Abstract
To manufacture beef tallow (BT)-based shortening and margarine with a reduced tendency to developing sandiness, BT/canola oil (CaO) blend (80:20 w/w), selected from the BT and CaO blends mixed in different ratios from 60:40 to 85:15 with 5% increments, was subjected to chemical interesterification (CIE) with sodium methoxide as the catalyst. The interesterified products were compared with the starting mixture in terms of solid fat content (SFC), and contents of high-melting point 1,3-disaturated long-chain fatty acid 2-monounsaturated long-chain fatty acid triacylglycerols (SUS TAGs) including 1,3-distearoyl-2-oleoyl-glycerol (StOSt), 1,3-dipalmitoy-2-oleoyl-glycerol (POP), and 1-palmitoyl-2-oleoyl-3-stearoyl-glycerol (POSt). Under the selected conditions: 60 °C, 0.6% CH3ONa, 90 min, the CIE product had a SFC profile that meets suggested bakery fat requirements, besides a content of SUS TAGs which is 22.14% lower than that of the non-interesterified blend. Also the fat produced had stable β′ polymorphs, crystal morphology, crystal sizes (<20 μm), and could resist temperature fluctuations. The CIE product obtained herein has an increased potential for manufacturing bakery shortenings and margarines with reduced graininess formation, increasing the possibilities for the commercial use of BT and CaO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Beef tallow (BT), a by-product of beef production, is an important animal fat widely used in the food industry. In particular, BT is utilized in bakery shortenings due to its advantageous properties—such as (1) high thermal and oxidative stability, (2) ideal plasticity at room temperature, and (3) typical aroma after baking, etc. However, in certain applications, BT-based plastic fats (BTPF) are also known to exhibit undesirable crystallization behaviors, among which growth of granular crystals with diameters up to 2–3 mm or above, a phenomenon also termed graininess formation, is one major drawback that impairs the consistency and plasticity of BTPF, e.g., shortenings and margarines. This phenomenon is especially troublesome in spring and autumn when the temperature fluctuates over a large range during BTPF handling, storage and transportation [1]. Prevention of the graininess formation has been a major focus in the specialty fats industry.
The mechanism of graininess formation in functional fats has been investigated by several groups. The transformation of palm oil into the β polymorph was suggested to be the reason for the formation of granular crystals in palm oil based margarine [2]. In a model fat blend consisting of POP and rapeseed oil, reproduced granular crystals showed higher POP content than their surrounding materials, and the most stable β 1 polymorph of POP was observed to contribute to the formation of granular crystals [3]; and in a margarine produced with excess palm oil, granular crystals showed POP as one of its major TAGs [4]. In a model margarine system composed of palm oil and tripalmitin (PPP), the agglomeration of high-melting point TAGs was found to lead to the formation of granular crystals [5]. The agglomerating of high-melting point TAGs, such as StOSt, POP, and POSt, together with forming of β-form crystals, was observed to occur in BT-based shortening with fluctuation of temperature, both in consequence causing the formation of granular crystals [1]. The above-cited reports show that high-melting SUS TAGs are β-tending and thus lead to the growth of granular crystals; however, few studies have dealt with the reduction of graininess formation in BTPF.
Interesterification (IE), which offers the possibility of manipulating the distribution and composition of fatty acids of TAGs to confer more desirable physicochemical properties on fats, has been widely used as a fat modification process to provide plastic fats of desirable qualities [6, 7]. The IE of BT and sunflower oil catalyzed by lipase or CH3ONa were found suitable for bakery shortening preparation [8, 9]. Two types of IE, i.e., chemical interesterification (CIE) and enzymatic interesterification (EIE), are available. CIE is a tried-and-true approach, as it has been around for a long time, and relevant industrial procedures and equipment are readily available. On the other hand, EIE reactions are more specific, require less severe reaction conditions and produce less waste compared with CIE, and may represent the way of the future. The purpose of IE is to obtain not only satisfactory melting properties but also suitable crystallization behavior for producing higher quality plastic fats with little sandy mouthfeel. Granular crystals easily develop in palm oil-based [5] and lard-based blends [10]. These undesirable characteristics in palm oil [11] and lard [10] are often reduced or greatly eliminated following appropriate IE of selected fat blends. To date, however, attempts have not yet been found regarding sandiness reduction in BT-based plastic fats via IE. Canola oil (CaO) as a major oil source which is low in saturated long-chain fatty acids such as palmitic and stearic acids. Since stearic and/or palmitic acid has been implied to be linked with graininess formation in plastic fats [1, 5], interesterifying BT by CaO is expected to help reduce the graininess formation.
