Abstract
To investigate the heat induced formation of α,β-unsaturated 4-hydroxy-aldehydes (4-hydroxy-2-trans-hexenal (HHE), 4-hydroxy-2-trans-octenal (HOE), 4-hydroxy-2-trans-nonenal (HNE) and 4-hydroxy-2-trans-decenal (HDE)) fatty acid methyl esters (FAMEs) of stearic, oleic, linoleic and linolenic acids were heated separately at 185°C for 0 to 6 hrs. The formation of 2,4-decadienal, a suspected intermediate in HNE formation, was also measured in these FAMEs. As expected methyl stearate (MS) and methyl oleate (MO) did not produce any of the α,β-unsaturated 4-hydroxy-aldehydes as a consequence of thermally induced lipid peroxidation. The formation of HHE was detected in both methyl linoleate (ML) and methyl linolenate (MLN), with concentration higher in MLN than in ML. The maximum HHE concentration was 3.99 μg HHE/g ML after 2 h and 50.78 μg HHE/g MLN after 4 h of heat treatment. HOE was detected in both ML and MLN, and the maximum concentration was 102.50 μg HOE/g ML after 6 h and 90.56 μg HOE/g MLN after 2 h of heating. HNE was found only in ML and its highest concentration was 84.82 μg HNE/g ML after 3 h of heating. HDE was not detected in any of the four heat treated FAMEs. 2,4-Decadienal was not found to be an intermediate in the formation of HNE in thermally induced oxidation of FAMEs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During free radical induced lipid peroxidation of polyunsaturated fatty acids, hydroperoxides are formed and decompose to a variety of secondary lipid peroxidation products such as the lipophilic aldehydes (alkanals, alkenals, alkadienals, and hydroxyl-alkenals). Among the lipophilic aldehydes, the α,β-unsaturated 4-hydroxy-aldehydes including 4-hydroxy-2-trans-hexenal (HHE), 4-hydroxy-2-trans-octenal (HOE), 4-hydroxy-2-trans-nonenal (HNE) and 4-hydroxy-2-trans-decenal (HDE) are of particular interest because of their reactivity with biomolecules and of their toxicity [1].
HNE is the most well-known compound among α,β-unsaturated 4-hydroxy-aldehydes and its toxicity is well established in the literature. It is related to atherosclerosis [2, 3], low density lipoprotein oxidation, stroke, Parkinson’s, Alzheimer’s and Huntington’s [4–8], liver [9] and other diseases. HNE is also able to modify proteins, nucleic acids and other biomolecules in vivo [10–13]. At cellular concentrations of 1–20 μM, HNE partially inhibits DNA and protein synthesis [1] and at >100 μM, acute effects were observed including inhibition of catabolic (i.e. mitochondrial respiration) and anabolic (i.e. DNA, RNA, protein synthesis) functions that led to cell death.
Recently, besides HNE the toxicity of HHE, HOE and HDE has also been investigated. HHE showed the cytotoxic effect on monocyte–macrophages [14] and stimulated the activity of hepatic phosphatidylinositol-4,5-bisphosphate-phospholipase C in vitro [15]. The incorporation of HHE into protein-linked carbonyl derivatives, a potential marker of oxidized proteins, has also been reported [16]. HOE exerted a significant inhibitory effect on DNA synthesis when added at concentrations of 100 μM or higher in isolated human hepatic stellate cells [17]. HOE significantly increased phospholipase-C activity between 10 pM and 10 nM concentrations in isolated plasma membranes from rat neutrophils [18]. Accelerated phosphatidyl-inositol-4,5-bisphosphate breakdown by HOE at concentrations ranging from 10−12 to 10−6 M in the liver membrane of male Wistar rats has also been reported [15]. HOE inhibited 71% of the respiration of hepatoma cells of male Wistar rats at level of 50 mmol aldehydes/106 cells [19] and showed cytotoxicity in human monocyte–macrophages [14]. HDE was detected during the oxidative damage of isolated rat liver mitochondria treated with iron/ascorbate [20]. The relative toxicity of the four α,β-unsaturated 4-hydroxy-aldehydes has been reported by some investigators [14, 15, 17–19]. Depending on the type of assay, the highest toxicity was found mostly for HNE and similar or somewhat lower toxicity is reported for HOE. However, Rossi et al. [15] reported that HOE was more toxic than HNE because it accelerated the breakdown of hepatic phosphatidylinositol-4,5-biphosphates more than HNE. HHE has been found to exhibit the lowest toxicity among the α,β-unsaturated 4-hydroxy-aldehydes.
