Abstract
The hydrophobation of rosmarinic acid with saturated aliphatic primary alcohols of various chain lengths (methanol to eicosanol) was achieved via an acid-catalyzed esterification in the presence of a highly acidic sulfonic resin. The resulting alkyl rosmarinates were isolated, characterized and their global free radical scavenging activity was determined by the 2,2-diphenyl-1-picrylhydrazyl method in the stationary state. Only the dodecyl ester showed a stronger activity than rosmarinic acid.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rosmarinic acid (RO) [(R)-a-[[3-(3,4-dihydroxyphenyl)-1-oxo-2E-propenyl]oxy]-3,4-dihydroxy-benzenepropanoic acid] is a polyphenolic compound present in high amount (≥3% w/w dry matter) in many herbal plants [1] of the Lamiaceae family such as rosemary (Rosmarinus officinalis), lemon balm (Melissa officinalis), sage (genus Salvia), oregano (Origanum vulgare) or savory (genus Satureja). Several biological activities have been described for rosmarinic acid, the most known being anti-inflammatory [2] antiviral [3] and antioxidative [4, 5]. Concerning the later activity, rosmarinic acid as well as its naturally occurring methyl, ethyl and butyl esters have shown strong free radical scavenging properties, especially towards superoxide anion [6]. These properties make them good candidates to replace some current synthetic antioxidants (i.e. butylated hydroxytoluene and butylated hydroxyanisole) in foods and cosmetics, which are increasingly controversial [7]. However, the implementation of such polar molecules in lipid-based systems (emulsion or other) is difficult and can lead to a decrease of their ability to counteract oxidation of unsaturated lipids at the oil–water interface [8, 9]. To solve this issue, one strategy consists of adjusting their polarity by the grafting of aliphatic chains of increasing length [10, 11]. Herein, we reported for the first time the synthesis and characterization (1H-13C-NMR and ESI-MS) of alkyl esters of rosmarinic acid with carbon chain lengths ranging from 1 to 20 carbon atoms (compounds R1–R20), and the evaluation of their global free radical scavenging activity by the well known 2,2-diphenyl-1-picrylhydrazyl (DPPH) method.
Materials and Methods
Materials
Sulfonic resin Amberlite® IR-120H, molecular sieve (3 Å), rosmarinic acid (96.5%), 2,2-diphenyl-1-picrylhydrazyl, n-butanol (99.8%), n-octanol (99%), n-dodecanol (99.5%), n-hexadecanol (99%), n-octadecanol (99%), n-eicosanol (98%) and solvents (analytical or HPLC grade) were purchased from Sigma-Aldrich (Saint Quentin, France). Silica (60–200 μm, pore size 60 Å) was from Acros organics (Geel, Belgium). Available immobilized lipase from C. Antarctica lipase B (Novozym® 435) was purchased from Novozymes A/S (Bagsvaerd, Denmark).
Methods
Chemical Synthesis and Purification of Alkyl Rosmarinates
The chemical esterification of rosmarinic acid (56 μmol) was carried out in sealed flasks each containing 5 mL of alcohol (methanol, 123.44 mmol; n-butanol, 54.64 mmol; n-octanol, 31.905 mmol; n-dodecanol, 22.46 mmol; n-hexadecanol, 16.95 mmol; n-octadecanol, 15.09 mmol and n-eicosanol, 13.6 mmol). The reaction mixtures were stirred (orbital shaker, 250 rpm, 55–70 °C) prior to the addition of the catalyst, the strongly acidic sulfonic resin Amberlite® IR-120H (5% w/w—total weight of both substrates) previously dried at 110 °C for 48 h. The water generated during the reaction was removed by absorption on molecular sieves (40 mg/mL) added to the medium. Samples (20 μL) were regularly withdrawn from the reaction medium then mixed with 980 μL of methanol, filtered (0.45 μm syringe filter Millex®-FH, Millipore Corporation Bedford, MA, USA) and finally analyzed by reverse phase HPLC with UV detection at 328 nm. After complete (4–21 days) conversion of rosmarinic acid into the corresponding ester the latter was purified in a two-step procedure. Firstly, a liquid–liquid extraction using hexane and acetonitrile was achieved to remove the excess of alcohol. Then, the remaining traces of the alcohol and rosmarinic acid were eliminated by flash chromatography on a CombiFlash® Companion® system (Teledyne Isco Inc., Lincoln, NE, USA). Separation was achieved on a silica column using an elution gradient of hexane and ether (20–100% in 35 min). The purity of the esters, obtained as pale yellow to yellow amorphous powders, was checked by HPLC and the absence of residual alcohol was confirmed by TLC.
