BTPP was prepared by refluxing 9.85 g (37.6 mmol) triphenyl phosphine with 4.8 g (38 ml mol) of benzyl chloride in 20 ml acetonitrile for 1 h. After cooling to room temperature, the product was precipitated by means of diethyl ether/ethyl acetate through dissolution/precipitation technique. The yield was 13.39 g (90%) of slightly hygroscopic white crystals.
Analysis of Jojoba Oil
-
Oil extraction: the Soxhlet extraction technique was utilised for extracting the oil from the dried jojoba seeds using redistilled n-hexane according to the method described by American Oil Chemists Society Methods AOCS [11]
-
Physical and chemical characteristics: Jojoba oil was analysed for its physical and chemical characteristics according to the American Oil Chemists Society Methods, (AOCS 1998) [11].
-
Fatty acid composition: Jojoba oil methyl esters were prepared according to AOCS method [12]. Determination of jojoba fatty acids composition was performed using a Hewlett Packard HP 6890 gas chromatograph, operated under the following conditions: Detector, flame ionisation (FID); column, capillary, 30.0 m × 530 μm, 1.0 μm thickness, polyethylene glycol phase (INNO Wax); N2 with flow rate, 15 ml/ min with average velocity 89 cm/s (8.2 psi); H2 flow rate, 30 ml/min; air flow rate, 300 ml/min; split ratio, 8:1, split flow, 120 ml/min; gas saver, 20 ml/min. Detector temperature, 280 °C; column temperature, 240 °C; injection temperature, 280 °C. Programmed temperature starting from 100 °C to reach a maximum of 240 °C was used for eluting the fatty acid methyl esters. The identification of the peaks was made as compared with chromatograms of standard fatty acids methyl esters (Sigma, USA).
FT-IR Analysis
The change in functional groups of oils were studied using FT-IR analysis, it was performed using a Mattson 5000 FTIR, USA spectrophotometer with a resolution of 4 cm−1.
Sulfation and Sulfitation Processes
Sulfation and/or sulfitation of the oil (80 g) were carried out in a three-necked flask fitted with a stirrer, a thermometer and also with an inlet for the addition of the reagents.
-
a.
Sulfating agent (H2SO4, 20% of the oil weight) was added dropwise at intervals with slow stirring while maintaining the temperature below 30 °C during the addition. The overall reaction time was 3 h.
-
b.
The sulfated oil was washed by 10% sodium chloride at ambient temperature and neutralised with ≈40% sodium hydroxide with agitation for 30–40 min.
-
c.
The sulfitation reaction was carried out using 40% sodium bisulfite (20% of the oil weight) and catalytic amount of PTC (12 mg of TEBA or BTPP). 80 g of the oil was weighed into the flask and the flask was dipped in a water bath, the temperature was adjusted to 50 °C with continuous stirring for 5 h.
-
d.
The sulfated and sulfited products so obtained were analysed by standard the ISI method [13] and official methods [14]; taking into consideration that the degree of conversion was estimated mostly on combined SO3 content.
-
e.
The product was prepared at pH 7 to a concentration of about 60–70% prior to its use as a fat-liquor.
Catalytic Efficiency of PTC
The catalytic efficiency “Ce” was calculated on the basis of the relation:
Fat-Liquoring Process
The leather pieces were first washed with water for about 15 min and the water drained off. Then the neutralisation process was carried out using 1% sodium formate and running the drum for 15 min at ≈10 rpm at 30 °C. Thereafter, 0.5 % sodium bicarbonate was added and the drum was run for an additional 10 min at the same speed. The leather pieces had a greenish blue colour with bromo cresol green throughout the whole thickness (pH 5.0–5.3). The neutralised leather pieces were washed with water and dyed with 5% acid dye for 30 min. Then, 6% fat-emulsion was added to the dyeing bath at room temperature. After complete addition of the fat liquor, the drum was run at ≈10 rpm for 40 min at 30 °C. The leather pieces were washed with water for about 10 min, removed from the drum, horsed up over-night, then sammed, set out and left to air dry by hanging up at room temperature for 2 days. The dried leather pieces were used for the various physical properties investigations.
Mechanical Measurements
Dumbbell-shaped specimens 50 mm in length and 4 mm (neck width) were used for the measurements of mechanical properties (tensile strength and elongation at break). The measured data are the average of four transverse and longitudinal measurements for each sample. These tests were carried out using an Instron Machine (model 1195) according to Egyptian standard methods, ES-123 [15]. The cross-head speed was controlled at 50 mm/min and the tests were done at room temperature (25 °C).
Scanning Electron Microscope
The experimental and control samples were prepared as circular samples (10 mm) and then subjected to sputter coating of gold ions to prepare a conducting medium (sputter coater-Edwards-Model S-150 A, Eng). A Jeol scanning microscope (Japan) JSM-T20 was used for the microscopic study.
Visual Properties and Outlooks
In addition to, the chemical analysis and physico-mechanical characters of the fat-liquored chrome tanned leather, the leather samples were investigated with respect to general appearance, colour shade and its evenness as well as to its handle softness and grain.
