Abstract
We have come up with a novel, integrated approach for making biodiesel by in-house producion of ethanol after fermentation of hexane extracted edible oil cake fiber. In addition, we have demonstrated how ethanol could be manufactured from commonly available oil cakes (such as canola, sunflower, sesame, soy, peanut) and dried distiller’s grains with solubles (DDGS). The edible oil cakes and DDGS were hexane extracted, ammonia fiber expansion pretreated, enzymatically hydrolysed and fermented to produce ethanol. From all the oil cakes tested in this work, DDGS and peanut oil cake showed the most promising results giving more than 180 g of glucose/kg of oil cake. These two feedstock’s were hydrolyzed at 15% solids loading and fermented by a native strain of Pichia stipitis. Most sugars were consumed during the first 24 h, with no pronounced inhibition of P. stipitis by the degradation products in the hydrolysate. Xylose consumption was more effective for peanut cake hydrolyzate compared to DDGS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With an ever increasing demand for petroleum, there has been growing interest in renewable feedstock for manufacturing bioethanol [1]. Several publications have addressed issues related to biofuel policies, challenges and opportunities facing the bioethanol industry, feasibility of a bioeconomy, and lignocellulosic feedstock availability. Biodiesel is another alternative fuel obtained by transesterification of triglycerides and low-boiling alcohols. In the United States, biodiesel is made from soybean oil, while in Europe; rapeseed (or canola) oil [2] is a common feedstock. In developing countries like India, Jatropha is a viable source of non-edible oil [3]. Biodiesel offers numerous advantages: (a) it is a renewable fuel, (b) domestic production reduces demand for diesel and (c) lowers net CO2 emissions compared to fossil fuels.
Biodiesel is a fatty acid based ester manufactured commercially via acid/base catalyzed transesterification of triglycerides and short chain alcohols [4]. The enzymatic approach using lipases for transesterification has been demonstrated as well. Detailed mechanistic understanding of the transesterification reaction has been addressed in previously published articles [4]. As more biodiesel plants are commissioned, the demand for methanol will precipitate price increases. There is a need to explore other alcohol feedstocks, ethanol being one possible candidate.
Currently, in the United States ethanol is made primarily from corn grain starch [5]. However, grain alone cannot meet future demands for ethanol, as 13% of the total corn produced in the US is already used for ethanol production. Thus, there is a considerable amount of research being done to produce ethanol from other sources (i.e. cellulose and hemicellulose) [6]. Bioethanol produced in an efficient and sustainable manner can offer numerous environmental and social advantages compared to fossil fuels. Ethanol can be blended in any proportion with conventional gasoline. Common blends include E10 (10% ethanol and 90% petroleum product), and E85 (85% ethanol and 15% conventional petroleum product) [7].
Ammonia fiber expansion (AFEX) is a novel pretreatment process that utilizes concentrated ammonia to pretreat lignocellulosics at lower severities compared to other pretreatments [6]. There are several physical and chemical modifications in the lignocellulosic ultra structure upon AFEX pretreatment. AFEX also demonstrates attractive economics compared to other pretreatment technologies based on a recent economic model [8] for producing bioethanol from corn stover.
In a conventional edible oil manufacturing facility, oil is removed from oil seeds by extrusion followed by extraction with hexane, leaving behind a protein and lignocellulosic rich oil cake. Commercially available oil seed cakes include canola, sunflower, sesame, peanut, and soybean. The oils extracted from these seeds are used for edible purposes; while, oil cakes are either used for special biotechnological applications [9], as animal feed, compost or land fill materials. Oil seed cakes/meals are rich in protein, fiber and other nutrients. The fiber consists of three major components: cellulose, hemicellulose and lignin. It might be possible to utilize the fiber rich portion of the oil cake to make bioethanol utilizing a suitable thermochemical pretreatment, enzymatic hydrolysis and fermentation process. There are several advantages of this integrated approach, which include, (1) readily available oil cake feedstock after extracting the oil reduces production cost, (2) excess ethanol present in the biodiesel can be left behind to help oxygenate it and (3) utilization of enriched oil cake protein meal after ethanol distillation as a value added product (e.g. animal feed). In addition there are a few disadvantages like (1) ethanol reacts much slower than methanol with the fatty acids, and this issue can be overcome by identifying novel catalysts in the future, and (2) the capital cost of making ethanol is currently more expensive than methanol. Though the cost of ethanol production is higher than methanol, with matured technologies the cost will become more competitive.
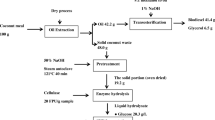
In this paper, we study the effect of varying AFEX pretreatment conditions on the cellulolytic digestibility of several oilseed cakes and dried distillers grains and solubles. The AFEX pretreated biomasses are enzymatically hydrolyzed to mixed sugars using synergistic combinations of cellulases, xylanases and other accessory enzymes. The mixed sugars are fermented to ethanol by a native yeast strain of Pichia stipitis and the resultant byproduct ‘enhanced cake’ rich in proteins could be used as animal feed or for making other valuable products. The efficiency of AFEX pretreatment and fermentation was evaluated based on the yield of free sugars and ethanol, respectively. A simplified mass balance for the in-house biodiesel process is also presented.
