Abstract
Ricinoleate, a monohydroxy fatty acid in castor oil, has many industrial uses. Dihydroxy and trihydroxy fatty acids can also be used in industry. We report here the identification of diacylglycerols (DAG) and triacylglycerols (TAG) containing trihydroxy fatty acids in castor oil. The C18 HPLC fractions of castor oil were used for mass spectrometry of the lithium adducts of acylglycerols to identify trihydroxy fatty acids and the acylglycerols containing trihydroxy fatty acids. Two DAG identified were triOH18:1–diOH18:1 and triOH18:0–OH18:1. Four TAG identified were triOH18:1–OH18:1–OH18:1, triOH18:0–OH18:1–OH18:1, triOH18:1–OH18:1–diOH18:1 and triOH18:0–OH18:1–diOH18:1. The structures of these two newly identified trihydroxy fatty acids were proposed as 11,12,13-trihydroxy-9-octadecenoic acid and 11,12,13-trihydroxyoctadecanoic acid. The locations of these trihydroxy fatty acids on the glycerol backbone were almost 100% at the sn-1,3 positions or at trace levels at the sn-2 position. The content of these acylglycerols containing trihydroxy fatty acids was at the level of about 1% or less in castor oil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
We have recently reported the identification of twelve diacylglycerols (DAG) and triacylglycerols (TAG) containing dihydroxy fatty acids in castor oil by mass spectrometry (MS) [1]. The structures of these novel dihydroxy fatty acids were proposed as 11,12-dihydroxy-9-octadecenoic acid (diOH18:1), 11,12-dihydroxy-9,13-octadecadienoic acid (diOH18:2) and 11,12-dihydroxyoctadecanoic acid (diOH18:0). The total acylglycerols containing dihydroxy fatty acids was about 2.5% of castor oil. Earlier, dihydroxystearic acid (diOH18:0) was identified in castor oil [2, 3]. We are reporting here the identification of DAG and TAG containing trihydroxy fatty acids (11,12,13-triOH18:1 and 11,12,13-triOH18:0) in castor oil (Figs. 1, 2). Trihydroxy fatty acids, 9,10,18-triOH18:1 and 9,10,18-triOH18:0, were reported earlier as the components of polymers of plant cutin and suberin [4, 5] and in Chamaepeuce seed oils [6]. In a microbial culture, ricinoleic acid could convert to 7,10,12-trihydroxy-8(E)-octadecenoic acid [7].
The structures of diacylglycerols and triacylglycerols containing trihydroxy fatty acids in castor oil. The calculated m/z of the lithium adducts of acylglycerols are also shown. The predominate regiospecific locations of the fatty acids on triacylglycerols are as shown. The structures of these fatty acids have been proposed as shown in Fig. 1
Ricinoleate (OH18:1), a monohydroxy fatty acid, has many industrial uses such as the manufacture of aviation lubricant, plastic, paint, nylons and cosmetics. Ricinoleate occurs as acylglycerols in castor oil, and about 70% of castor oil is triricinolein (triricinoleoylglycerol) [8]. Castor oil is the only commercial source of ricinoleate. The presence of a hydroxyl group on a fatty acid drastically changes the physical properties of the oil, e.g., viscosity, pour point, melting point, heat of fusion, solubility, crystal structure, and polymorphism [9]. Because of the physical and chemical changes from the normal fatty acids, many industrial uses of ricinoleate have been found. Trihydroxy fatty acids and TAG containing trihydroxy fatty acids have different physical properties from those of ricinoleate and dihydroxy fatty acids, and can be used to produce bio-based products similar to those of ricinoleate with different physical properties. Both dihydroxy fatty acids and trihydroxy fatty acids have not been used in industry yet as there has been no practical source, but in the future they may be isolated from castor oil or produced by a transgenic oil seed plants.
Regiospecific isomers of TAG also affect the physical properties for industrial uses. After the identification of acylglycerols containing dihydroxy fatty acids in castor oil [1], we reported the ratios of regiospecific isomers of TAG containing dihydroxy fatty acids [10]. The ratios of the TAG regioisomers reported included the TAG of AAB type and ABC type with two and three different fatty acids, respectively. We report here the identification of acylglycerols containing trihydroxy fatty acids in castor oil as well as the regiospecific quantification of the TAG containing trihydroxy fatty acids.
