Abstract
Three commercial immobilized lipases, Lipozyme RM IM, Lipozyme TL IM and Novozym 435, were screened for the production of monoacylglycerols (MAG) by glycerolysis of camellia oil in a solvent medium of tert-butyl alcohol. Novozym 435 showed the best performance and was selected to catalyze the glycerolysis reaction. Different reaction conditions for the batch reaction, substrate mole ratio, substrate concentration and temperature, were investigated. The optimal reaction conditions were determined as 6:1 mole ratio of glycerol to camellia oil at 40% (w/v) of substrate concentration in tert-butyl alcohol at a reaction temperature of 50 °C. Under these optimal conditions, the conversion rate of camellia oil was 98.7% (10 h), and the mixture of acylglycerols contained 82.0% of MAG. A packed-bed reactor (PBR) system with 4.5 g Novozym 435 was employed in continuous production. The resulting product mixture of acylglycerols contained 80.74% of MAG and was obtained at a flow rate of 0.25 mL/min of substrates. The long-term operation of the PBR system gave an average productivity of 0.698 kg MAG/(kg enzyme h) after 38 days of operation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It has been estimated that approximately 200,000–250,000 metric tons of emulsifiers are produced per year worldwide, of which monoacylglycerols (MAG) account for approximately 75% of the total [1]. Commercial MAG are widely used in the food, pharmaceutical and cosmetic industries. Almost all of these MAG have saturated fatty acids in their chemical structure because their chemical manufacture involves processing at high temperature with hydrogenated oil and fats [2]. With the appearance of a commercial GMO (glycerol monooleate) product, it was found that MAG with unsaturated fatty acids have different characteristics. For an example, unsaturated glycerol monooleate has a destabilization power better than that of saturated MAG [3]. Also, unsaturated glycerol monooleate has more potential applications for the production of ice cream, because of good melting resistance [4], and in other food processing and industries. Moreover, unsaturated MAG is better for people’s health than saturated MAG. Recently, more attention has been focused on the production of new MAG with unsaturated fatty acids.
MAG have been produced mainly by chemical methods at high temperatures which has some drawbacks such as dark-colored by-products and an undesirable flavor. More importantly, unsaturated fatty acids, which have beneficial health and functional properties, may be destroyed during the high temperature process [5, 6]. Compared to the chemical process, enzymatic catalysis for the modification of fats and oils is a good way of solving the problems. The enzymatic reaction could be performed under mild conditions effectively, and specifically afford a product with a higher yield and a better quality [1, 7]. Different enzymatic reactions, such as alcoholysis, esterification and glycerolysis, have been used to produce MAG with unsaturated fatty acids [1, 2, 8–13]. The enzymatic glycerolysis reaction seems to be a very attractive process, not only in terms of the production of heat-sensitive compounds but also in terms of higher MAG contents [1].
For glycerolysis reactions, a solvent-free system needs fewer purification steps but the yield is usually lower due to poor miscibility between the oil and glycerol phases. Solvent systems provides better miscibility and thus enhance mass transfer, which increases the yield of MAG. tert-Butanol is a good solvent in such applications, with advantages such as better solubility with glycerol, it does not react with the substrates and has a high reaction efficiency [1].
The aim of this research was to develop an efficient process for producing MAG with a high content of oleic acid by the glycerolysis reaction in tert-butanol with camellia oil. Camellia oil is produced from the seed of Camellia oleifera Abel which is naturally cultivated in China. It is a good source of oleic acid which accounts for approximately 76% of the total fatty acids in contrast to other oil sources such as sunflower oil and rapeseed oil. Three immobilized lipases, Novozym 435 (from Candida antarctica), Lipozyme RM IM (from Rhizomucor miehei) and Lipozyme TL IM (from Thermomyces lanuginose), were screened. Different reaction conditions for the batch reaction, the substrate mole ratio, the substrate concentration and temperature, were investigated. A continuous operation of MAG production using a packed-bed reactor (PBR) was also explored.