The toxicity of these reactive aldehydes is based on their structure which provides high reactivity to other compounds. The C2 to C3 position of the double bond and a hydroxyl group in C4 position easily form Michael adducts with thiol groups of proteins [10, 21] and the aldehyde group at the C1 position can react with the amino group of proteins or DNA [21]. The hydroxyl group attached to the C4 position provides not only more reactivity but more stability to Michael adducts than the non-hydroxylated alkenals [21]. The increase in chain length from six to nine carbons has been shown to increase apoptotic induction and toxicity of alkenals explaining the high toxicity of HNE [22].
Recent experiments reported from this laboratory revealed the formation of HNE in heat treated vegetable oils and their incorporation into fried food [23, 24]. Several of the other toxic α,β-unsaturated 4-hydroxy-aldehydes were also identified in heat treated oils recently from this laboratory [25].
In the present study fatty acid methyl esters (FAMEs) such as methyl stearate (MS), methyl oleate (MO), methyl linoleate (ML) and methyl linolenate (MLN) were thermally treated at frying temperatures to follow the formation of α,β-unsaturated 4-hydroxy-aldehydes such as HHE, HOE, HNE, HDE. The precursory role of 2,4-decadienal for HNE formation was also investigated.
Materials and Methods
Chemicals and Materials
FAMEs, such as MS, MO, ML and MLN were purchased from Nu-Chek Prep, Inc (Elysian, MN). 2, 4-Dinitrophenylhydrazine (DNPH), 2,4-decadienal and hexanal standards were purchased from Sigma (St Louis, MO, USA). HHE and HNE standards were obtained from Cayman Chemical Co. (Ann Arbor, MI, USA). HOE and HDE were received as a gift from the Department of Biochemistry, University of Graz (Graz, Austria). Hydrochloric acid was obtained from J.T. Bakers Inc. (Phillipsburg, NJ, USA), HPLC-grade methanol from Fisher Scientific (Fair Lawn, NJ, USA), and HPLC-grade water, n-hexane and dichloromethane from EMD Chemicals, Inc. (Gibbstown, NJ, USA). Silica gel plates (20 × 20 cm, 250 μm layer, Al SIL G) for thin layer chromatography (TLC) and No.1 filter paper were purchased from Whatman Ltd. (Kent, England).
Preparation of DNPH Reagent
The reagent was prepared daily, according to the method of Seppanen and Csallany [25], by combining 10 mg DNPH, recrystallized three times from methanol, with 20 ml 1 N HCl at 50 °C for about 1 h. After cooling, the mixture was extracted four times with HPLC-grade hexane in a separatory funnel to remove impurities. The aqueous purified DNPH reagent was used immediately.
Preparation of Thermally Oxidized FAMEs
Three grams of each FAME, MS, MO, ML and MLN were placed in 25 × 125 mm open test tubes, and continuously heated for 0–6 h at 185 °C (frying temperature). For each FAME, a total of 12 open test tubes were heated simultaneously and two test tubes, as duplicates for each heating time period, were removed for analysis after 0, 1, 2, 3, 4 and 6 h of heating. The formation of HHE, HOE, HNE and HDE was analyzed after 0, 1, 2, 3, 4 and 6 h of heating and the formation of 2,4-decadienal after 0, 1, 3 and 6 h of heating.
Preparation of Hexanal-DNPH Standard
Pure hexanal-DNPH was prepared by the method developed in this laboratory [26]. The mixture of 800 mg of recrystallized DNPH, 80 ml methanol, 2 ml 6 N hydrochloric acid and 1 ml pure hexanal, was heated for 10 min at 60 °C, and placed in an ice bath for crystallization. For purification, the hexanal-DNPH crystals were filtered through Whatman No.1 filter paper and they were recrystallized two more times from about 50 ml of methanol. After the final crystallization, the hexanal-DNPH was dried in a desiccator for 3 days and the product was kept at −20 °C in an air tight container.