Experimental Procedures
Materials
Castor oil and lithium acetate were obtained from Sigma (St. Louis, MO, USA). HPLC and GC grade methanol and 2-propanol (Burdick & Jackson) for LC–MS were purchased from VWR International (West Chester, PA, USA). High purity nitrogen for LC–MS was acquired from Praxair (Oakland, CA, USA). Research grade (99.999%) helium (Praxair) was used as a collision gas.
HPLC Fractionation of the Molecular Species of AG in Castor Oil
The fractionation of the molecular species of acylglycerols (AG) in castor oil was as previously reported [8]. Chromatographic fractionation was performed using a Waters HPLC (Waters Associate, Milford, MA, USA) and a C18 analytical column (Gemini, 250 × 4.6 mm, 5 μ, C18, Phenomenex, Torrance, CA, USA). One milligram of castor oil in ethanol (50 μl) was chromatographed at 22 °C (room temperature) with a linear gradient from 100% methanol to 100% 2-propanol in 40 min, at a 1 ml/min flow rate, and detected at 205 nm (Fig. 3). One-half minute fractions were collected and corresponding fractions were pooled from 15 HPLC runs. Fractions eluted before triricinolein (retention time 9.7 min) were used for MS studies. The final methanol sample solutions were prepared for direct infusion into the mass spectrometer by combining approximately one-fourth of each HPLC fraction (dried) with 50 μl of a methanol solution of 100 mM lithium acetate and diluting to a total volume of 250 μl.
Electrospray Ionization Mass Spectrometry
An LCQ Advantage quadrupole ion-trap mass spectrometer with Xcalibur 1.3 software (ThermoFinnigan, San Jose, CA, USA) was utilized for MS analysis of the various molecular species of AG. The infusion at a 2.5 μl/min flow rate from a syringe pump produced stable singly charged lithiated parent ions which were subsequently fragmented for MS2 and MS3 analysis. ESI source conditions were as follows: 50 arbitrary units (au) nitrogen sheath gas flow rate, 4.5 kV spray voltage, 250 °C ion-transfer capillary temperature, 1.5 m/z isolation width, 100–1,500 m/z mass range, 5 min acquisition time, 38 V capillary voltage and normalized collision energy ranging 35–40% for MS2 and MS3.
Results and Discussion
Castor oil was fractionated (0.5 min/fraction) by reversed-phase C18 HPLC (Fig. 3) and the HPLC fractions #8–19 (retention time 3.5–9.5 min) that eluted before triricinolein (retention time about 9.7 min) were used for MS studies. DAG containing trihydroxy fatty acids were in the HPLC fractions #8–11 (retention time 3.5–5.5 min) and TAG containing trihydroxy fatty acids were in the HPLC fractions #11–20 (retention time 5.0–10 min) according to the MS studies of individual fractions. The MS1 of each HPLC fraction was studied and the major ions were further studied with MS2 and MS3. Figure 4 is the MS2 spectrum of m/z 691.5, the lithium adducts of DAG, trihydroxyoleoyl-ricinoleoyl-glycerol (triOH18:1–OH18:1, non-stereospecific) and di(dihydroxyoleoyl)-glycerol (diOH18:1–diOH18:1, non-stereospecific), from HPLC fraction #9 (retention time 4.0–4.5 min). The spectrum (Fig. 4) shows the fragment ions from the neutral losses of ricinoleate, dihydroxyoleate and trihydroxyoleate as [M + Li − OH18:1]+ at m/z 393.3, [M + Li − diOH18:1]+ at m/z 377.3 and [M + Li − triOH18:1]+ at m/z 361.3 respectfully. The three free fatty acids are shown as [triOH18:1 + Li]+ at m/z 337.3, [diOH18:1 + Li]+ at m/z 321.2 and [OH18:1 + Li]+ at m/z 305.3 (barely detected). Trihydroxy fatty acid, [triOH18:1 + Li]+ at m/z 337.3, was the major fragment ion. Two other prominent ions were [M + Li − C6H13CHO]+ at m/z 577.4 from the loss of an aldehyde and [M + Li − C5H11CH=C=O]+ at m/z 579.4 from the loss of a ketene, and were recently reported (Figs. 3, 4, 5, 7, 8 of Ref. [1]). The fragment ions from the loss of C6H13CHO and C5H11CH=C=O are also shown in Fig. 5. The fragment ion of acid anhydride from the loss of glycerol backbone of DAG was detected as [M + Li − C3H4O]+ at m/z 635.5. The proposed fragmentation pathway of the acid anhydride was recently reported (Fig. 4b, c of Ref. [11]).