Experimental Procedures
Materials
Novozym 435 (from Candida antarctica), Lipozyme RM IM (from Rhizomucor miehei) and Lipozyme TL IM (from Thermomyces lanuginose) were kindly provided by Novozymes A/S (Bagsvaerd, Denmark). Isopropyl alcohol (99.8%), n-hexane (97%) and tert-butanol (99%) were purchased from the Guangzhou Chemical Reagent Factory (Guangzhou, China). Methanolic boron trifluoride solution (12–15% as BF3) was obtained from Sigma–Aldrich. Camellia oil was purchased from Kerry Oils & Grains Ltd (Shenzhen, China): 0.06% water; 0.05% FFA; peroxide value 0.3 mequiv/kg; fatty acids C16:0, 8.238%; C18:0, 2.079%; C18:1, 76.206%; C18:2, 9.636%; and others, 3.841% (w/w). The glycerol (99.5%) contained 0.2% water.
Glycerolysis Reaction
Batch Reaction
The glycerolysis experiments were carried out in a 50-mL conical flask with stirring in an orbital shaking water bath at 180 rpm. The reaction mixture was composed of triacylglycerols (camellia oil), tert-butyl alcohol, glycerol, and lipase. Three commercially available immobilized lipases, Novozym 435, LypoZyme RM IM, and Lipozyme TL IM were screened for the production of MAG by incubating the lipases with a 4:1 M ratio of glycerol and camellia oil at a 40% (w/v) of total substrate concentration in the tert-butyl alcohol at 50 °C. The reaction temperature (40, 50, and 60 °C), the substrate concentration (30, 40, and 50%, w/v, based on total substrates) and molar ratio of glycerol/camellia oil (3:1, 4:1, 5:1, 6:1; 7:1) were altered to study their effects on the conversion rate of camellia oil and the MAG content in the acylglycerols when Novozym 435 was selected as the catalyst. Aliquot fractions (0.10 mL) of reaction mixture were periodically removed from the reaction for HPLC analysis. Each experiment was prepared in triplicate, and the results were expressed as means ± standard deviation.
Continuous Reaction by PBR
The continuous glycerolysis experiments were carried out by PBR. The Novozym 435 was packed into a column (20 × 1 cm, i.d.). The reaction mixture was prepared as 40% (w/v) of glycerol and camellia oil (6:1 mole ratio) in tert-butyl alcohol, and was constantly mixed by magnetic stirring, and reaction temperature was held at 50 °C. The reaction mixture was added to the bottom of the column at a flow rate of 0.25 mL/min. The product was removed at the top of the column. Aliquot fractions (0.10 mL) of reaction mixture were periodically removed from the reaction for HPLC analysis.
After continuous process of 38 days, the reaction was suspended and the reaction mixture was collected. The tert-butanol was removed by rotatory evaporator (0.1 MPa, 60 °C, RE-52A, Shanghai Yarong Company). The glycerol was removed by molecular distillation (MD—S80 short path falling film distiller, Handway Co. Ltd). The evaporating and feed temperatures were 100 and 60 °C, respectively, the feed flow rate was 1.5 mL/min, the condenser temperature was set at 40 °C, and the residual pressure was 8 Pa. The composition of the separated acylglycerols was analyzed by HPLC analysis.
HPLC Analysis of Acylglycerols in the Reaction Mixture
A 0.1-mL sample of the reaction mixture was transferred into a centrifuge tube, and 1 mL of n-hexane and isopropyl alcohol (10:1 v/v) was added and mixed by vortex. The mixture was centrifuged at 10,000×g for 1 min to remove the glycerol. 10 μL of supernatant was used for HPLC analysis.
A Waters 2695 HPLC with a refractive index detector was used to analyze the MAG and the TAG contents in the mixture of acylglycerols produced by the glycerolysis reaction. A Phenomenex 00G-4274-E0 column (Phenomenex Corporation, 4.6 mm i.d. × 250 mm, 5 μm particle size) was used. The mobile phase was n-hexane and isopropyl alcohol (10:1 v/v). The flow rate was 1 mL/min. Peaks in HPLC were evaluated by comparison of their retention times with those of known standards. Peak percentages and areas were calculated using Waters 2695 integration software. Analysis was carried out in triplicate, and the values were of the average of triplicate measurements of the duplicate samples ± standard deviation. The MAG content in the mixture of acylglycerols was presented in a form of percentage in the acylglycerols, and the conversion rate of TAG was calculated (Eq. 1):
Analysis of Fatty Acid Compositions of MAG
The identification of acylglycerols by TLC was carried out on GF254 silica gel plates (100 × 200 mm, 0.20–0.25 mm of thickness, Branch of Qingdao Haiyang Chemical Plant, Qingdao) activated by heating at 105 °C for 30 min. A 100-μL sample was dissolved in 900 μL of chloroform/methanol (2:1 v/v) and was mixed by vortex, and then 200 μL of the mixture was spotted onto the plate. The plate was developed for 35 min in a mixture of benzene/chloroform/acetic acid (50:20:0.7, v/v/v) as the developing solvent [2]. After development and drying, spots of each lipid were examined under 254-nm UV light.