Preparation of DNPH Derivatives of α,β-Unsaturated 4-Hydroxy-Aldehydes from Thermally Oxidized FAMEs
The preparation of DNPH derivatives of aldehydes, including HHE, HOE, HNE, HDE and 2,4-decadienal from heat treated FAMEs, was conducted as described by the method of Seppanen and Csallany [25]. One gram of the previously heat treated or unheated FAMEs was reacted overnight at room temperature with 5 ml freshly prepared DNPH reagent. The DNPH derivatives were extracted with 10 ml of 75:25 (v/v) methanol:water from the FAMEs. This procedure was repeated two more times and the methanol: water extracts were combined. The DNPH derivatives were re-extracted with 10 ml of dichloromethane three times from the combined methanol: water extracts and concentrated with N2 gas to about one ml.
Thin Layer Chromatography
The whole sample (about 1 mL) of the concentrated DNPH derivatives in dichloromethane was applied on two TLC plates and developed in dichloromethane to polar and nonpolar aldehydes, and to osazone regions (Rf = 0.25, 0.75 and 0.50, respectively). The polar fraction from the TLC plate, which included HHE, HOE, HNE and HDE, and the nonpolar fraction from the TLC plate, which included the 2,4-decadienal, were cut into small pieces and extracted three times with 10 mL of methanol. The combined methanol extracts were centrifuged at 1,360×g for 15 min to precipitate the residual silica. The clarified supernatant fractions were concentrated under N2 gas to the exact volume of 1 mL and 50 μL aliquots were injected into the HPLC column in duplicate for the analysis.
HPLC Analysis
The HPLC system consisted of a solvent delivery system (9050, Varian, Walnut Greek, CA, USA), a sample injector (712 WISP, Waters, Milford, MA, USA), Ultrasphere ODS HPLC column (5 × 4.6 mm, 25 cm), (Beckman, Fullerton, CA, USA) and a UV–VIS detector (9010, Varian). The integration of peaks shown on chromatograms was conducted using Star Workstation software (Varian, Walnut Greek, CA, USA) installed on the computer connected to the UV–VIS detector. For polar aldehydes, an isocratic elution with a mixture of 50:50 (v/v) methanol: water was used for 10 min, the gradient elution increased to 100% of methanol in 20 min, and 100% of methanol was used for an addition of 10 min. The nonpolar aldehydes were analyzed identically as the polar aldehydes but the elution of compounds started with a mixture of 75:25 (v/v) methanol:water. The absorbance of the polar and nonpolar aldehydes was monitored at 378 nm. Rejection of peaks was set to 2,000 area counts. The detection limit of the HPLC system was 12 ng hexanal measured as DNPH derivatives per 50 μl injection.
The peaks of the HHE, HOE, HNE, HDE and 2,4-decadienal, from the HPLC chromatograms, were identified by comparing the retention time of each isolated compound with the retention time of the peak of pure standard. Identification of each isolated compound was also carried out by co-chromatography with pure standards in four different polarity solvent systems as described in the method developed in this laboratory [26]. Solvent systems used for co-chromatography for DNPH derivatives of HHE, HOE, HNE and HDE were methanol: water, 45:55, 50:50, 55:45 and 60:40 (v/v), and for DNPH derivative of 2,4-decadienal were methanol: water, 65:35, 70:30, 75:25 and 80:20 (v/v). The percent recovery of added pure aldehyde-DNPH from the co-chromatography was calculated from the increase in the peak area due to the added pure aldehyde-DNPH to the original peak area of the isolated aldehyde-DNPH from the heat treated FAMEs. The recovery for the procedure was examined by adding 10 μg pure HNE to 1 g of unheated methyl oleate and the recovery of the whole procedure including derivatization with DNPH, prepurification on TLC and quantitation by HPLC was found to be 74.8%. Unheated methyl oleate was used as a control and did not contain any HNE. Aldehyde-DNPH derivatives were quantified by comparing their peak area with the area of known amount of pure hexanal-DNPH standard and expressed as μg hexanal equivalent/g FAME. One ng of pure hexanal was measured to be equivalent of 4,500 area counts by the HPLC system used in the experiment. The isolated α,β-unsaturated 4-hydroxy-aldehyde concentrations were calculated from the hexanal equivalents based on the molecular weight differences between hexanal and the aldehyde measured. The aldehyde concentrations for HHE, HOE and HNE were expressed as μg aldehyde/g FAME.