The objective of this study was to reduce the formation of granular crystals in BT-based plastic fats by decreasing the content of SUS TAGs, notably StOSt, POP, POSt, through IE of BT with CaO. This study continues previous work performed by Jin et al. [1]. The appropriate conditions of interesterification was obtained by monitoring SFC and the reduction rate of SUS TAGs. Also, the polymorphic behavior, crystal morphology, and crystal network of CIE products under temperature fluctuations were investigated.
Experimental Procedures
Materials
BT was imported from Australia. CaO was purchased from a local supermarket. Both BT (palmitic acid (26.96%), stearic acid (20.49%)) and CaO (palmitic acid (4.79%), stearic acid (trace)) were kept at 4 °C in the dark until used. The catalyst, CH3ONa, was stored at room temperature in a desiccator to avoid decomposition. Supelco 37 component FAME Mixture was purchased from Sigma-Aldrich China (Shanghai, China). All other reagents and solvents were of analytical or chromatographic grade to suit analytical requirements.
Fatty Acid Composition
Fatty acid methyl esters (FAME) were prepared according to the AOCS Official Method Ce 2-66 [12] and subsequently analyzed with GC-14B gas chromatography (GC) equipped with a flame ionization detector (Shimadzu, Tokyo, Japan) and a fused-silica capillary column (CP-Sil88, 100 m × 0.25 mm × 0.2 mm). The temperature of the injection port and detector were all set at 250 °C. The column was heated to 120 °C, and held for 3 min, then increased to 175 °C at a rate of 8 °C/min, and held for another 28 min, and at 3 °C /min to 215 °C, held for 20 min. The fatty acid species was identified using the retention time of a FAME standard solution.
Blend Preparation
BT was melted at 60 °C in an oven prior to use. The liquefied BT and CaO were mixed with BT in proportions ranging from 60 to 85% (w/w) with 5% increments. The following determinations were carried out—moisture by oven method, and acid (AV) and peroxide (PV) values by AOCS Official Methods [12]. Values obtained at different times were related to the one of original blend. The selected blend was the BT/CaO 80:20 (reaction substrate). Its moisture, PV and AV were 0.03%, 2.1 meq/kg and 0.04%, respectively. The catalysts for CIE are extremely sensitive to moisture; therefore fat or oil should contain <0.01% (w/w) water. FFA and peroxides also impair catalyst performance, and their levels should be maintained at <0.05% (w/w) [13]. Therefore, the blended substrate was dried under vacuum immediately before reaction, and catalyst dosage was moderately increased.
Chemical Interesterification
Portions (100 g each) of the BT/CaO blends in the appropriate ratio as chosen in Blend Preparation were dried under vacuum at 90 °C for 30 min. After thermal equilibration of each sample at 60, 75 or 90 °C, the catalyst (0.4, 0.6, or 1.0% CH3ONa, w/w) was added and reaction was followed under vacuum conditions with continuous stirring for 30, 60, and 90 min. The reaction was stopped and then the interesterified blends were vacuum-dried according to the method reported [14]. Experimental design and the number of CIE products are shown in Table 1. Non-interesterified blend, used as the control, was numbered 0. The blends, interesterified and non-interesterified, were held at 4 °C for following determinations.
SFC
Following the AOCS Official Method Cd 16b-93 [12], the SFC of the samples was determined on a PC120 pulsed nuclear magnetic resonance (pNMR) spectrometer (Bruker, Karlsruhe, Germany). The sample was placed in the NMR tube and melted at 80 °C for 30 min, tempered at 0 °C for 90 min, and then kept at the desired temperature for 30 min before measurements were recorded.
TAGs Composition
TAGs were separated by reversed-phase high-performance liquid chromatography (HPLC). A Nova-pak RP-C18 column (150 mm × 4.6 mm, particle size 4 μm) (Waters, Milford, USA) with acetonitrile/dichloromethane (65:35, v/v) as the eluent at a flow rate of 1.0 mL/min and an evaporative light scattering detector (ELSD) was used. TAGs were identified by high-performance liquid chromatography–atmospheric pressure chemical ionization mass spectrometry (HPLC/APCI-MS). The HPLC conditions were same as described before. A Platform ZMD 4000 (Waters, Milford, USA) mass spectrometer (MS) equipped with an APCI interface was run at an APCI source block and probe temperatures of respectively 100 and 400 °C and an MS multiplier voltage of 700 V. The measurement range was between m/z 250 and 1,200.