HPLC Monitoring of Rosmarinic Acid Conversion
Monitoring of ester formation was carried out with a ACE® 5 C18 reversed phase column (5 μm, 250 × 4.6 mm, 100 Å) (ACT, Aberdeen, Scotland) using a Dionex Ultimate 3000 HPLC system (Dionex, Jouy En Josas, France). Peak integration was performed using Chromeleon software (Version 6.8). Briefly, gradient elution was performed using methanol and phosphoric acid 3 mM at 1 mL/min and 25 °C, in linear gradients from 0/100 (v/v) to 100/0 (v/v) for 30 min, then 100/0 (v/v) for 10 min and back to 0/100 (v/v) in 5 min. Rosmarinic acid (R0) and its esters (R1–R20) were detected under UV light at 328 nm and their retention time, in minutes, were (R0): 19.6, (R1): 21.4, (R4): 25.4, (R8): 29.6, (R12): 32.5, (R16): 34.4, (R18): 35.5 and (R20): 36.7 . As the alkyl rosmarinate were the only product of the reaction between rosmarinic acid and the alcohol, the molar yield of the ester formed was defined as follows:
It was calculated from the calibration curves established from purified esters. The reaction was stopped when the molar yield of the ester was at a maximum.
ESI-MS, 1H-NMR and 13C-NMR Characterization
Pure esters were first characterized by electrospray ionisation-mass spectrometry (ESI-MS) in negative mode using a mass spectrometer composed of a quadrupole ion trap mass analyzer, an external atmospheric pressure ion source and an integrated syringe pump (Finnigan LCQ Serie MS detector, San Jose, CA, USA). Methanolic solutions of esters (100 mg/L) were directly injected into the ESI-source (infusion mode) at a 5-μL/min flow rate. The zoom scan mode was used for an accurate determination of the parent ion m/z, while fragment ions were obtained in MS2 mode. Basic operating conditions in the negative mode were the following: collision energy of 30–35%, ionization voltage of 4.5 kV, capillary temperature of 300 °C and a sheath gas (nitrogen) flow of 20 arbitrary units.
1H- and 13C-NMR spectra were recorded in DMSO with a Bruker DRX 400 spectrometer at 400 and 100 MHz respectively. In the case of alkyl rosmarinates represented as RCO–O–αCH2–βCH2–(CH2)n−3–CH3, three types of methylene were distinguished in the alkyl chain for the attribution of chemical shifts: –αCH2– and –βCH2– correspond to methylene in alpha- and beta-position related to oxygen respectively, and –(CH2)n−3– correspond to the other methylene groups, where n is the total number of carbon of the alkyl chain.
Radical Scavenging Activity Assessment by the DPPH Method and Determination of Stationary Parameters
Basically, the DPPH method consists in measuring the ability of a molecule to reduce the 2,2-diphenyl-1-picrylhydrazyl radical (DPPH·) in methanol and its subsequent bleaching at 515 nm. The radical scavenging activity of rosmarinic acid and its esters against DPPH· was performed spectrometrically at 515 nm and 20 °C, as described by Brand-Williams et al. [12] and recently applied to chlorogenate fatty esters by López Giraldo et al. [13]. Briefly, the absorbance decay was monitored on solutions consisting in 1,950 μL methanolic DPPH· solution (60 μM) and 50 μL methanolic solution of phenolic compound (14.6–585 μM). The absorbance decay was monitored each minute until it reached a steady state (14 h for rosmarinic acid and 6 h for its esters) that corresponds to the complete consumption of the antioxidant. In absence of antioxidant (blank), the spontaneous DPPH· bleaching during the experiment appeared to be low and did not exceed 3–4% and 6–7% after 6 and 14 h respectively. However, a correction of this intrinsic DPPH· bleaching was systematically made in order to avoid any bias. The global radical scavenging activity was defined as the total number of reduced DPPH· at stationary state (n ss) and expressed as a mol DPPH· radical reduced by a mol of tested compound. It was calculated as 1/(2 × EC50) where EC50 corresponds to the amount of antioxidant necessary to reduce the initial DPPH· concentration by 50%. All determinations were done in triplicate.