Statistical Analysis
The average and standard error of the mean (SEM) were calculated for each heating time period, and for each α,β-unsaturated 4-hydroxy-aldehyde and 2,4-decadienal.
Results and Discussion
The present study was conducted to investigate how the levels of unsaturation of fatty acids are involved in the formation of the lipophilic aldehydes (HHE, HOE, HNE and HDE) in thermally induced lipid peroxidation processes. The FAMEs tested have different levels of unsaturation and they are the main ingredients in various edible oils and fats. Since the fatty acids are mostly in esterified form in oils and fats, four different FAMEs (stearic, oleic, linoleic and linolenic) were selected to represent saturated, monounsaturated ω9, polyunsaturated ω6 and ω3 fatty acids, the major components of fats and oils. Pure methyl esters of the above fatty acids were heated in duplicates at 185 °C for 0–6 h and the formations of HHE, HOE, HNE and HDE were investigated using an HPLC method developed in this laboratory [25]. Each duplicate heated sample was analyzed by duplicate injections to the HPLC system. In total four replicates of data were produced and used for the statistical analysis. In addition, the formation of 2,4-decadienal was similarly investigated since it was suspected to be a possible precursor in the formation of HNE.
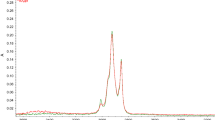
Typical HPLC chromatograms of the polar α,β-unsaturated 4-hydroxy-aldehydes, HHE, HOE, and HNE, isolated from heat treated ML and MLN are shown in Fig. 1. The retention times were 25.8 ± 0.16 min for HHE, 29.3 ± 0.10 min for HOE, and 30.3 ± 0.12 min for HNE. It can be seen that in heat treated ML the major peak was HNE, followed by HOE and a very small peak of HHE, however, HDE was found not to be present. In heat treated MLN the major peak was HOE and a relatively large peak of HHE was present. The major peak which is close to HNE was found not to be HNE by co-chromatography using several different polarity solvent systems. MLN oxidation also did not produce any HDE. Figure 2 shows the HPLC chromatograms of 2,4-decadienal, a nonpolar lipophilic aldehyde formation due to heat treatment in ML and in MS, with retention time 25.1 ± 0.04 min.
HPLC chromatograms of the DNPH derivatives of polar aldehydes and related carbonyl compounds extracted from a methyl linoleate and b methyl linolenate heated at 185 °C for 3 h and c polar aldehydes standards. HHE: DNPH derivative of 4-hydroxy-2-trans-hexenal, HOE: DNPH derivative of 4-hydroxy-2-trans-octenal, HNE: DNPH derivative of 4-hydroxy-2-trans-nonenal
Table 1 shows the presence of HHE, HOE, HNE, HDE and 2,4-decadienal in FAMEs due to heat treatment at 185 °C between 0 and 6 h. HHE and HOE formations were detected in ML and MLN, however, HNE formation was detected only in ML. 2,4-Decadienal was found in MS and ML. In MO, no HHE, HOE, HNE, HDE and 2,4-decadienal formations were detected between 0 and 6 h of heat treatment at 185 °C. No HDE formation was detected in any of four FAMEs tested due to heat treatment at 185 °C between 0 and 6 h.
The purity of DNPH derivatives of HHE, HOE, HNE and 2,4-decadienal isolated by HPLC from the heated FAME samples was tested by co-chromatography in three to four different polarity solvent systems according to Seppanen and Csallany [25] and the percent recoveries of the added pure aldehyde standards are shown in Table 2. The results demonstrate that the aldehyde standards and the aldehydes derived from the heated FAME were co-eluting in the different polarity solvent systems from the HPLC column and the percentage recoveries of added pure standards were around 100% in most solvent systems.