The MAG band were scraped off the plate and methylated according to ISO 5509:2000(E) (Animal and vegetable fats and oils—preparation of methyl esters of fatty acids). The sample was introduced into a 50-mL flask, then 4 mL of 0.5 mol/L methanolic sodium hydroxide solution was added and boiled under reflux for about 10 min, after an addition of 5 mL of methanolic boron trifluoride solution (12–15% as BF3). The boiling was continued for a further 3 min. Two milliliters of isooctane was added to the boiling mixture at the top of the condenser. The flask was removed immediately. Twenty milliliters of saturated sodium chloride solution was added to the flask. The flask was covered and was shaken vigorously for at least 15 s. More saturated sodium chloride solution was added to bring the liquid level of the mixture to the neck of the flask. After the separation of the two phases (the isooctane phase and the saturated sodium chloride solution phase), 1 mL of the upper isooctane layer was transferred to a 4-mL vial and a small amount of anhydrous sodium sulfate was added to remove any traces of water.
A Hewlett-Packard 7890 GC was used to analyze the fatty acid composition of methyl esters that was produced in esterification. The products were separated on a FFFAP column (PERMABOND-FFFAP DF-0.25, 25 m × 0.25 mm i.d., Macherey-Nagel, Germany) using nitrogen as the carrier gas. A temperature program was used by keeping the samples in a column oven at 150 °C for 2 min. The temperature was then increased to 230 °C at 10 °C/min and held for 8 min for a total run time of 18 min. The split ratio was 20:1. The injector and the flame ionization detector temperature were set at 250 and 300 °C, respectively. Nonadecanoic acid (19:0) (Sigma–Aldrich) was used as the standard for qualitative determination of the fatty acids.
Results and Discussion
Screening of Lipases
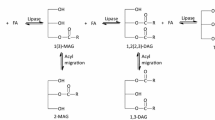
Three commercially available immobilized lipases, Lipozyme TL IM, Novozym 435 and Lipozyme RM IM, were used to produce MAG by the means of glycerolysis of camellia oil. Table 1 shows the effects of different enzymes on the content of MAG and TAG at the different reaction time. Conversion rates of TAG, 98.66, 53.10 and 69.22%, were obtained from Novozym 435, Lipozyme RM IM and Lipozyme TL IM, respectively, after 60 h of incubation. It was obvious that Novozym 435 had stronger activity in the glycerolysis reaction than the other two immobilized lipases. Novozym 435 provided the best performance for the production of MAG (1-MAG was predominant). In particular, more 1,3-DAG was formed using Novozym 435 as the catalyst than those using the other two enzymes, perhaps due to its nonspecific characteristics. The catalysis efficiency of lipases is also related to the support material for immobilizing them in the reaction system [11]. Novozym 435 and Lipozyme RM IM that are immobilized on resin, probably providing a more stable reaction system than that of Lipozyme TL IM, which was immobilized in silica granulates. Therefore, Novozym 435 was selected for the subsequent studies.