Ion trap mass spectrum of ESI-MS2 of DAG mixture, trihydroxyoleoyl-ricinoleoyl-glycerol (triOH18:1–OH18:1) and di(dihydroxyoleoyl)-glycerol (diOH18:1–diOH18:1), [M + Li]+ at m/z 691.5 (collision energy 38%) from HPLC fraction #9 of castor oil. triOH18:1 [trihydroxyoleate], diOH18:1 [dihydroxyoleate], OH18:1 [ricinoleate], C5H11CH=C=O [ketene], C6H13CHO [aldehyde]
Ion trap mass spectrum of ESI–MS3 of trihydroxyoleate [triOH18:1 + Li]+ at m/z 337.3 (collision energy 40%). This was from [triOH18:1 + Li]+ at m/z 337.3 shown in Fig. 4. triOH18:1 [trihydroxyoleate], C5H11CH=C=O [ketene], C6H13OCHO [aldehyde]. For proposed fragmentation pathways, see Fig. 6 a, b, c
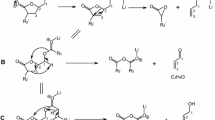
Figure 5 is the MS3 spectrum of trihydroxyoleate [triOH18:1 + Li]+ at m/z 337.3 from Fig. 4. The fragment ion, [triOH18:1 + Li − C6H13OCHO]+ at m/z 207.2 was from the loss of a hydroxyl aldehyde after the cleavage between C-11 and C-12. The proposed fragmentation pathway is shown in Fig. 6a. This is the same as our recently proposed fragmentation pathway of a vicinal dihydroxy fatty acid, diOH18:1, from the loss of an aldehyde, [diOH18:1 + Li − C6H13CHO]+ at m/z 207.1 (Fig. 6a of Ref. [1]). Since both fragment ions from triOH18:1 and diOH18:1 were the same at m/z 207, the third hydroxyl group on triOH18:1 was at one of the carbon atoms from C-13 to C-18 (Fig. 6a). The third hydroxyl group did not directly involve in the proposed fragmentation mechanism. The fragment ion in Fig. 5, [triOH18:1 + Li − C5H11CH=C=O]+ at m/z 225.1, was from the loss of a ketene as shown in the proposed fragmentation pathway of Fig. 6b. The pathway was similar to our recently proposed pathway of [diOH18:1 + Li − C5H11CH=C=O]+ at m/z 209.2 (Fig. 6b of Ref. [1]). However the third hydroxyl group of triOH18:1 at C-13 position migrated from C-13 to C-11 as shown in Fig. 6b instead of a hydrogen atom of diOH18:1 migrating from C-13 to C-11 (Fig. 6b of Ref. [1]). The proposed fragmentation pathway of m/z 195.1 in Fig. 5 is shown as Fig. 6c, [triOH18:1 + Li − C6H12O – CH2=C=O]+ at m/z 195.1, from the losses of an aldehyde and a ketene. The fragment ions from the loss of an aldehyde were common for the unsaturated hydroxy fatty acids and AG containing unsaturated hydroxy fatty acids (Figs. 3, 4, 5, 7 of Ref. [1]; Figs. 3, 5, 6, 7 of Ref. [11]; Figs. 2a, b, c; 5a, b, c, d (loss of 114, C7H14O) of Ref. [12]). The fragment ions from the loss of a ketene were also common (Figs. 3, 4, 5, 8 of Ref. [1]; Figs. 3, 5, 6, 7 of Ref. [11]). We propose that the third hydroxyl group of triOH18:1 was at position C-13 as shown in Fig. 1c. The proposed fragmentation pathways (Fig. 6) made the proposed structures of fatty acids possible including the numbers and locations of the hydroxyl group and double bonds. It is impossible to propose the structures of fatty acids if the fragmentation pathways cannot be proposed from the precursor ions to fragment ions. We depended on the proposed fragmentation pathways, Fig. 6b, c to propose the location of the third hydroxyl group at the C-13 position.