The formation of HHE in ML and MLN heated at 185 °C between 0 and 6 h are shown in Fig. 3. The HHE concentrations in ML were relatively consistent between 1 and 6 h of heating times and the highest concentration (3.99 μg HHE/g FAME) was achieved by 2 h. The HHE concentrations in MLN increased up to 4 h of heating and it was remained about the same up to 6 h. HHE reached maximum concentration 50.78 μg HHE/g FAME at 4 h of heating and its formation was 12.7 times higher than in ML. Although HHE was detected in both ML and in MLN, its formation is primarily originated from the three double bonds containing ω3 FAME. The ω3 fatty acids including linolenic and docosahexaenoic acids (DHA) have been reported as precursors of HHE in the literature. The presence of HHE in autoxidized MLN was reported by Nakamura et al. [27] and Van Kuijk et al. [28] reported the formation of HHE from oxidized docosahexaenoic acid (DHA). However, this is the first time that HHE has been reported to form not only from ω3, but also from ω6 fatty acids such as ML. In the present study, 1.07–3.99 μg HHE/g FAME was detected in heated ML between 1 and 6 h of heat treatment. The relatively low formation of HHE from linoleic acid may be one reason why it has not been previously reported.
Figure 4 shows the formation of HOE in heated ML and MLN at 185 °C between 0 and 6 h. HOE concentrations in ML increased up to 3 h of heating then decreased at 4 h, however it reached a maximum concentration of 102.50 μg HOE/g FAME at 6 h. In MLN HOE concentration reached a maximum of 90.56 μg HOE/g FAME after 2 h of heating, then decreased gradually to 59.44 μg HOE/g FAME at 6 h. The formation of HOE from 2-octenal was reported by Grein et al. [29]; however, the formation of HOE, a α,β-unsaturated 4-hydroxy-aldehyde, during heat induced lipid peroxidation of ML and MLN is reported for the first time in the present study. The fact that in heat treated polyunsaturated fatty acids containing oils, HOE is formed in similar concentration and it possess similar toxicity to HNE is significant because of their combined toxicity.
The formation of HNE, the most investigated α,β-unsaturated 4-hydroxy-aldehyde, was detected only in heat treated ML. Concentrations between 0 and 6 h of heating at 185 °C are presented in Fig. 5. The HNE concentration reached a maximum after 3 h of heating to the level of 84.82 μg HNE/g FAME then decreased to about 65 μg HNE/g FAME after 6 h of heating. The formation of HNE from ω6 fatty acids including linoleic acid and arachidonic acid has been reported and confirmed in the literature [30, 31]. Investigations from this laboratory have reported its formation in high PUFA vegetable oils due to heat treatments [23]. Under specific conditions 2,4-decadienal has been found to be an intermediate precursor for the formation of HNE. Grein et al. [29] reported the formation HNE from 2,4-decadienal in water-mediated oxidation under oxygen atmosphere. Spiteller et al. [32] reported the transformation of 2,4-decadienal to HNE in the presence of m-chloroperbenzoic acid.
The formation of 2,4-decadienal in thermally treated methyl esters of stearic, oleic, linoleic and linolenic acids was also investigated in the present study. Among the four FAMEs tested, 2,4-decadienal was formed only in heat treated MS and ML at 185 °C (Table 1). The overall formation was much lower in MS than in ML (Fig. 6). In both MS and ML, the concentrations reached a maximum after 1 h of heating at 185 °C and the concentrations were reduced to very low levels by heating up to 6 h. Maximum concentration in ML was 14.31 μg 2,4-decadienal/g FAME which was 3.0 times higher than in MS. Although 2,4-decadienal was found in both MS and ML. HNE was found only in ML. Since MS did not contain any HNE due to heat treatment this seems to indicate that 2,4-decadienal is not an intermediate of HNE formation during a thermally induced lipid peroxidation process of fatty acids.