Quantitative determination of individual TAGs in fat blends was performed using HPLC following the procedures of Chen et al. [15]. The reduction rate of SUS TAGs (StOSt, POP, POSt) were calculated as the ratio of the decrease in the area percentages of the above mentioned species to the corresponding initial area percentage [15]:
Graininess Resistance Under Temperature Cycling
The melted (80 °C for 30 min) interesterified and non-interesterified BT/CaO fat blends were stored in a KBF115 climatic chamber (Binder, Tuttlingen, Germany) in which the temperature was held at 5 °C for 12 h and 20 °C for another 12 h. This temperature program was repeated each day as a cycle. After each cycle, the fat samples were examined using X-ray diffraction and polarized light microscopy.
Crystal Polymorphism by X-ray Diffraction
The polymorphic forms of fat crystals in the blends were determined by D8 Advance X-ray diffraction (Bruker, Karlsruhe, Germany), using Cu–Ka radiation with a Ni filter (k = 1.54056 Å, voltage 40 kV, current 40 mA, fixed 1.0, 1.0, and 0.1 mm divergence, anti-scatter and receiving slits, respectively). The short spacing were observed in the 2θ range of 10–30 o, and the scan rate was set at 2°/min. The samples were analyzed at ambient temperature, and duplicate analysis was carried out.
Crystal Morphology by Polarized Light Microscopy
The morphology of crystallized samples was observed using polarized light microscopy (DMRX, Leica, Germany) with a Canon A640 digital camera attached (Canon, Tokyo, Japan). A small amount of crystallized sample after each temperature cycle was placed on a glass slide and covered with a glass cover slip. The 1.80 cm × 1.80 cm cover slip was then squeezed (2.5 kgf) to produce a film of uniform thickness and avoid the introduction of air bubbles into the sample. The photomicrograph of the crystal was taken at 400× magnification and each photomicrograph represents a typical field. All handling was performed at ambient temperature.
Statistical Analysis
Statistical analysis was performed by the Statistical Analysis System software (SAS, Cary. NC). Analysis of variance (ANOVA) with Duncan’s multiple range test was performed. Differences were considered significant at P <0.05. Although no standard errors are shown in Table 1 and 2 and Fig. 1, the standard deviations in the results varied between 0.004 and 0.870 for all of the experiments conducted.
Results and Discussion
Selection of the Substrate Blend
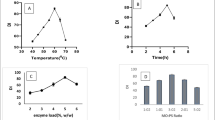
The plasticity range of fats can be typically reflected by SFC. Normally, a broad plastic range is required for a bakery shortening. The plasticity of shortening helps air incorporation to increase the volume during the creaming stage in cake batter and cookie dough formation. The typical ranges for bakery fat are 23–40% at 10 °C, 16–30% at 21.1 °C, 13–27% at 26.7 °C, 9–21% at 33.3 °C, and 3–11% at 40 °C [16]. Fig. 1 shows SFC profiles of BT/CaO blends in different proportions showing increased SFC with the increasing amounts of BT in the blends, as expected. Interesterified fats tend to show lower SFC values than the physical blends [9], hence, BT/CaO 80:20, 75:25 and 70:30 fat blends, which gave SFC values that are slightly higher than that of typical commercial bakery fats, were taken as appropriate. Although the three blends had desirable SFC, the BT/CaO 80:20 blend was selected because it maintained the unique pleasant flavor of BT. This flavor was less typical with 25 or 30% CaO.
SFC Changes of the Reaction Products
The SFC of fats is responsible for functional characteristics in plastic fats, including their general appearance, ease of packing, spreadability, oil exudation and organoleptic properties. For example, the SFC values of fats given between 4 and 10 °C determine the ease of spreading of fats at refrigeration temperature, that at 25 °C determine the stability and resistance to oil exudation at room temperature, and that between 33 and 38 °C influence mouth feel or waxy senses [17].