Proposed fragmentation pathways of trihydroxy fatty acids. a [triOH18:1 + Li − C6H13OCHO]+ at m/z 207.2 shown in Fig. 5. b [triOH18:1 + Li − C5H11CH=C=O]+ at m/z 225.1 shown in Fig. 5. c [triOH18:1 + Li − C6H12O – CH2=C=O]+ at m/z 195.1 shown in Fig. 5. d [triOH18:0 + Li − C7H14O2]+ at m/z 209.2 shown in Fig. 7. e [triOH18:0 + Li − C7H16O2]+ at m/z 207.2 shown in Fig. 7
Figure 7 is the MS3 spectrum of trihydroxystearate [triOH18:0 + Li]+ at m/z 339.3. This was from the base ion m/z 339.3 of the MS2 spectrum of m/z 693.5 (not shown), the lithium adducts of trihydroxystearoyl-ricinoleoyl-glycerol (triOH18:0–OH18:1) and dihydroxyoleoyl-dihydroxystearoyl-glycerol (diOH18:1–diOH18:0) from HPLC fraction #9 (retention time 4.0–4.5 min). The fragment ion at m/z 209.2 in Fig. 7 was proposed as [triOH18:0 + Li − C7H14O2]+ from the loss of an aldehyde (aldehyde group at C-12) as shown in Fig. 6d. The fragment ion at m/z 207.2 in Fig. 7 was proposed as [triOH18:0 + Li − C7H16O2]+ from the loss of an alcohol (hydroxyl group at C-12) as shown in Fig. 6e. Two other prominent fragment ions were shown as [triOH18:0 + Li − H2O]+ at m/z 321.2 and [triOH18:0 + Li − H2O − H2O]+ at m/z 303.3. We have recently reported the mass spectrum of 11,12-diOH18:0, [diOH18:0 + Li]+ at m/z 323.3 (Fig. 9 of Ref. [1]). No cleavage of the carbon–carbon bond was detected. However, Fig. 7 of triOH18:0 showed the carbon–carbon bond cleavage. The third hydroxyl group at the C-13 position facilitated the cleavage between C-11 and C-12. We proposed the third hydroxyl group at the C-13 position as shown in Fig. 1d. This is the first report of trihydroxy fatty acids with the three hydroxyl groups attached to the three adjacent carbon atoms.
Ion trap mass spectrum of ESI–MS3 of trihydroxystearate [triOH18:0 + Li]+ at m/z 339.3 (collision energy 40%). This was from the base ion m/z 339.3 of the MS2 spectrum of m/z 693.5 (not shown), the lithium adducts of trihydroxystearoyl-ricinoleoyl-glycerol (triOH18:0–OH18:1) and dihydroxyoleoyl-dihydroxystearoyl-glycerol (diOH18:1–diOH18:0) from HPLC fraction #9. triOH18:0 [trihydroxystearate], C7H14O2 [aldehyde], C7H16O2 [alcohol]. For proposed fragmentation pathways, see Fig. 6 d, e
These two trihydroxy fatty acids with the three hydroxyl groups attached to the three adjacent carbon atoms showed very low abundances of the fragment ions by three sequential dehydrations, e.g., [triOH18:1 + Li − H2O − H2O − H2O]+ at m/z 283.3 (Fig. 5), and [triOH18:0 + Li − H2O − H2O − H2O]+ at m/z 285.4 (Fig. 7). However, the abundances of both [triOH18:1 + Li − H2O]+ at m/z 319.3 (Fig. 5) and [triOH18:0 + Li − H2O]+ at m/z 321.2 (Fig. 7) from one dehydration were very high (base ions). The abundances of [diOH18:1 + Li − H2O − H2O]+ at m/z 285.3 (Fig. 5 of Ref. [1]), [diOH18:2 + Li − H2O − H2O]+ at m/z 283.2 (Fig. 8 of Ref. [1]) and [diOH18:0 + Li − H2O − H2O]+ at m/z 287.3 were low (Fig. 9 of Ref. [1]). The abundances of [diOH18:1 + Li − H2O]+ at m/z 303.2 (Fig. 5 of Ref. [1]), [diOH18:2 + Li − H2O]+ at m/z 301.2 (Fig. 8 of Ref. [1]) and [diOH18:0 + Li − H2O]+ at m/z 305.2 (Fig. 9 of Ref. [1]) were high. The fragment ions by three sequential dehydrations were not detected for the dihydroxy fatty acids (Figs. 5, 8, 9 of Ref. [1]).
