Abstract
The effect of chlorophyll in photo-oxidation of virgin coconut oil (VCO) during production and storage was studied. Photo-oxidation during VCO production was performed under accelerated conditions using fluorescent lights (4,000 lux) for 8 h. Peroxide values (PVs) and chlorophyll contents of the samples were measured at 1 h intervals. To compare the photo-oxidation during storage, VCO samples were separately stored under ambient and normal room light intensity (380–400 lux) for up to 4 months and PVs were measured weekly. The results indicated that relatively low light intensity during the settling stage of VCO production had no significant effect on photo-oxidation. Photo-oxidation of VCO, however, was observed during storage when exposed to high intensity fluorescent light. The degradation of chlorophyll content in VCO was evident during exposure to a high fluorescent light intensity which was accompanied by high lipid peroxide accumulation. A significant negative correlation between PV and chlorophyll content was found in VCO without light protection. The storage of VCO significantly increased the PV after 10 weeks. This study confirmed that chlorophyll which is naturally present in VCO even at a very low level (less than 0.1 ppm) could initiate a photo-oxidation reaction leading to quality deterioration during prolonged storage or display at retailers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the Philippine National Standards, virgin coconut oil (VCO) is defined as an oil that is obtained from the fresh, mature kernel of the coconut by mechanical or natural means, with or without the use of heat, without undergoing chemical refining, bleaching or deodorizing, and which does not lead to an alteration of the nature of the oil [1]. The common methods to produce VCO, especially in Indonesia, are wet-milling methods which do not involve thermal or chemical treatment. The absence of heating and chemical treatment of the oil allows it to have a unique taste and has been claimed to have numerous beneficial health effects. Thus, VCO production has shown dramatic growth in the market.
Some of the VCO products are packed in transparent plastic bottles only and some others with additional light protection, i.e. sealed paper boxes. During prolonged storage or display at retailers, VCO may undergo quality deterioration leading to rejection by consumers especially due to the presence of a rancid flavor and odor. In our previous study, we found that within the period before the expiration date, 11 out of 18 commercial brands of VCO marketed by retailers around Yogyakarta-Indonesia fell short of the quality standards. Objectionable odor and taste were clearly detected by panelists on samples having a peroxide value (PV) of 1.0 mequiv/kg or higher.
Hydrolysis and oxidation are the two main reactions which could result in the deterioration of oils. According to Scrimgeour [2], hydrolysis can be catalyzed by an acid, base, or lipase, but it also occurs as an uncatalyzed reaction between fats and water dissolved in the fat phase at suitable temperatures (~250 °C) and pressures (2–6 MPa). However, oxidation is mostly responsible for much more of the deterioration of fats and oils than hydrolysis [3]. Oxidation reactions influence the chemical, sensory and nutritional properties of edible oils and thus play an important role in determining their use and shelf-life [4]. Ultimately, this oxidative deterioration could lead to significant losses for producers, retailers, and consumers.
Oxidation reactions can be initiated by either diradical triplet oxygen or non-radical singlet oxygen. The singlet oxygen can be formed in foods from triplet oxygen by photosensitized reactions [5]. The triplet oxygen oxidation also known as auto-oxidation through the free radical chain reaction via the attack on the alpha methylene of the carbon double bonds of unsaturated fatty acids is a slow process. On the other hand, the singlet oxygen oxidation also known as photo-oxidation involves the direct attack of the extremely electrophilic singlet oxygen on the unsaturated fatty acids, resulting in the generation of peroxy radicals and, ultimately, hydro-peroxides is a very fast reaction. The reaction rate of the photo-oxidation is at least 1,000–1,500 times faster than auto-oxidation [4].
Photo-oxidation occurs when there is light, triplet oxygen and photo-sensitizer [5]. Chlorophyll and its derivatives are common sensitizers that act as promoters of photo-oxidation in vegetable oils [6]. After absorption of energy from light, chlorophyll can transfer it to triplet oxygen to form the more reactive singlet oxygen, which subsequently reacts with not only unsaturated fatty acids, but also with other electron-rich food components including vitamins and amino acids [5]. Lee et al. [7] reported that vegetable oils containing natural sensitizers, such as chlorophyll at 0.065–1.33 ppm, can produce singlet oxygen and initiate the photo-oxidation reaction. These oxidation products catalyze the oxidation chain reaction, resulting in the oils’ quality deterioration. The objective of this study was to determine the presence of chlorophyll and its effect on photo-sensitized oxidation of VCO during production and storage.