Table 2 shows the SFCs of the substrate (the control) and 27 interesterified products as a function of temperature, respectively. Generally, all the interesterified products exhibit lower SFC values than the starting BT/CaO 80:20 blend, a tendency consistent with the reports of Rodríguez et al. [9]. Comparison of SFC results of interesterified products revealed that SFC gradually decreased with an increase in the amount of catalyst under otherwise same IE conditions (figures not shown), which are due to the decrease in the proportion of high melting TAGs in the IE products. Similar results showing that the levels of high melting TAGs were reduced after IE have also been reported [6, 7]. In the present study, all the interesterified products had broad plastic ranges and SFC values that closely match those of commercial bakery fats (Table 2), whose typical SFC ranges has been mentioned above; and thus can be used for manufacture of bakery shortenings and margarines. Equally critical, besides SFC, is the crystal habit of certain TAG. So, we analyzed the TAG changes of the reaction products.
TAG Changes of the Reaction Products
Figure 2 shows the TAG profiles of BT/CaO blends before (the control) and after CIE (interesterified product) corresponding to treatment 1 (Table 2). The change in TAG profiles is less obvious, but the relative concentrations of several TAGs increased while others were observed to decrease. The results obtained were similar to previous findings by Chen et al. [15]. This change indicates that CIE has occurred in all reaction products.
The molecular structures of the TAG species present in both the control fats and the interesterified fats were determined using reversed-phase HPLC/APCI-MS, which gives very useful information not only on which fatty acids are present in a TAG molecule, but also on the specific distribution of fatty acids in a TAG, conventionally determined by enzymatic hydrolysis procedures followed by GC analysis. Under reversed-phase chromatographic conditions, TAGs are separated on the basis of their equivalent carbon number, ECN (ECN = CN-2 DB, where CN is the number of carbons and DB the number of double bonds). Peak identification can be performed by combining ECN values with the information from the MS spectrum. The MS spectra of TAGs showed sodium adducts of the molecular ions [M + Na]+ and one fatty acyl moiety cleaved ions [M−(R–COO)]+ ([DG]+). Regarding the [DG]+ ions, their intensity changes mainly in relation to the position occupied by the fatty acid that is removed. The less abundant [DG]+ ion corresponds to the loss of the fatty acid from the sn-2 position because this is energetically less favorable than losing a fatty acid from the sn-1 or sn-3 position. For instance, POP would be expected to yield [PO]+ and [PP]+ ions in a two-to-one ratio, regardless of the positional placement of palmitic or oleic acid on the glycerol backbone. An actual proportion of the [PP]+ ion to [PO]+ ion substantially less than 1:2, or 50%, indicates that [PP]+ ion is energetically disfavored and that the TAG is the 1,3-isomer [18]. The mass spectra of various TAGs showed relative abundance of [DG]+ species in consistence with rules above (spectra for individual TAGs not shown for sparing page space), which were also similar to those reported previously [18, 19]. The main TAG species identified in the control and interesterified product (examplified by treatment 1) had been assigned to the peaks in Fig. 2.
The reduction rate of SUS TAGs (StOSt, POP, POSt) after CIE are showed in Table 1. The fat under 60 °C, 0.6% CH3ONa interesterified 90 min (corresponding CIE product 6 shown in Table 2) showed the highest reduction rate (22.14%), giving a more advantageous TAG composition for formulating reduced graininess plastic fats, because these high-melting point TAGs are one of the key factors for the graininess formation in shortenings and margarines [1, 5]. Randomization also led to a rise in SUS TAGs in some interesterified products (especially in CIE product 21, SUS TAGs increased by 4.02%) but generally IE resulted in a lower content of high melting point SUS TAGs than the control, as in the results obtained by Norizzah et al. [6]. The agglomeration of high-melting TAGs led to the formation of granular crystals in shortenings and margarines as crystal nuclei. Once nucleation occurs, other high-melting TAGs join together and crystals grow to form larger crystals. When the fat crystal size in plastic fats equals or exceeds the sensory threshold, detectably grainy crystals will occur. So, on the premise of maintaining a certain solid fat content, lower high-melting TAGs making the TAG composition of base stocks more reasonable will help the manufacture of shortenings and margarines with a reduced tendency to sandiness development.