We have identified DAG and TAG containing trihydroxy fatty acids as shown in Fig. 2. DAG, both triOH18:1–OH18:1 and triOH18:0–OH18:1, were identified in the HPLC fractions 8–9 (retention time 3.5–4.5 min). TAG, triOH18:1–OH18:1–OH18:1, was identified in the HPLC fractions 11–18 (retention time 5.0–9.0 min), triOH18:0–OH18:1–OH18:1 in fractions 11–16 (retention time 5.0–8.0 min), triOH18:1–OH18:1–diOH18:1 in fractions 11–17 (retention time 5.0–8.5 min), triOH18:0–OH18:1–diOH18:1 in fractions 11–13 (retention time 5.0–6.5 min). This HPLC system did not separate TAG containing trihydroxy fatty acids well. This HPLC system also did not separate TAG containing dihydroxy fatty acids well [1]. Therefore, the estimation of the content of DAG and TAG containing trihydroxy fatty acids is difficult. We have estimated the content of DAG and TAG containing dihydroxy fatty acids and trihydroxy fatty acids as about 2.5% in castor oil using HPLC and ELSD detector [8]. The content of DAG and TAG containing trihydroxy fatty acids was about 1% or less in castor oil. These TAG containing polyhydroxy fatty acids are more polar than that of triricinolein (retention time about 9.7 min, in fraction 20) and were eluted before triricinolein in the C18 reversed-phase HPLC as expected.
AG in castor oil are complicated due to different numbers and locations of hydroxyl group and double bond on the fatty acid chains, the difference in chain length and the different location of fatty acids on glycerol backbone. The AG containing trihydroxy fatty acids in castor oil are not limited to the list in Fig. 2. Some minor MS1 ions of the lithium adducts of AG from each HPLC fractions were not further studied for MS2 and MS3 due to the abundances were too low for further studies. The minor MS1 ions with the possible AG are as follows: Fraction 8 (retention time 3.5–4.0 min), m/z 707.6, triOH18:1–diOH18:1; Fractions 11-14 (retention time 5.0–7.0 min), m/z 1003.6, triOH18:1–OH18:1–triOH18:1 or triOH18:1–diOH18:1–diOH18:1; Fraction 15 (retention time 7.0–7.5 min), m/z 1019.3, triOH18:1–diOH18:1–triOH18:1; Fractions 16–17 (retention time 7.5–8.5 min), m/z 1021.3, triOH18:0–diOH18:1–triOH18:1; Fraction 18 (retention time 8.5–9.0 min), m/z 1003.4, triOH18:1–OH18:1–triOH18:1 or triOH18:1–diOH18:1–diOH18:1; Fraction 19 (retention time 9.0–9.5 min), m/z 1005.4, triOH18:0–OH18:1–triOH18:1 or triOH18:1–diOH18:1–diOH18:0.
MS is a power identification method. Earlier, molecular species of TAG in castor oil were identified by MS of trimethylsilyl derivatives [13] or underivatized TAG [2, 3, 12], but not both. We used underivatized AG for the MS identification here and earlier [1, 14]. The use of underivatized AG saves a step of derivatization and avoids the fragment ions in the mass spectra from the derivative portion of the molecules such as trimethylsilyl group.
Hydroperoxides were the primary oxidation products formed from the autoxidation of TAG [15]. Polyunsaturated fatty acids can be converted by the reaction with oxygen to hydroperoxy fatty acids by autoxidation, such as the conversion of linoleic acid to 13-hydroperoxy-9,11-octadecadienoic acid (13-HPODE), 9-HPODE, 11-HPODE, 8-HPODE and 14-HPODE [16, 17]. The hydroperoxy fatty acids from unsaturated fatty acids were multiple isomers. Hydroperoxy fatty acids may be decomposed to their corresponding hydroxy fatty acids. The dihydroxy fatty acids identified recently [1] and trihydroxy fatty acids identified here in castor oil were not isomers. Epoxy fatty acids were also the autoxidation products of unsaturated fatty acids and might be decomposed to their corresponding vicinal dihydroxy fatty acids. The location of vicinal dihydroxyl groups should be the same as the double bond of the original unsaturated fatty acids. However, the double bonds at the C-9 position were intact for the polyhydroxy fatty acids. Likely, these polyhydroxy fatty acids identified in castor oil were not the autoxidation products of fatty acids and were biosynthesized from ricinoleic acid. The formation of these polyhydroxy fatty acids are not clear, however, they are the constituents of castor oil for industrial uses and it is important to have their structures elucidated.