Materials and Methods
Materials
A freshly prepared VCO obtained from a local VCO producer was used in this experiment. It was made from the fresh, mature kernel of coconut (12–14 months), which was grated and made into coconut milk obtained by adding boiled water (1:1). The coconut milk was left to settle for 1 h to separate the cream from the skim. The cream was taken, stirred, and left for 5 h to allow the formation of a layer of oil between the dregs and the water. The oil was then removed and left to settle for 24 h, after which it was filtered to separate the gum from the oil (VCO). The VCO was used without any further refining process. A chlorophyll a standard was purchased from Sigma Chemical Company (St. Louis, MO, USA) and all chemicals used were of analytical grade.
Photo-oxidation Test during VCO Production
To evaluate the effect of photo-oxidation during VCO production, especially during settling which is used to allow oil separation overnight (24 h), two different jars were used, one transparent and the other wrapped in aluminum foil. After filtration to remove the gum from the oil, a portion of the VCO product was placed in a number of transparent serum bottles with rubber caps, while another portion was protected from light by wrapping the serum bottles with aluminum foil. Photo-oxidation was performed under accelerated condition using fluorescent lights with an intensity of approximately 4,000 lux and samples were exposed to that light for up to 8 h at room temperature (30 ± 1 °C). PVs and chlorophyll contents of the samples were measured at 1 h interval. PVs were determined according to the method II.D.13 proposed by the IUPAC Official Method 2.501 [8], and chlorophyll contents of the samples were measured at 663.8 nm using a spectrophotometer (UV-1650 PC, Shimadzu, Japan). The chlorophyll a standard was used to calculate the measurement [9].
Photo-oxidation Test During VCO Storage
To evaluate the effect of photo-oxidation during VCO storage at room temperature (30 ± 1 °C), two sets of VCO samples were separately stored either in the dark in a cupboard or in a normal room light intensity (380–400 lux) for a period of 4 months. Each set consisted of 51 VCO samples (15 ml) in 20 ml transparent serum bottles with rubber caps. The head space in the bottles was just air without introducing new air during storage. Triplicate samples of each set were taken for PVs analysis weekly.
Statistical Analysis
Triplicate samples of VCO for each treatment were taken. Each sample was analyzed individually in duplo. Statistical analyses of the data were performed by one-way analysis of variance (ANOVA) and Duncan’s multiple range tests using Microsoft Excel 2007. A linear regression analysis was used to find correlation coefficients between PVs and chlorophyll contents in VCO photo-oxidized at 4,000 lux.
Results and Discussion
Photo-oxidation during VCO Production and Chlorophyll a Content
Photo-oxidation is a reaction that initiates quality deterioration in fatty products, including VCO. Intense light exposure in photo-oxidation induces an increased rate of peroxide formation in the oil [10]. The PV is a measure of the concentration of peroxides and hydro-peroxide forms in the initial stage of lipid oxidation. The number of peroxides present in vegetable oils reflects its oxidative level and thus its tendency to become rancid [4, 11, 12].
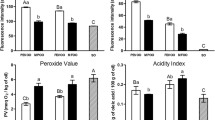
Figure 1 shows that the effect of light exposure in PV changes. Light exposed VCO showed very significantly higher PV (P < 0.01) than the light protected samples, both at the settling stage with (treatment B) or without (treatment D) light protection. Light exposure, however, had no significant effect on PV changes (P > 0.05) in light protected VCO samples (treatment A and C).
Pattern of PV changes in VCO exposed to fluorescent light (4,000 lux) for up to 8 h. (Samples from a jar which was wrapped in aluminum foil during settling and placed in serum bottles with A or without B light protection; Samples from transparent jar during settling were placed in serum bottles with C or without D light protection)
In this experiment, the settling stage was performed at room temperature with light intensity at about 380–400 lux. A short duration (24 h) of exposure to relatively low light intensity (less than 400 lux) during the settling stage of VCO production was not capable of significantly initiating the photo-oxidation reaction. The PV of the VCO obtained from the settling stage without light protection, i.e. transparent jar was 0.12 ± 0.022 mequiv/kg. It was not significantly different (P > 0.05) from the VCO obtained from the light protected jar, i.e. the jar which was wrapped in aluminum foil, which was 0.11 ± 0.001 mequiv/kg. It only takes approximately 3 h of light exposure (4,000 lux) on freshly prepared VCO without light protection to increase the PV to 1.0 mequiv/kg, while the light protected samples never reached that value.