The Effect of Reaction Conditions on the Production of MAG by a Batch Reaction
Substrate Molar Ratio
The molar ratio of reaction substrates is a crucial factor affecting the total glycerolysis reaction. Molar ratios of glycerol to camellia oil of 3:1, 4:1, 5:1, 6:1 and 7:1 were studied for their effects on the production of MAG. The total amount of reaction substrate (10 g, camellia oil and glycerol) was kept constant in 25 mL tert-butyl alcohol. The amount of camellia oil and glycerol was changed according to the different substrate molar ratio. The reaction was carried out at 50 °C with 0.5 g of Novozym 435. The results are shown in Fig. 1. The MAG content in the mixture of acylglycerols was increased as the substrate molar ratio (oil/glycerol) increased from 3:1 to 7:1. Only a slight increase (~1%) was achieved when the molar ratio changed from 6:1 to 7:1. When the reaction was conducted at the substrate molar ratio of 6:1, the conversion rate of camellia oil was 98.02% and the corresponding content of MAG in the mixture of acylglycerides was 82.03% after a reaction period of 10 h. The content of MAG decreased as the substrate molar ratio decreased although the conversion rate of camellia oil changed less (<0.4%), and the content of DAG (diacylglycerols) increased as the amount of glycerol in the reaction system increased, which is consistent with the reports by Yamane et al. [14]. Yamane et al. [14] found that the main product of glycerolysis was DAG at low molar ratio of glycerol to palm olein of 1:2. This means that the enhancement of glycerolysis reaction from TAG to MAG needs excess glycerol compared to the glycerolysis reaction from TAG to DAG. However, it was difficult to use glycerol at too high concentration because its viscosity resulted in a pressure-drop in the PBR reaction process. In the latter experiments, the optimal molar ratio of glycerol to camellia oil was fixed at 6:1.
Substrate Concentration
The effect of substrate concentration in tert-butyl alcohol on the production of MAG was also investigated. The substrate concentration in tert-butyl alcohol was set as 30, 40 and 50%, respectively. The total reaction substrate (camellia oil and glycerol) was kept constant in different volumes of tert-butyl alcohol, and the reaction was carried out at 50 °C with 0.5 g of Novozym 435. It can be seen from Fig. 2 that the content of MAG in the mixture of acylglycerols increased as the substrate concentration was increased from 20 to 50%, but little increment was observed from the substrate concentration of 40% increasing to 50%. A high substrate concentration was found better for product separation. However, turbidity of the fluid in the reaction mixture was observed when the substrate concentration was increased to 50%. Therefore, a 40% substrate concentration in tert-butyl alcohol was the optimum at which the corresponding conversion rate was 98.7% and the content of MAG was 82.0% after a 10-h reaction time.
Temperature
Temperature played a very important role in the reaction process. Glycerol has a low miscibility with oils and fats. At high temperatures, the viscosity could be reduced, and also the substrate diffusion or its solubility could be improved. However, if the temperature is set too high, enzyme denaturation can happen. Therefore, an optimal temperature should be selected. The effect of temperature on the MAG production was evaluated. The temperature was set at 40, 50 and 60 °C, respectively. The reaction was performed with 0.5 g of Novozym 435 at the optimal conditions as mentioned previously. The results are shown in Fig. 3. The content of MAG in the mixture of acylglycerols was increased as the temperature increased, but it increased less when the temperature was over 50 °C. The MAG content was 82.10% after 8 h of reaction.
Continuous Glycerolysis in PBR
The optimal conditions for the production of MAG were determined from the batch reactions: 40% of substrate concentration (w/v) in tert-butyl alcohol, 6:1 mol ratio of glycerol to camellia oil, and 50 °C reaction temperature. Under these conditions, a continuous production of MAG was performed by PBR at different flow rates. The results are listed in Table 2. The MAG content in the mixture of acylglycerols was changed with the increment of reaction mixture’s flow rate. When the flow rate of reaction mixture was set as 0.25 mL/min, the highest MAG content of 80.74% was obtained. A lower amount of MAG content was observed when the flow rate of reaction mixture was increased from 0.1 to 0.25 mL/min. It indicated that a short reaction time is beneficial without reducing the TAG conversion/MAG formation significantly. However, the MAG content decreased with a further increase in the flow rate of the reaction mixture, presumably due to an insufficient reaction time for the enzyme and substrates.
Long-term production of MAG was carried out by PBR and the results are shown in Fig. 4. It was shown that the activity of Novozym 435 was unchanged during the continuous operation process of 38 days. 3,545.8 g of acylglycerols was obtained when the tert-butanol and glycerol were removed by rotatory evaporator and molecular distillation. The content of MAG in the acylglycerols was 80.73% by HPLC analysis, and a higher average productivity of 0.698 kg MAG/(kg Enzyme h) was obtained compared to prior reports [15, 16].
The reaction products were separated by thin layer chromatography to obtain MAG. The fatty acid profiles of MAG were determined by GC as 9.59% of C16:0, 2.36% of C18:0, 73.97% of C18:1 and 10.68% of C18:2. The results indicated that MAG with a high content of oleic acid (73.97%) could be efficiently produced by the above proposed process. Further optimization of the PBR process is being carried out to address scale-up problems of the process.