We have recently showed that an estolide, (12-ricinoleoylricinoleoyl)-diricinoleoyl-glycerol (RRRR), in castor oil was naturally occurring, by the biosynthesis of RRRR from radiolabeled triricinolein in castor microsomal incubation [14]. To prove that the trihydroxy fatty acids are occur naturally, a similar biosynthetic study is needed. For the biosynthetic study, the trihydroxy fatty acids need to be isolated from castor oil and the HPLC system needs to be developed for the radiochromatogram. Earlier we used radiolabelled ricinoleate for the castor microsomal incubation [18]. The radiochromatogram (Fig. 2b of Ref. [18]) of the AG showed the radioactivity at the HPLC retention times of 5–7.5 min corresponding to the region of AG containing di- and trihydroxy fatty acids. It is likely that the AG containing di- and trihydroxy fatty acids are naturally occurring compounds.
Figure 8 is the MS2 spectrum of m/z 971.6, the lithium adducts of TAG, trihydroxyoleoyl-diricinoleoyl-glycerol (triOH18:1–OH18:1–OH18:1) and di(dihydroxyoleoyl)-ricinoleoyl-glycerol (diOH18:1–diOH18:1–OH18:1), from fraction #12 (retention time 5.5–6.0 min). The ion m/z at 971.6 was the most abundant ion (MS1, not shown) in HPLC fraction 12. The spectrum (Fig. 8) shows the fragment ions from the neutral losses of ricinoleate, dihydroxyoleate and trihydroxyoleate as [M + Li − OH18:1]+ at m/z 673.5, [M + Li − diOH18:1]+ at m/z 657.5 and [M + Li − triOH18:1]+ at m/z 641.5 respectfully, in the order of their decreasing abundances which is the same as that of Fig. 4. The three free fatty acids are shown as [triOH18:1 + Li]+ at m/z 337.3, [diOH18:1 + Li]+ at m/z 321.2 and [OH18:1 + Li]+ at m/z 305.3 at very low relative abundances (Fig. 8). However, as shown in Fig. 9, the MS3 spectrum of [M + Li − OH18:1]+ at m/z 673.5 from Fig. 8, these free fatty acid lithium adducts were prominent fragment ions.
Ion trap mass spectrum of ESI–MS2 of TAG mixture, trihydroxyoleoyl-diricinoleoyl-glycerol (triOH18:1–OH18:1–OH18:1) and di(dihydroxyoleoyl)-ricinoleoyl-glycerol (diOH18:1–diOH18:1–OH18:1), [M + Li]+ at m/z 971.6 (collision energy 36%) from HPLC fraction #12 of castor oil. triOH18:1 [trihydroxyoleate], diOH18:1 [dihydroxyoleate], OH18:1 [ricinoleate], C6H13CHO [aldehyde]
Ion trap mass spectrum of ESI–MS3 of [M + Li − OH18:1]+ at m/z 673.4 from Fig. 7. triOH18:1 [trihydroxyoleate], diOH18:1 [dihydroxyoleate], OH18:1 [ricinoleate], C6H13CHO [aldehyde]
Figure 9 is the MS3 spectrum of [M + Li − OH18:1]+ at m/z 673.5 from Fig. 8, the MS2 spectrum of the lithium adducts of triOH18:1–OH18:1–OH18:1 and diOH18:1–diOH18:1–OH18:1 at m/z 971.6 (base ion, MS1) from the HPLC fraction 12 (retention time 5.5–6.0 min). Figure 9 shows the fragment ions from the loss of fatty acid as α,β-unsaturated fatty acid specific at the sn-2 position, e.g., [M + Li − OH18:1 − OH18:1 + 2]+ at m/z 377.2 and [M + Li − OH18:1 − diOH18:1 + 2]+ at m/z 361.2 [11, 19]. The loss of triOH18:1 as α,β-unsaturated triOH18:1 specific at the sn-2 position was not detected as [M + Li − OH18:1 − triOH18:1 + 2]+ at m/z 345.2. In the molecule of triOH18:1–OH18:1–OH18:1, triOH18:1 was not detected at the sn-2 position and was all at the sn-1,3 positions.