This result indicated that light exposure with relatively high intensity was very effective at initiating photo-oxidation. Oil oxidation is accelerated by light, especially in the presence of sensitizers such as chlorophylls. Choe and Min [6] reported that sensitizers in the singlet state absorb light energy very rapidly, in picoseconds, and become excited. Excited singlet sensitizers can return to their ground state via emission of light, internal conversion or intersystem crossing results in excited triplet state of sensitizers. The excitation energy of triplet sensitizers can be transferred onto adjacent triplet oxygen to form more active singlet oxygen. Electrophilic singlet oxygen can directly react with high-electron-density double bonds producing both conjugated and nonconjugated hydro-peroxides [5, 6, 13, 14]. This was the reason for the increasing PVs in VCO samples without light protection. The serum bottle which was unprotected from light enables the sensitizer to absorb light energy, initiate oxidation, and produce hydro-peroxides. The quantity of hydro-peroxides formed during the photo-oxidation is directly proportional to the total amount of light absorbed [4, 10].
Figure 1 also shows that the PVs decreased after 5 h of fluorescent light exposure in treatment D (VCO samples in the serum bottles without light protection from the settling stage with transparent jar). In this condition, hydro-peroxides will have decomposed into secondary oxidation products. If the rate of hydro-peroxide decomposition is greater than that of hydro-peroxide formation in the oil, it could have very low hydro-peroxide content. The oil at the end of the oxidation period could have a low PV although it is highly oxidized and has a rancid flavor [11, 12]. In this result, the low rate of hydro-peroxide formation may be due to the degradation of chlorophyll content that acts as sensitizer in VCO samples.
Figure 2 shows that the degradation of chlorophyll a content in VCO samples exposed to relatively high light intensity (4,000 lux) at room temperature. According to Byun et al. [15], the major chlorophyll pigments in oils, about 90%, are a-type included pheophytin a and pyropheophytin a. That chlorophyll and its derivatives act as sensitizers to produce singlet oxygen [6] which initiates the photo-oxidation reaction and causes hydro-peroxide production. Consequently, light exposure in VCO samples could increase PVs (Fig. 1) or decrease the chlorophyll contents (Fig. 2).
Degradation of chlorophyll a content in VCO exposed to fluorescent light (4,000 lux) for up to 8 h. (Samples from the jar which wrapped in aluminum foil during settling and placed in the serum bottles with A or without B light protection; Samples from transparent jar during settling and placed in the serum bottles with C or without D light protection)
The chlorophyll content in VCO although at a relatively low level, i.e. 0.098 ± 0.001 ppm and 0.097 ± 0.001 ppm in VCO samples from the settling stage in jars with and without light protection, respectively, was able to initiate the photo-oxidation reaction. As reported by Lee et al. [7], the chlorophyll content at 0.065–1.33 ppm in vegetable oils can produce singlet oxygen and initiate the photo-oxidation reaction. Kochevar and Redmond in Choe and Min [6] reported that a sensitizer molecule may generate 103–105 molecules of singlet oxygen before becoming inactive. The highly reactive singlet oxygen then probably attacks the double bond between the fifth and sixth carbon of chlorophyll a, resulting in a subsequent shift of the position of the double bond and the formation of hydro-peroxides, which are then further cleaved through oxygen–oxygen linkage to form degradation products [16]. These degradation products were no longer detected as chlorophyll a, so that only a minor amount of chlorophyll a was detected after VCO was exposed to a relatively high intensity of fluorescent light (Fig. 2).
In the earlier stages of exposure, the degradation of chlorophyll a takes place at a slower rate and accelerates as the oil oxidizes due to the presence of hydro-peroxide free radicals [17]. Figure 2 shows that the chlorophyll a content changes during light exposure with relatively high light intensity (4,000 lux) for up to 8 h. The amount of chlorophyll a decreased on increasing the duration of light exposure in unprotected VCO samples. In these samples, chlorophyll decreased no more than 5.65% after 1 h exposure to light and decreased 18.82, 31.37 and 57.97% after 2, 3 and 4 h exposure with light, respectively. Chlorophyll degraded faster with longer light exposure. According to Byun et al. [15], the degraded chlorophyll could not catalyze the photo-oxidation of light exposed samples.
Chen and Huang [16] reported that chlorophyll a degradation fits the first-order model in a cold chamber (−5.4 °C) at 2,000 lux light intensity. The degradation rate of that model was very fast as shown by k value [0.084 h−1]. In comparison with the said study, this experiment which was conducted with a 4,000-lux light intensity exposure of VCO samples at room temperature resulted in a similar trend. The chlorophyll a content of VCO samples without light protection was no longer measurable after a 5-h exposure to relatively high light intensity. This caused a decrease in the PVs as shown in Fig. 1, whereas, the correlation between the degradation of chlorophyll a and peroxide content in VCO samples exposed to fluorescent light is shown in Fig. 3.