Results and Discussion
Synthesis of Alkyl Ester of Rosmarinic Acid
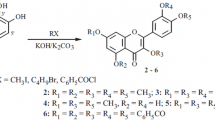
In a first attempt, rosmarinic acid or its methyl ester were enzymatically esterified following a procedure already used in a previous work [10] for the esterification of chlorogenic acid (5-caffeoyl quinic acid, 5-CQA). However, none of the corresponding esters was obtained whatever the alcohol used and the reaction conditions. The reason for such a failure was attributed to the putative inhibition of the enzyme due to steric hindrance at the catalytic site. Then, alkyl rosmarinates were obtained by acid catalyzed esterification of rosmarinic acid with the corresponding saturated aliphatic primary alcohols (Fig. 1) in the presence of a highly acidic sulfonic resin. The reaction was achieved at low temperature (55–70 °C) to avoid rosmarinic degradation, in an excess of the alcohol that played, when melted, both the role of substrate and solvent. The reaction was stopped after 4–21 days, when the molar yield of the ester has reached its maximum: (R1): 98.5%, (R4): 99.3%, (R8): 99.5%, (R12): 94.4%, (R16): 81.6%, (R18): 99.0% and (R20): 99.0%. It corresponded to the quasi complete conversion of rosmarinic acid, except for hexadecyl rosmarinate where the maximum acid conversion was 82%. After the purification steps, the purity of esters was at least 96% and only traces of rosmarinic acid were detected by HPLC. The remaining 4% were attributed to the impurities already present in the starting material (rosmarinic acid, 96.5% purity) or to their derivatives, that could not be eliminated by flash chromatography.
NMR and MS Characterization of Alkyl Rosmarinates
Rosmarinic acid and its esters were first characterized by 1H NMR and 13C NMR (Table 1). Data obtained for rosmarinic acid, and its methyl and butyl esters were highly consistent with those reported in the literature [14–16]. For alkyl chains of more than four carbon atoms, 1H NMR of –αCH2–, –βCH2– and other methylene group give multiplets with chemical shifts of 4.07–4.03, 1.54–1.51 and 1.37–1.23 ppm respectively. On the other hand, 13C-NMR chemical shifts of the same methylene were respectively 64.6–64.3, 31.3–31.1 and 29.3–22.1 ppm.
ESI-MS spectra (full scan mode) of both rosmarinic acid and its esters (Table 2) showed two peaks corresponding to the molecular ion of the deprotonated compound [MM–H] and its adduct [2MM–H]. The MS2 fragmentation of rosmarinic acid molecular ion (358.97) lead to three peaks at 197, 179 and 161 (m/z) corresponding to the deprotonated form of 3-(3,4-dihydroxyphenyl)lactic and caffeic acids and their dehydrated forms, respectively (Fig. 2; Table 2). These results were in agreement with the fragmentation scheme proposed by Møller et al. [17].
MS2 fragmentation scheme of rosmarinic acid as proposed by Møller et al. [17]
In case of alkyl rosmarinates, fragmentation of molecular ion always leads to only two peaks at 179 and 135 (m/z) whose correspond to the deprotonated form of caffeic acid and its residue after decarboxylation, respectively (Fig. 3; Table 2). The differences in fragmentation between rosmarinic acid and its esters may be due to the higher weakness of the ester bond at carbon 9 compared to carbon 9′.
As a consequence, the fragmentation of molecular ion of each ester theoretically led to the breaking of this weaker ester bond into the deprotonated form of caffeic acid and 3-(3,4-dihydroxyphenyl) lactates. However, the fragment containing the alkyl chain was not detected under our working conditions.
Radical Scavenging Properties of Alkyl Rosmarinates
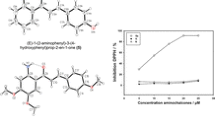
In terms of antioxidant properties, we reported in Table 3 the global free radical scavenging activity (n ss) of rosmarinic acid and its ester determined by the DPPH method. The global free radical scavenging activity was defined as the total mole of DPPH reduced by mole of antioxidant at stationary state. Surprisingly, it appeared that only dodecyl rosmarinate (R12) with a n ss value of 12.36 ± 0.19 could reduce more DPPH· than rosmarinic acid (n ss = 9.78 ± 0.06).
Indeed, in a similar work on chlorogenic acid (5-CQA) and its alkyl esters [13] it was found that all chlorogenates exhibit higher n ss values (6.2–8.5) than 5-CQA (4.9). However, it is worth mentioning that rosmarinic acid reached a steady state in 14 h, whereas all esters reached it in 6 h. Interestingly, such a strong influence of the esterification on the DPPH· bleaching was already observed with chlorogenic acid and its alkyl ester [13] and protocatechuic acid and its methyl ester [18]. In addition, it can be observed that all n ss values were greater than 4 which is the number of available phenolic hydroxyl groups present in each tested molecule and known as being responsible for the antiradical activity. These results suggest, as proposed by other authors [19] the possible nucleophilic methanol regeneration of oxidized forms (quinone) of antioxidant into new reducing phenolic compounds and its combination with dimerization phenomena. In the future, further investigations on structure of reaction products will be required to determine the part of each mechanism involved according to alkyl chain length.