Figure 10 is the MS3 spectrum of [M + Li − OH18:1]+ at m/z 675.4 from the lithium adducts of triOH18:0–OH18:1–OH18:1 (non-stereospecific) and diOH18:1–diOH18:0–OH18:1 (non-stereospecific) at m/z 973.6 from the HPLC fraction 13 (retention time 6.0–6.5 min). The ion m/z at 973.6 was the most abundant ion (MS1, not shown) in HPLC fraction 13 (retention time 6.0–6.5 min). The MS2 spectrum of this ion (not shown) showed the neutral losses as [M + Li − triOH18:0]+ at m/z 641.4, [M + Li − diOH18:0]+ at m/z 657.4, [M + Li − diOH18:1]+ at m/z 659.4 and [M + Li − OH18:1]+ at m/z 675.4. Figure 10 shows the fragment ions from the loss of fatty acid as α,β-unsaturated fatty acid specific at the sn-2 position, e.g., [M + Li − OH18:1 − OH18:1 + 2]+ at m/z 379.2 and [M + Li − OH18:1 − diOH18:0 + 2]+ at m/z 361.2. The loss of triOH18:0 as α,β-unsaturated triOH18:0 specific at sn-2 was not detected as [M + Li − OH18:1 – triOH18:0 + 2]+ at m/z 345.3. In the molecule of triOH18:0–OH18:1–OH18:1, triOH18:0 was not detected at the sn-2 position and was all at the sn-1,3 positions.
Ion trap mass spectrum of ESI–MS3 of [M + Li − OH18:1]+ at m/z 675.4 from the lithium adducts of triOH18:0–OH18:1–OH18:1 and diOH18:1–diOH18:0–OH18:1 at m/z 973.6 from the HPLC fraction 13 of castor oil (MS1 and MS2 spectra, not shown). diOH18:1 [dihydroxyoleate], OH18:1 [ricinoleate], C6H13CHO [aldehyde], triOH18:0 [trihydroxystearate]
There are other examples (spectra not shown) which showed that the trihydroxy fatty acids were not detected (or only as a trace ) at the sn-2 position of TAG in castor oil: (1) [M + Li − OH18:1 − triOH18:1 + 2]+ at m/z 361.2 was not detected in the MS3 spectrum of [M + Li − OH18:1]+ at m/z 689.5. M was the mixture of triOH18:1–OH18:1–diOH18:1 and diOH18:1–diOH18:1–diOH18:1 (lithium adducts at m/z 987.6) from the HPLC fraction 12 (retention time 5.5–6.0 min). (2) [M + Li − diOH18:1 − triOH18:1 + 2]+ at m/z 345.2 was not detected in the MS3 spectrum of [M + Li − diOH18:1]+ at m/z 673.4. M was the mixture of triOH18:1–OH18:1–diOH18:1 and diOH18:1–diOH18:1–diOH18:1 (lithium adducts at m/z 987.6) from the HPLC fraction 12 (retention time 5.5–6.0 min). (3) [M + Li − diOH18:1 − triOH18:0 + 2]+ at m/z 345.2 was not detected in the MS3 spectrum of [M + Li − diOH18:1]+ at m/z 675.4. M was the mixture of triOH18:0–OH18:1–diOH18:1 and diOH18:1–diOH18:1–diOH18:0 (lithium adducts at m/z 989.5) from the HPLC fraction 13 (retention time 6.0–6.5 min). (4) [M + Li − OH18:1 − triOH18:0 + 2]+ at m/z 361.2 was detected in trace only in the MS3 spectrum of [M + Li − OH18:1]+ at m/z 691.4. M was the mixture of triOH18:0–OH18:1–diOH18:1 and diOH18:1–diOH18:1–diOH18:0 (lithium adducts at m/z 989.5) from the HPLC fraction 13 (retention time 6.0–6.5 min).
We have identified DAG and TAG containing trihydroxy fatty acids in castor oil as shown in Fig. 2. The structures of the trihydroxyl fatty acids were proposed as 11,12,13-triOH18:1 and 11,12,13-triOH18:0 (Fig. 1). The content of DAG and TAG containing trihydroxy fatty acids was about 1% of castor oil or less. The regiospecific studies of the TAG containing trihydroxy fatty acids in castor oil showed that 100% or almost 100% of trihydroxy fatty acids were at the sn-1,3 positions. Ricinoleate was predominately at the sn-2 position of the TAG containing dihydroxy fatty acids and ricinoleate in castor oil [10]. The normal fatty acid was predominately at the sn-2 position of diricinoleoyl-acyl-glycerols in castor oil [11].