Correlation between PV and chlorophyll a content in VCO exposed to fluorescent light (4,000 lux) for up to 8 h. (Samples from the jar which wrapped in aluminum foil during settling and placed in the serum bottles with (a) or without (b) light protection; Samples from transparent jar during settling and placed in the serum bottles with (c) or without (d) light protection)
Figure 3 shows the negative slopes of a simple linear correlation between PV and chlorophyll a content in the VCO during the treatment. The slope in Fig. 3a is not significantly different from zero at the 5% probability level so that it could be declared equal to zero. It can be stated that the PV changes were not accompanied by the changes of chlorophyll a content. However, the slopes in Fig. 3c and b or d were significantly different from zero at 5 and 1% probability levels, respectively. The greater slopes (Fig. 3b and d) indicated the stronger impact. There was strong evidence that the VCO with a high PV also has a low chlorophyll a content, and vice versa. Under this condition, it is the decreasing chlorophyll content and its possible degradation which has a strong sensitizing effect on photo-oxidation that is supposed to cause the increased PV.
Chlorophyll a content decreased after prolonged exposure to light. Chen and Huang [16] reported that in the model system containing trans-β-carotene and fatty acid ester exposed to high light intensity (5,000 lux), chlorophyll a degraded and formed chlorophyll a isomer I and chlorophyll a isomer II. Those isomers could act as sensitizers. This fact may occur in VCO exposed to fluorescent light (4,000 lux) so that the PVs increased in spite of decreases in chlorophyll a content. In this experiment, the half-life of chlorophyll a was approximately 4 h (Fig. 2). Before becoming inactive, chlorophyll a produced singlet oxygen rapidly. This singlet oxygen attacked the unsaturated fatty acid and formed hydro-peroxides. These hydro-peroxides could act as pro-oxidants [11] that cause the oxidation chain reaction.
Although the chlorophyll a content decreased, the PVs increased with a very significant correlation (α < 0.01) as shown in Fig. 3b and d. The rate of chlorophyll degradation was influential in increasing the PVs with correlation coefficient (r) of (−) 0.961 (Fig. 3b) and (−) 0.952 (Fig. 3d). On the whole, Fig. 3 shows that prevention from light exposure after the settling stage, especially during prolonged storage or display for sale at retailers, can protect VCO products from photo-oxidation and inhibit quality deterioration.
Photo-oxidation during VCO Storage
Figure 4 shows the pattern of PV changes during VCO storage under ambient (dark) storage and normal room light intensity (380–400 lux). There were no significant changes in PV until after 10 weeks of the storage period. The storage of VCO in normal light intensity at room temperature significantly (P < 0.01) increased the PV after 10 weeks of storage. At the end of the storage period (16 weeks) the PV reached 0.49 ± 0.01 mequiv/kg and 1.19 ± 0.01 mequiv/kg in VCO samples stored in the dark and normal room light intensity, respectively. Our previous study (data not shown) showed that the objectionable odor and taste were clearly detected by panelists on samples having PV of 1.0 mequiv/kg or higher. These results show that light exposure to VCO increases the formation rate of oxidation products and initiates quality deterioration.
The chlorophyll in light-exposed VCO samples absorbed the light energy and became excited. The excited chlorophyll changed triplet oxygen to singlet oxygen. The singlet oxygen attacked the electron-rich fatty acid and formed hydro-peroxides at the double bonds [5, 6]. In the earlier storage period, low light intensity (380–400 lux) and low chlorophyll content in VCO samples (0.098 ppm) resulted in a slow photo-sensitized process and thus slower PV changes. The hydro-peroxide produced in that process could act as a pro-oxidant which resulted in the chain reaction of free radical peroxide formation [11]. Consequently, the PVs increased, especially in the light-exposed VCO stored at room temperature with a significant PV increase after 10 weeks of storage.
Under ambient storage, the rise of PV in VCO samples existed after 4 weeks of storage. Maybe this is the induction period which takes place for the initiation step of auto-oxidation. Before being stored in the dark, the VCO was exposed to light that illuminated the processing room during production. This enabled the formation of singlet oxygen, which produced hydro-peroxide and initiated the oxidation chain reaction (auto-oxidation).
During storage under light, chlorophyll is a strong pro-oxidant which acts as a sensitizer to produce singlet oxygen [18], but under dark conditions it acts as an antioxidant which contributes hydrogen atoms to free radicals [6, 13, 19]. It was noted that the PV of VCO samples subjected to normal room light intensity (380–400 lux) during prolonged storage increased at a significantly (P < 0.01) higher rate as compared with those with ambient (dark) storage.