In conclusion, saturated and monounsaturated FAMEs such as MS and MO model systems due to heat treatment at 185 °C did not produce any of the four α,β-unsaturated 4-hydroxy-aldehydes, HHE, HOE, HNE and HDE. However, heat induced lipid peroxidation of ML (an ω6 FAME) produced HHE, and about equal concentration of HOE and HNE. Heat treatment of MLN (an ω3 FAME) produced HHE and HOE where HOE concentration was found to be at much higher concentration than HHE. It was found in the present experiment that 2,4-decadienal due to heat treatment of FAMEs is not a likely precursor for HNE.
The fact that HNE and HOE are produced in similar concentrations from heated ML and the fact that they both are highly toxic compounds make it important to measure their formation together from high PUFA ω6 oils. With reference to the present findings, it is believed that the selection of vegetable oils used for thermal processing should be relatively low in ω6 and ω3 fatty acids for human consumption, since these α,β-unsaturated aldehydes are readily absorbed from the diet and therefore their toxicity is related to public health.
References
Esterbauer H (1993) Cytotoxicity and genotoxicity of lipid oxidation products. Am J Clin Nutr 57:779S–786S
Grootveld M, Atherton MD, Sheerin AN, Hawkes J, Blake D, Richens TE, Silwood CJL, Lynch E, Claxson AWD (1998) In vivo absorption, metabolism, and urinary excretion of α,β-unsaturated aldehydes in experimental animals. Relevance to the development of cardiovascular diseases by the dietary ingestion of thermally stressed polyunsaturate-rich culinary oils. J Clin Invest 101:1210–1218
Kritchevsky D (1991) Dietary fat and experimental atherosclerosis. Int J Tissue React 13:59–65
Keller JN, Mark RJ, Bruce AJ, Blane E, Rothstein JD, Uchida K, Waeg G, Mattson MP (1997) 4-Hydroxynonenal, an aldehydic product of membrane lipid peroxidation, impairs glutamate transport and mitochondrial function in synaptosomes. Neuroscience 80:685–686
Subramanian R, Roediger F, Jordan B, Mattson MP, Keller JN, Waeg G, Butterfield DA (1997) The lipid peroxidation product, 4-hydroxy-2-trans-nonenal, alters the coformation of cortical synaptosomal membrane proteins. J Neurochem 69:1161–1169
Owen AD, Schapira HA, Jenner P, Marsden CD (1997) Indices of oxidative stress in Parkinson’s disease, Alzheimer’s disease and dementia with Lewy bodies. J Neural Trans Suppl 51:167–173
Mark RJ, Lovell MA, Markebery WR, Uchida K, Mattson MP (1997) A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. J Neurochem 68:255–264
Mattson MP (1997) Central role of oxyradicals in the metabolism of amyloid beta-peptide cytotoxicity. Alzheimer’s Dis Rev 2:1–14
Parola M, Robino G (2001) Oxidative stress-related molecules and liver fibrosis. J Hepat 35:297–306
Witz G (1989) Biological interactions of α,β-unsaturated aldehydes. Free Rad Biol Med 7:333–349
Comporti M (1993) Lipid peroxidation: biopathological significance. Molec Aspects Med 1:199–207
Kanazawa E, Ashida H (1991) Target enzymes on hepatic dysfunction caused by dietary products of lipid peroxidation. Arch Biochem Biophys 288:71–78
Kaneko T, Kaji K, Matsuo M (1988) Cytotoxicities of a linoleic acid hydroperoxide and its related aliphatic aldehydes toward cultured human umbilical vein endothelial cells. Chem Biol Interact 67:295–304
Müller K, Hardwick SJ, Marchant CE, Law NS, Waeg G, Esterbauer H, Carpenter KLH, Mitchinson MJ (1996) Cytotoxic and chemostatic potencies of several aldehydic components of oxidized low density lipoprotein for human monocyte-macrophages. FEBS Lett 388:165–168