Results and Discussion
Characterisation of Oil
Physical and chemical properties of the oil are shown in Table 1. Local jojoba oil has a relatively high iodine value (IV) 86.0 mg I2/g oil, saponification value (SV) 92.0 mg KOH/g oil and ester value (EV = SV − AV) 91.0 mg KOH/g oil. The other characteristics like the unsaponifiable matter, the acid value (AV) and the acetyl number were relatively low. These data indicated that the oil has a relatively high ratio of unsaturation centres and ester groups, while it has a low ratio of free fatty acids (difference between SV and AV). These results are in agreement with the previous studies reported by Allawzi et al. [16].
Fatty Acid Composition
The identified various fatty acids can be divided into two main groups as illustrated in Table 2. As shown in Table 2, the unsaturated fatty acids content is about 97% of the total acid content, where eicos-11-enoic is the main constituent in this group (64.64%). It is also clearly seen that, the saturated fatty acid content was found to be 2.27% of the total acid content and the ratio of the saturated to unsaturated acids was found to be (1:43). These results are in a good accordance with the previous results reported by Moustafa [17] and Binman et al. [18].
FT-IR
FT-IR spectra of jojoba oil show stretching and bending absorption peaks at 3,003 and 721 cm−1 which correspond to olefinic (=CH) group. The spectra also show other stretching absorption bands at 1,648, 1,739 and 1,172 cm−1 which correspond to (C=C) bond, C=O and C–O of ester group respectively. The bands of CH3 and CH2 groups appear at 2,925 and 2,855 cm−1 (Fig. 1a).
Sulfation/Sulfitation Reaction
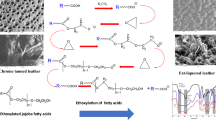
It should be noted that SO3 content of the resulting constituents is of great significance, so that the degree of conversion was estimated mostly as SO3 content. In spite of the SO3% content obtained by H2SO4 (13.3%) is higher than that obtained by NaHSO3 (3.2%, without catalyst), but it has an undesirable odour and colour. Therefore, the present work aims at improving the sulfitation reaction by using PTC. It is clearly seen from Fig. 2 that based on the combined SO3% content, the efficiency of sulfitation reaction can be arranged in the following descending order: BTPP > TEBA > non-catalysed system.
Sulfitation reaction mechanism is different in its chemistry to sulfonation reaction and the sodium salt of sulfonic acid is produced as opposed to a sulfate ester. The mechanism of sulfitation reaction is carried out by first partially oxidising the system by blowing air or aeration to increase the reactive centres which easily react with NaHSO3, as illustrated in Schemes 1, 2.
It can be mentioned from Scheme 2 that, the hydrophilicity of the sulfited product by NaHSO3/PTC system increased due to the formation of hydroxyl groups (OH), as confirmed by FT-IR (Fig. 1b).
Mechanism of PTC
It is obvious from the obtained results shown in Fig. 3 that the combined SO3 is improved in the presence of PTC than that in its absence (non-catalysed system).
It is also clear that the catalytic efficiency “Ce” of BTPP is higher than TEBA, as shown in Table 3.
The main principle of PTC system is the continuous formation of lipophilic ion pairs (Q+/HSO3 −) of sulfite anions derived from NaHSO3 with the lipophilic cations (Q+) supplied by the PT-catalyst. These anions (Q+/HSO3 −) are able to enter the organic media (oil) where the required reaction takes place. Due to the lipophilicity of the catalyst, the surface tension of the phase-boundary between the two separated phases [19, 20] are reduced, as shown in Scheme 3.
It was found that BTPP is more effective than TEBA. This could be attributed to the superior thermal stability of BTPP than TEBA. The markedly higher stability of BTPP is based on the great stability brought by the extensive delocalisation of electrons by resonance through three phenyl rings (12 resonating structures). On the other hand, the positive charge in TEBA is only localised on the nitrogen atom (Fig. 3).
FT-IR of the Sulfited Product
Figure 1b showed characteristic absorption peaks at 1,175 and 1,213 cm−1 corresponding to symmetric –SO3H, and at 1,010 and 1,055 cm−1 corresponding to asymmetric –SO3H vibrations. It can also be seen from Fig. 1b that, a broad band appeared at 3,447 cm−1 which corresponds to the resulting hydroxyl group.
Strength Properties of Fat-liquored Leather
Fat-liquoring process was carried out on neutralised leather using about 5% per 100 g leather. Strength properties have been given the greatest consideration in evaluating fat-liquored leather because they gives an indication of fibre lubricity. The mechanical properties were evaluated according to the Egyptian standard specification of leather [15].
It is clearly seen from Fig. 4 that the tensile strength and elongation at break of treated leather via jojoba fat liquor were relatively higher than that of commercial fat-liquor (TRUPONOL® SF, oxidised sulfited fatty acid ester, pH 6.3) commonly used in Egyptian tanneries.
The enhancement in mechanical properties of treated leather is due to a good lubrication of fibres. The sulfited portion of oil during liquoring of chrome tanned leather is chemically bound to the leather fibres, i.e. interacts with active centres in the collagen molecules of leather fibres, while the emulsified portion is mainly located between the fibres.