Polymorphism
The control and CIE products 6 and 21 were each crystallized under temperature cycles, and their polymorphisms were characterized by X-ray diffraction spectra. Polymorphic forms were determined from d spacings. The short spacings of the β′ form are at 4.2 and 3.8 Å and that of the β form is at 4.6 Å. Levels of β′ and β crystals in mixtures were estimated by the relative intensity of the short spacings at 3.8, 4.2 and 4.6 Å [20]. The polymorphic forms of the control, and CIE products 6 and 21 were analyzed each after 1 and 14 temperature cycles, respectively. The control crystallized in a mixture of β and β′ form appearing at 4.53, 4.19 and 3.81 Å, respectively, after cycle 1 (Table 3). CIE product 6 consisted mainly of the β′ form together with a small content of β form crystals after temperature cycle 1 and 14. CIE product 6 contained more of the β′ form than CIE product 21. According to Chu et al. [7], POP, PPP are β-tending TAGs and as transesterification reduced POP and PPP levels in the blend, β′ crystals formation was promoted. Table 3 shows that CIE product 6 had stable polymorphic forms under temperature fluctuations. It is likely that the reduction of SUS TAGs (StOSt, POP, POSt) after CIE favored stabilization of the polymorph in β′ form in the present study. The form of crystal is important in bakery fats such as shortening fats. The β′ form is the most desirable because this small needle-like polymorph gives good aeration to bakery dough and smooth texture to the bakery products. Processors also prefer this form because it is structurally stable and maintains small-to-moderate crystal sizes allowing for soft and smooth products. The β polymorph is undesirable because it leads to the development of large crystals and sand-like texture. Therefore, CIE product 6 having mostly β′ form crystals could be essential to avoid a sandy mouth feel and be used as an alternative for bakery fats.
Crystal Morphology
Crystal sizes are essential for final product consistency and acceptability, with smaller crystals leading to firmer products, whereas larger size crystals ranging between 30 and 140 μm are reported to produce a sandy mouth feel [3]. Another study [4] reported that crystal aggregates become visible to the naked eye and can be perceived in the mouth when they reach sizes between 100 and 300 μm. The microstructures of the control and CIE products 6 and 21 crystallized and stored under temperature cycles 1, 3, 7, 14 were each visualized by polarized light microscopy (Fig. 3) in order to assess the effects of temperature fluctuations on fat-crystal network structure in terms of crystal network density, crystal morphology, and crystal sizes, etc. The control contained well-organized spherulites with needle-shaped crystals oriented radially from the center which were more tightly packed with less space between adjacent crystals than CIE products 6 and 21 under temperature cycle 1. On continuing temperature cycling, the crystals in CIE product 6 displayed more discrete morphology when compared to those in the control and CIE product 21. After cycle 7, the control showed a number of small evenly distributed crystals interspersed with large crystals (of approximately 20–30 μm). In addition, large crystals ranging between 25 and 40 μm are spread in the CIE product 21 after cycle 7 and 14. The CIE product 6 showed more discrete crystal morphology and small crystals (no more than 20 μm) in all the temperature fluctuation cycles. Therefore, CIE product 6 had a more stable crystal morphology and stable sizes and hence was resistant to temperature fluctuations, which can prevent the agglomeration of crystals that form granular crystals. These smaller crystal sizes and sparser spatial distributions of mass within the networks in CIE product 6 compared with the control sample was a result of SUS TAGs (StOSt, POP, POSt) reduction after CIE. Other CIE products with lowered content of high melting point SUS TAGs also have stable β′ polymorphs, small crystal sizes during temperature cycling (data not shown) and exhibited SFC profiles and plasticity suitable for use in bakery plastic fats.
Conclusions
In conclusion, BT/CaO blends subjected to CIE, under proper conditions, can be altered in their TAGs composition and physical properties compared to the control blend (non-interesterified). Their contents of SUS TAGs (StOSt, POP, POSt) were reduced; their SFC profiles matched recommended values more closely; and their stability of β′ polymorphs, smaller crystal sizes and sparser spatial distributions of mass within the fat crystal networks could resist temperature fluctuations. All the changes combined could assist in eliminating the profound graininess formation phenomena in BT-based shortenings and margarines. The plastic fat obtained via controlled CIE maintained satisfactory quality parameters, such as consistency and plasticity, while helping to avoid problems of trans fat which is usually associated with conventional plastic fats based on hydrogenated oils. These characteristics increase the possibilities for expanding the commercial use of BT and CaO.