References
Damstrup ML, Jensen T, Sparsø FV, Kiil SZ, Jensen AD, Xu X (2005) Solvent optimization for efficient enzymatic monoacylglycerol production based on a glycerolysis reaction. J Am Oil Chem Soc 82(8):559–564
Pawongratn R, Xu X, H-Kittikun A (2007) Synthesis of monoacylglycerol rich in polyunsaturated fatty acids from tuna oil with immobilized lipase AK. Food Chem 104(1):251–258
Pelan BMC, Watts KM, Campbell IJ, Lips A (1997) The stability of aerated milk protein emulsions in the presence of small molecule surfactants. J Dairy Sci 80(10):2631–2638
Zhang Z, Goff HD (2005) On fat destabilization and composition of the air interface in ice cream containing saturated and unsaturated monoglyceride. Int Dairy J 15(5):495–500
Sonntag NOV (1982) Glycerolysis of fats and methyl esters—status, review, and critique. J Am Oil Chem Soc 59(10):795A–802A
Noureddini H, Harkey DW, Gutsmanc MR (2004) A continuous process for the glycerolysis of soybean oil. J Am Oil Chem Soc 81(2):203–207
Peng L, Xu X, Tan T (2000) Enzymatic production of high quality monoacylglycerols. In: Mohan RM (ed) Research advances in oil chemistry. GRN, Calcutta, pp 53–78
Muñío MM, Esteban L, Robles A, Hita E, Jiménez MJ, González PA, Camacho B, Molina E (2008) Synthesis of 2-monoacylglycerols rich in polyunsaturated fatty acids by ethanolysis of fish oil catalyzed by 1, 3 specific lipases. Process Biochem 43(10):1033–1039
Esteban L, Muñío MM, Robles A, Hita E, Jiménez MJ, González PA, Camacho B, Molina E (2009) Synthesis of 2-monoacylglycerols (2-MAG) by enzymatic alcoholysis of fish oils using different reactor types. Biochem Eng J 44(2–3):271–279
Ghamgui H, Miled N, Rebaï A, Karra-chaâbouni M, Gargouri Y (2006) Production of mono-olein by immobilized Staphylococcus simulans lipase in a solvent-free system: optimization by response surface methodology. Enzyme Microb Techol 39(4):717–723
Yang TK, Rebsdorf M, Engelrud U, Xu XB (2005) Monoacylglycerol synthesis via enzymatic glycerolysis using a simple and efficient reaction system. J Food Lipids 12(4):299–312
Esmelindro ÂFA, Fiametti KG, Ceni G, Corazza ML, Treichel H, Oliveira D, Oliveira JV (2008) Lipase-catalyzed production of monoglycerides in compressed propane and AOT surfactant. J Supercrit Fluids 47(1):64–69
Ferreira-Dias S, Correia AC, Baptista FO, Fonseca MMR (2001) Contribution of response surface design to the development of glycerolysis systems catalyzed by commercial immobilized lipases. J Mol Catal B Enzym 11(4–6):699–711
Yamane T, Kang ST, Kawahara K, Koizumi Y (1994) High-yield diacylglycerol formation by solid-phase enzymatic glycerolysis of hydrogenated beef tallow. J Am Oil Chem Soc 71(3):339–342
H-Kittikun A, Kaewthong W, Benjamas C (2008) Continuous production of monoacylglycerols from palm olein in packed-bed reactor with immobilized lipase PS. Biochem Eng J 40(5):1525–1530
Kaewthong W, Sirisansaneeyakul S, Prasertsan P, H-Kittikun A (2005) Continuous production of monoacylglycerols by glycerolysis of palm olein with immobilized lipase. Process Biochem 40(5):1525–1530
Acknowledgments
This work was made possible with funding provided by the National Natural Science Foundation of China (20706021), the Research Fund for the Doctoral Program of Higher Education (20070561073) and the National Key Technology R&D Program 2006BAD27B04.
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Zeng, Fk., Yang, B., Wang, Yh. et al. Enzymatic Production of Monoacylglycerols with Camellia Oil by the Glycerolysis Reaction. J Am Oil Chem Soc 87, 531–537 (2010). https://doi.org/10.1007/s11746-009-1533-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-009-1533-x