Rossi MA, Fidale F, Garramone A, Esterbauer H, Dianzani MU (1990) Effect of 4-hydroxyalkenals on hepatic phosphatidylinositol-4, 5-bisphosphate-phospholipase C. Biochem Phamarcol 39:1715–1719
Yamada S, Funada T, Shibata N, Kobayashi M, Kawai Y, Tatsuda E, Furuhata A, Uchida K (2004) Protein-bound 4-hydroxy-2-hexenal as a marker of oxidized n-3 polyunsaturated fatty acids. J Lipid Res 45:626–634
Robino G, Parola M, Marra F, Caligiuri A, De Franco RMS, Zamar E, Bellomo G, Gentilini R, Pinzani M, Dianzani MU (2000) Interaction between 4-hydroxy-2, 3-alkenals and the platelet-derived growth factor-β receptor. J Biol Chem 275:40561–40567
Rossi MA, Di Mauro D, Dianzani MU (1993) Action of lipid peroxidation products on phosphoinositide specific phospholipase C. Molec Aspects Med 14:273–279
Canuto RA, Biocca ME, Muzio G, Garcea R, Dianzani MU (1985) The effect of various aldehydes on the respiration of rat liver and hepatoma AH-130 cells. Cell Biochem Funct 3:3–8
Reinheckel T, Noack H, Lorenz S, Wiswedel I, Augustin W (1998) Comparison of protein oxidation and aldehyde formation during oxidative stress in isolated mitochondria. Free Rad Res 29:297–305
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehydes and related aldehydes. Free Rad Biol Med 11:81–128
Haynes RL, Szweda L, Pickin K, Welker ME, Townsend AJ (2000) Structure-activity relationships for growth inhibition and induction of apoptosis by 4-hydroxy-2-nonenal in raw 254.7 cells. Mol Pharmacol 58:788–794
Seppanen CM, Csallany AS (2002) Formation of 4-hydroxynonenal, a toxic aldehydes, in soybean oil at frying temperature. JAOCS 79:1033–1038
Seppanen CM, Csallany AS (2004) Incorporation of the toxic aldehydes 4-hydroxy-2-trans-nonenal into food fried in thermally oxidized soybean oil. JAOCS 81:1137–1141
Seppanen CM, Csallany AS (2001) Simultaneous determination of lipophilic aldehydes by high-performance liquid chromatography in vegetable oil. J Am Oil Chem Soc 78:1253–1260
Kim S-S, Gallaher DD, Csallany AS (1999) Lipophilic aldehydes and related carbonyl compounds in rat and human urine. Lipids 34:489–495
Nakamura T, Toyomizu M, Magamoto T (1977) Lipid degradation products capable of reacting with amino acid—identification of 4-hydroxy-2-hexenal, 9-formyl methyl-8-noneonate, and 10-formyl methyl-9-decenoate from autoxidized methyl linolenate. Bull Japan Soc Sci Fish 43:1097–1104
Van Kuijk FJ, Holte LL, Dratz EA (1990) 4-Hydroxyhexenal: a lipid peroxidation product derived from oxidized docosahexaenoic acid. Biochim Biophys Acta 1043:116–118
Grein B, Huffer M, Scheller G, Schreier P (1993) 4-Hydroxy-2-alkenals and other products formed by water-mediated oxidative decomposition of α,β-unsaturated aldehydes. J Agric Food Chem 41:2385–2390
Esterbauer H (1982) Aldehydic products of lipid peroxidation. In: McBrien DGH, Slater TF (eds) Free radicals lipid peroxidation and cancer. Academic Press, London, pp 101–128
Poli G, Dinzani MU, Cheeseman KH, Slater TF, Lang J, Esterbauer H (1985) Separation and characterization of the aldehydic products of lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in isolated rat hepatocytes and rat liver microsomal suspensions. Biochem J 227:629–638
Spiteller P, Kern W, Reiner J, Spiteller G (2001) Aldehydic lipid peroxidation products derived from linoleic acid. Biochim Biophys Acta 1531:188–208
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Han, I.H., Csallany, A.S. Formation of Toxic α,β-Unsaturated 4-Hydroxy-Aldehydes in Thermally Oxidized Fatty Acid Methyl Esters. J Am Oil Chem Soc 86, 253–260 (2009). https://doi.org/10.1007/s11746-008-1343-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-008-1343-6