In conclusion, this work relates the first heterogeneous synthesis of a homologous series of alkyl rosmarinates of chain lengths ranging from 1 (methyl) to 20 (eicosyl) carbon atoms and their full characterization. In terms of global radical scavenging properties according to the DPPH method at the stationary sate, it shows that the grafting of an alkyl chain to rosmarinic acid does not necessarily lead to an increase of its stoichiometry against DPPH· (except for dodecyl), despite the drastic acceleration of the free radical scavenging process.
References
Lamaison JL, Petitjean-Freytet C, Carnat A (1991) Medicinal Lamiaceae with antioxidant properties, a potential source of rosmarinic acid. Pharm Acta Helv 66:185–188
Osakabe N, Takano H, Sanbongi C, Yasuda A, Yanagisawa R, Inoue K, Yoshikawa T (2004) Anti-inflammatory and anti-allergic effect of rosmarinic acid (RA); inhibition of seasonal allergic rhinoconjunctivitis (SAR) and its mechanism. Biofactors 21:127–131
Swarup V, Ghosh J, Ghosh S, Saxena A, Basu A (2007) Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitis. Antimicrob Agents Chemother 51:3367–3370
Laguerre M, López-Giraldo LJ, Lecomte J, Baréa B, Cambon E, Tchobo PF, Barouh N, Villeneuve P (2008) Conjugated autoxidizable triene (CAT) assay: a novel spectrophotometric method for determination of antioxidant capacity using triacylglycerol as ultraviolet probe. Anal Biochem 380:282–290
Exarchou V, Nenadis N, Tsimidou M, Gerothanassis IP, Troganis A, Boskou D (2002) Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage, and summer savory. J Agric Food Chem 50:5294–5299
Choudhary MI, Begum A, Abbaskhan A, Ajaz A, Shafique-Ur-Rehman, Atta-Ur-Rahman (2005) Phenyl polypropanoids from Lindelofia stylosa. Chem Pharm Bull 53:1469–1471
Iverson F (1995) Phenolic antioxidants: health protection branch studies on butylated hydroxyanisole. Cancer Lett 93:49–54
Decker EA, Warner K, Richards MP, Shahidi F (2005) Measuring antioxidant effectiveness in food. J Agric Food Chem 53:4303–4310
Frankel EN, Meyer AS (2000) The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J Sci Food Agric 80:1925–1941
López Giraldo LJ, Laguerre M, Lecomte J, Figueroa-Espinoza MC, Barouh N, Baréa B, Villeneuve P (2007) Lipase- catalyzed synthesis of chlorogenate fatty esters in solvent-free medium. Enzyme Microb Technol 41:721–726
Figueroa-Espinoza MC, Villeneuve P (2005) Phenolic acids enzymatic lipophilization. J Agric Food Chem 53:2779–2787
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 28:25–30
López Giraldo LJ, Laguerre M, Lecomte J, Figueroa-Espinoza MC, Baréa B, Weiss J, Decker EA, Villeneuve P (2009) Kinetic and stoichiometry of the reaction of chlorogenic acid and its alkyl esters against the DPPH radical. J Agric Food Chem 57:863–870
Lu Y, Foo LY (1999) Rosmarinic acid derivatives from Salvia officinalis. Phytochemistry 51:91–94
Fecka I, Turek S (2008) Determination of polyphenolic compounds in commercial herbal drugs and spices from Lamiaceae: thyme, wild thyme and sweet marjoram by chromatographic techniques. Food Chem 108:1039–1053
Huang H, Chao QR, Tan RX, Sun HD, Wang DC, Ma J, Zhao SX (1999) A new rosmarinic acid derivative from Isodon oresbius. Plant Med 65:92–93
Møller JKS, Catharino RR, Eberlin MN (2007) Electrospray ionization mass spectrometry fingerprinting of essential oils: spices from the Labiatae family. Food Chem 100:1283–1288
Saito S, Kawabata J (2006) DPPH (=2, 2-diphenyl-1-picrylhydrazyl) radical-scavenging reaction of protocatechuic acid (=3, 4-dihydroxybenzoic acid): difference in reactivity between acids and their esters. Helv Chim Acta 89:1395–1407
Saito S, Kawabata J (2005) Effects of electron-withdrawing substituents on DPPH radical scavenging reactions of protocatechuic acid and its analogues in alcoholic solvents. Tetrahedron 61:8101–8108
Acknowledgments
Luis Javier López Giraldo thanks the support from Programme Alβan, the European Union Programme of High Level Scholarships for Latin America, scholarship N°. E05D055786CO.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lecomte, J., Giraldo, L.J.L., Laguerre, M. et al. Synthesis, Characterization and Free Radical Scavenging Properties of Rosmarinic Acid Fatty Esters. J Am Oil Chem Soc 87, 615–620 (2010). https://doi.org/10.1007/s11746-010-1543-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-010-1543-8