Scanning Electron Microscopy
SEM looks deeply into hide fibre structure and shows the effect of fat liquor on fibre and grain surface. SEM of the cross-section (500×) of leather fibre before and after fat liquoring showed a significant lubrication of fibre bundles; Fig. 5a, b. Also, the grain surface (100×) of the fat liquored leather exhibits a soft grain without any fatty-spew; Fig. 6a, b.
Visual Properties and Outlook
Leather, especially that used in footwear and leather clothes is in great demand for its unique properties with regard to the comfort to the wearer. Footwear designers and all leather industries always look first at the appearance of the leather. So in addition to the analytical data outlined and discussed above, the fundamental criteria for the evaluation of a fatty matter and its suitability for leather fat-liquoring are mainly shown from the visual properties of the treated leather [6], including its handle, firmness of grain, shade of colour and its uniformity as shown in Table 4.
Conclusions
The results from this work demonstrate that:
-
Phase transfer catalysis enhances the sulfitation process better than the traditional one in the absence of PTC.
-
Phosphonium type PTC has a higher activity than the ammonium type PTC.
-
It is recommended to use NaHSO3 as a good sulfiting agent in the presence of PTC.
-
An improvement in the texture and strength properties of fat-liquored leather using sulfited jojoba oil has been achieved.
References
Burgess D (1993) General aspects of fat-liquoring: an introduction to the application and chemistry of fat-liquoring. J Soc Leather Tech Chem 78:39–43
Kronick PL (Haverford PA) (1998) Use of polymerizable oil for leather fat liquor. US Patent 5, 853, 427
Alexander KTW, Convington AD, Stosic RG (1993) The production of soft leather. Part 3. Measuring softness. J Am Leather Chem Assoc 88:254–269
Kulkarni AS, Khotpal RR, Mehtarl RL, Bhakare HA (1991) Studies in sulphation and sulphonation of some semi-drying oils. Paint India 7:41–42
Lorant D, Heorghe S, Ewalld M (1980) Structura Chimica Si Compozitia Trigliceridelor Sulfonate-Sulfatate, Cu Trioxid De Sulf Gazos. Rev. Chem. Bucharest. Revista Dechimie 31(1):14–20
Nashy EHA, EL-Sayed NH, El-Morsy SS (2002) Studies on water dispersible products based on sulfitation of wool wax by trichlorosilyl sulphite reagent. J Soc Leather Tech Chem 89:212–218
Hamm DJ (1984) Use of jojoba oil in foods. J Food Sci 49:419–426
Magdassi S, Shani A (1990) Surface activity of quaternary ammonium salts derived from jojoba oil. J Am Oil Chem Soc 67(9):605–606
Bhatia V, Alka Chaudhry G, Siva S, Bisht R, Meenu K (1990) Modification of jojoba oil for lubricant formulation. J Am Oil Chem Soc 67(1):1–7
Katoh M, Tayuchi M, Kunimoto T (1998) Current Status of Jojoba Oil Utilization on Cosmetics in Japan. In: Proceeding of the seventh international conference on jojoba and its uses. Phoenix, Arizona, USA, January, 17–22
Firestone D (1998) Official methods and recommended practices of the American Oil Chemists Society. The fifth edition includes all changes 1993–1997, Edition Analytical methods, Champaign-Illinois
Official and Tentative Methods of the American Oil Chemists Society (1985) Third edition. American Oil Chemists Society. 508 South Sixth Street, Champaign-Illinois
Indian Standard Institute (ISI) (1971) Specification for sulfated oils for leather fat-liquoring, No. IS: 6357
Official Methods Analysis of Association of Official Analytical Chemists, (1990) Published by the Association of Official Analytical Chemists, 15th edn. Nineteenth Street, Suite 210, Arlington, Virginia, USA
Egyptian Standard Specifications (1986) Physical methods of leather. E.S 123
Allawzi M, Abu-Arabi MK, Al-Zoubi HS, Tamimi A (1998) Physico-chemical characteristics and thermal stability of Jordanian Jojoba oil. J Am Oil Chem Soc 75(1):57–62
Moustafa N (1989) Chemical and biological evaluation of some new oily seeds. Ph.D. Thesis degree in Agric, Cairo University, Cairo
Binman S, Belfer S, Shani A (1996) Functionalization at the double-bond region of Jojoba Oil 7-chemical binding of jojoba liquid was to polystyrene resins. J Am Oil Chem Soc 73(9):1075–1081
Bokman F, Bohman D, Siegbahn HOG (1992) Acta Chem Scand 46:403–405
Makosza M, Fedorynski M (1987) Adv Catalysis 35:375–422
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nashy, ES.H.A., Megahed, M.G. & Abd EL-Ghaffar, M.A. Preparation of Fat-Liquor Based on Jojoba Oil Under Phase Transfer Catalysis. J Am Oil Chem Soc 88, 1239–1246 (2011). https://doi.org/10.1007/s11746-011-1778-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-011-1778-z