References
Lin JT, Arcinas A, Harden AH (2009) Identification of acylglycerols containing dihydroxy fatty acids in castor oil by mass spectrometry. Lipids 44:359–365
Stübiger G, Pittenauer E, Allmaier G (2003) Characterization of castor oil by on-line and off-line non-aqueous reversed-phase high-performance liquid chromatography-mass spectrometry (APCI and UV/MALDI). Phytochem Anal 14:337–346
Weber H, Scholten F, Leinz G (2006) Triacylglycerol analysis of castor oil by online HPLC–ESI/MSn. Dtsch Lebensm Rundsch 102:239–245
Espelie KE, Dean BB, Kolattukudy PE (1979) Composition of lipid-derived polymers from different anatomical regions of several plant species. Plant Physiol 64:1089–1093
Graca J, Pereira H (2000) Methanolysis of bark suberins: analysis of glycerol and acid monomers. Phytochem Anal 11:45–51
Mikolajczak KL, Smith CR (1967) Optically active trihydroxy acids of Chamaepeuce seed oils. Lipids 2:261–265
Kim H, Kuo TM, Hou CT (2000) Production of 10,12-dihydroxy-8(E)-octadecenoic acid, an intermediate in the conversion of ricinoleic acid to 7,10,12-trihydroxy-8(E)-octadecenoic acid by Pseudomonas aeruginosa PR3. J Ind Microbiol Biotechnol 24:167–172
Lin JT, Turner C, Liao LP, McKeon TA (2003) Identification and quantification of the molecular species of acylglycerols in castor oil by HPLC using ELSD. J Liq Chromatogr Relat Technol 26:773–780
Foubert I, Dewettinck K, Van de Walle D, Dijkstra AJ, Quinn PJ (2007) Physical properties: Structural and physical characteristics. In: Gunstone FD, Harwood JL, Dijkstra AJ (eds) The lipid handbook, vol 8, 3rd edn. CRC Press, Taylor & Francis Group, Boca Raton, pp 535–590
Lin JT (2009) Ratios of regioisomers of triacylglycerols containing dihydroxy fatty acids in castor oil by mass spectrometry. J Am Oil Chem Soc 86:1031–1035
Lin JT, Arcinas A (2007) Regiospecific analysis of diricinoleoylacylglycerols in castor (Ricinus communis L.) oil by electrospray ionization-mass spectrometry. J Agric Food Chem 55:2209–2216
Byrdwell WC, Neff WE (1998) Analysis of hydroxyl-containing seed oils using atmospheric pressure chemical ionization mass spectrometry. J Liq Chromatogr Relat Technol 21:1485–1501
Hogge LR, Taylor DC, Reed DW, Underhill EW (1991) Characterization of castor bean neutral lipids by mass spectrometry/mass spectrometry. J Am Oil Chem Soc 68:863–868
Lin JT, Arcinas A, Harden LA, Fagerquist CK (2006) Identification of (12-ricinoleoylricinoleoyl)diricinoleoylglycerol, and acylglycerol containing four acyl chains, in castor (Ricinus communis L.) oil by LC–ESI–MS. J Agric Food Chem 54:3498–3504
Byrdwell WC, Neff WE (1999) Non-volatile products of triolein produced at frying temperatures characterized using liquid chromatography with online mass spectrometric detection. J Chromatogr A 852:417–432
Garscha U, Nilsson T, Oliw EH (2008) Enantiomeric separation and analysis of unsaturated hydroperoxy fatty acids by chiral column chromatography–mass spectrometry. J Chromatogr B 872:90–98
Schneider C, Pratt DA, Porter NA, Brash AR (2007) Control of oxygenation in lipoxygenase and cyclooxygenase catalysis. Chem Biol 14:473–488
Lin JT, Chen JM, Liao LP, McKeon TA (2002) Molecular species of acylglycerols incorporating radiolabeled fatty acids from castor (Ricinus communis L.) microsomal incubations. J Agric Food Chem 50:5077–5081
Hsu FF, Turk J (1999) Structural characterization of triacylglycerols as lithiated adducts by electrospray ionization mass spectrometry using low-energy collisionally activated dissociation on a triple stage quadrupole instrument. J Am Soc Mass Spectrom 10:587–599
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lin, JT., Chen, G.Q. Acylglycerols Containing Trihydroxy Fatty Acids in Castor Oil and the Regiospecific Quantification of Triacylglycerols. J Am Oil Chem Soc 87, 1371–1379 (2010). https://doi.org/10.1007/s11746-010-1617-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-010-1617-7