References
Jin Q, Gao H, Shan L, Liu Y, Wang X (2007) Study on grainy crystals in edible beef tallow shortening. Food Res Int 40:909–914
Ishikawa H, Mizuguchi T, Kondo S (1980) Studies on granular crystals growing in palm oil. J Jpn Oil Chem Soc 29:235–242
Watanabe A, Tashima I, Matsuzakj N, Kurashige J, Sato K (1992) On the formation of granular crystals in fat blends containing palm oil. J Am Oil Chem Soc 69:1077–1080
Miura S, Konishi H (2001) Crystallization behavior of 1,3-dipalmitoyl-2-oleoyl-glycerol and 1-palmitoyl-2,3-dioleoylglycerol. Eur J Lipid Sci Technol 103:804–809
Tanaka L, Miura S, Yoshioka T (2007) Formation of granular crystals in margarine with excess amount of palm oil. J Am Oil Chem Soc 84:421–426
Norizzah AR, Chong CL, Cheow CS, Zaliha O (2004) Effects of chemical interesterification on physicochemical properties of palm stearin and palm kernel olein blends. Food Chem 86:229–235
Chu BS, Ghazali HM, Lai OM, Cheman YB, Yusof S (2002) Physical and chemical properties of a lipase–transesterified palm stearin/palm kernel olein blend and its isopropanol-solid and high melting triacylglycerol fractions. Food Chem 76:155–164
Foglia TA, Petruso K, Feairheller SH (1993) Enzymatic interesterification of tallow–sunflower oil mixtures. J Am Oil Chem Soc 70:281–285
Rodríguez A, Castro E, Salinas MC, López R, Miranda M (2001) Interesterification of tallow and sunflower oil. J Am Oil Chem Soc 78:431–436
Lutton E, Mallery M, Burgers J (1962) Interesterification of lard. J Am Oil Chem Soc 39:233–235
Laning S (1985) Chemical interesterification of palm, palm kernel and coconut oils. J Am Oil Chem Soc 62:400–407
Firestone D (2004) Official methods and recommended practices of the American Oil Chemists’ Society, 5th edn. American Oil Chemists’ Society, Champaign
Marangoni AG, Rousseau D (1995) Engineering triacylglycerols: the role of interesterification. Trends Food Sci Tech 6:329–335
Ribeiro APB, Grimaldi R, Gioielli LA, Gonçalves LAG (2009) Zero trans fats from soybean oil and fully hydrogenated soybean oil: physico–chemical properties and food applications. Food Res Int 42:401–410
Chen CW, Chong CW, Ghazali HM, Lai OM (2007) Interpretation of triacylglycerol profiles of palm oil, palm kernel oil and their binary blends. Food Chem 100:178–191
List GR, Warner K, Pintauro P, Gil M (2007) Low-trans shortening and spread fats produced by electrochemical hydrogenation. J Am Oil Chem Soc 84:497–501
Aguedo M, Hanon E, Danthine S, Paquot M, Lognay G, Thomas A, Vandenbol M, Thonart P, Wathelet JP, Blecker C (2008) Enrichment of anhydrous milk fat in polyunsaturated fatty acid residues from linseed and rapeseed oils through enzymatic interesterification. J Agric Food Chem 56:1757–1765
Byrdwell WC (2001) Atmospheric pressure chemical ionization mass spectrometry for analysis of lipids. Lipids 36:327–346
Heidi L, Suomela J-P, Kallio H (2007) Quantification of triacylglycerol regioisomers in oils and fat using different mass spectrometric and liquid chromatographic methods. Rapid Commun Mass Spectrom 21:2361–2373
Lee JH, Akoh CC, Lee K-T (2008) Physical properties of trans-free bakery shortening produced by lipase-catalyzed interesterification. J Am Oil Chem Soc 85:1–11
Acknowledgments
This work was supported by the National High Technology Research and Development Program (863 Program) of China (Contract No. 2010AA101506). The authors gratefully acknowledge Mr. Tao Guan Jun of State Key Laboratory of Food Science and Technology, Jiangnan University, for technical assistance during the HPLC/APCI-MS analysis.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Meng, Z., Liu, Y., Shan, L. et al. Reduction of Graininess Formation in Beef Tallow-Based Plastic Fats by Chemical Interesterification of Beef Tallow and Canola Oil. J Am Oil Chem Soc 87, 1435–1442 (2010). https://doi.org/10.1007/s11746-010-1627-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-010-1627-5