Conclusions
A short (overnight) duration of exposure to relatively low light intensity (less than 400 lux) during the settling stage of VCO production had no significant effect on photo-oxidation. Photo-oxidation of VCO, however, was observed during storage at room temperature and when it was exposed to high intensity (4,000 lux) fluorescent light. The degradation of chlorophyll content in VCO was evident during exposure to a high fluorescent light intensity which was accompanied by high lipid peroxides accumulation. A significant negative correlation between PV and chlorophyll content was found during the storage of VCO without light protection. The storage of VCO in the room light (380–400 lux) at room temperature (30 ± 1 °C) significantly increased the PV after 10 weeks of storage.
References
Bureau of Product Standards (BPS) (2004) Philippine National Standards: virgin coconut oil. Philippines, Department of Trade and Industry. http://www.bps.dti.gov.ph/splash.html
Scrimgeour C (2005) Chemistry of fatty acid. In: Shahidi F (ed) Bailey’s industrial oil and fat products, vol 5, 6th edn. Wiley, Hoboken, pp 1–43
List GR, Wang T, Shukla VKS (2005) Storage, handling and transport of oils and fats. In: Shahidi F (ed) Bailey’s industrial oil and fat products, vol 5, 6th edn. Wiley, Hoboken, pp 191–229
Anwar F, Chatha SAS, Hussain AI (2007) Assessment of oxidative deterioration of soybean oil at ambient and sunlight storage. Grasas y Aceites 58(4):390–395
Min DM, Boff JM (2002) Chemistry and reaction of singlet oxygen in food. Comp Rev Food Sci and Food Saf 1:58–72
Choe E, Min DB (2006) Mechanisms and factors for edible oil oxidation. Comp Rev Food Sci and Food Saf 5:169–186
Lee KH, Jung MY, Kim SY (1997) Quenching mechanism and kinetics of ascorbyl palmitate for the reduction of photosensitized oxidation of oils. J Am Oil Chem Soc 74(9):1053–1057
IUPAC (1992) Standard methods for the analysis of oils, fats and derivatives. Pergamon, New York
AOCS (2004) Official methods and recommended practices of the AOCS, 5th edn. AOCS Press, Champaign
Rahmani M, Csallany AS (1998) Role of minor constituents in the photo-oxidation of virgin olive oil. J Am Oil Chem Soc 75(7):837–843
Kim HJ, Hahm TS, Min DB (2007) Hydro-peroxide as a prooxidant in the oxidative stability of soybean oil. J Am Oil Chem Soc 84:349–355
Marina AM, Man YBC, Nazimah SAH (2009) Chemical properties of virgin coconut oil. J Am Oil Chem Soc 86:301–307
Chen BH, Liu MH (1998) Relationship between Chlorophyll a and β-carotene in a lipid-containing model system during illumination. Food Chem 63(2):207–213
Decker EA (1998) Strategies for manipulating the prooxidative/antioxidative balance of food to maximize oxidative stability. Trends Food Sci Tech 9:241–248
Byun M-W, Jo C, Lee K-H, Kim K-S (2002) Chlorophyll breakdown by gamma irradiation in a model system containing linoleic acid. J Am Oil Chem Soc 79(2):145–150
Chen BH, Huang JH (1998) Degradation and isomerization of chlorophyll a and β-carotene as affected by various heating and illumination treatments. Food Chem 62(3):299–307
Thron M, Eichner K, Ziegleder G (2001) The influence of light of different wavelengths on chlorophyll-containing foods. LWT Food Sci Technol 34:542–548
Fakourelis N, Lee EC, Min DB (1987) Effects of chlorophyll and β-carotene on the oxidation stability of olive oil. J Food Sci 52(1):234–235
Lee J, Lee Y, Choe E (2007) Temperature dependence of the autoxidation and antioxidants of soybean, sunflower, and olive oil. Eur Food Res Technol 226:239–246
Acknowledgments
This work was financially supported by research grant in 2008–2009 from the Ministry of Research and Technology the Republic of Indonesia and authors greatly appreciate the support.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Rukmini, A., Raharjo, S. Pattern of Peroxide Value Changes in Virgin Coconut Oil (VCO) Due to Photo-Oxidation Sensitized by Chlorophyll. J Am Oil Chem Soc 87, 1407–1412 (2010). https://doi.org/10.1007/s11746-010-1641-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-010-1641-7