Experimental Procedures
Materials
All seeds (canola, sunflower, sesame, peanut, and soybean) were purchased from a local market except canola seeds which were obtained from Michigan State University Agronomy Research Center (MSU-ARC). DDGS was a gift from Big River Resources LLC, West Burlington, Iowa.
Oilseed Extrusion
The seeds were pre-milled using a laboratory blender prior to extrusion. The five oilseeds were extruded using a TäbyPress TYP 20 (Electrolux, Sweden). The press was equipped with different bit sizes for varying particle size seeds. The desired moisture content for the seeds was 7% prior to extrusion. The extruded oil cakes were frozen (−20 °C) until further analysis.
Oil Cake Hexane Extraction
The residual oil was extracted from the extruded oil cake using an accelerated solvent extraction (ASE) system (Dionex ASE 200, Sunnyvale, CA). The method used was based upon a modified procedure developed by Dionex. About 10 g of extruded cake was placed in the extraction cell, fitted with a cellulose filter disk. The following conditions were set on the ASE system: oven temperature (105 °C), the static time (10 min), flush volume (70%), purge time (60 s), static cycles (2) and pressure (1,000 psi) during each extraction. After the extraction process, hexane was recovered using a rotary evaporator (Rotavapor R, Buchi, Flawil, Switzerland) and reused. The extracted oil cake was air dried overnight to remove residual hexane.
Compositional Analysis
The oilseeds and cakes were analyzed for their glucan (cellulose and starch), xylan, arabinan, lignin and ash content. Composition analyses of the samples was performed according to the NREL Laboratory analytical procedure (LAP): “preparation of samples for compositional analysis” and “determination of structural carbohydrates and lignin in biomass” [10]. The moisture content in biomass was measured by an infrared moisture balance (Denver Instrument, IR-30). Monomeric sugar concentration for compositional analysis was determined by a high performance liquid chromatography (HPLC) using a Bio-Rad Aminex HPX-87H column.
Some of the analyses were conducted by Dairy One Forage Laboratory (Ithaca, NY) [11]. Crude fat (Extraction by Soxtec HT6 System using anhydrous diethyl ether, followed by crude fat residue determined gravimetrically after drying); protein (Kjeldahl method); starch (free sugars were extracted by soxhlet extraction using water and solids filtered prior to starch analysis. Solid residues are thermally solubilized using an autoclave, and incubated with glucoamylase enzyme to hydrolyze starch to produce dextrose. The dextrose was analyzed using a YSI glucose analyzer. Starch content was determined by multiplying dextrose by 0.9); water soluble carbohydrates (Samples incubated with water in a 40 °C bath extracting water soluble carbohydrates (WSC) comprised of simple sugars and fructan. WSC were determined after acid hydrolysis with sulfuric acid and colorimetric reaction with potassium ferricyanide); other feed values like acid detergent fibers (ADF) (24 h replicated samples were individually weighed into filter bags and digested for 75 min in 2 L of ADF solution in ANKOM A200 Digestion Unit. Samples are rinsed three times with boiling water in filter bags followed by an acetone rinse and drying at 100 °C for 2 h.), neutral detergent fibers (NDF) (24 h replicate samples were individually weighed into filter bags and digested for 75 min in 2 L of NDF solution in ANKOM A200 digestion unit. Four milliliters of alpha amylase and 20 g sodium sulfite are added to the samples at the start of digestion. Samples are rinsed three times with boiling water. Alpha amylase is added to the first two rinses. Water rinses are followed by an acetone rinse and drying at 100 °C for 2 h). Mineral analysis [analyzed using a Thermo Jarrell Ash IRIS Advantage HX Inductively Coupled Plasma (ICP) Radial Spectrometer].
AFEX Pretreatment
AFEX pretreatment was performed in 22-mL stainless steel high-pressure vessels in a high-throughput manner, with five simultaneous reactions. The biomass with varying moisture (40–80%) (kg water/kg dry biomass) was added to the reactor, sealed and degassed using a vacuum pump to create negative pressure. Liquid ammonia (1 kg of ammonia/kg of dry biomass) was charged to the reactor vessel. The temperature was raised on a heating block and maintained at necessary temperature (i.e. 70–100 °C) for 5 min residence time before explosively releasing the ammonia. The instantaneous pressure drop in the vessel caused the ammonia to vaporize, causing an explosive decompression of the biomass and considerable fiber disruption. The pretreated material was allowed to stand under the hood overnight to remove residual ammonia and stored in a freezer until further use.
Enzymatic Hydrolysis
The enzymatic hydrolysis procedure was based upon the LAP-009 protocol from the National Renewable Energy Laboratory [10]. The hydrolysis was carried out for 5 wt.% of dry biomass (i.e. 0.75 g for 15 mL reaction volume) conducted in a 20 mL glass vial. The volume was adjusted to 15 mL after addition of buffer, antibiotics and enzymes (assuming 1 g/mL density for biomass). Citrate buffer (1 M) was added to adjust final pH to 4.8. Tetracycline and cycloheximide loadings were based on the LAP-009 protocol. Spezyme CP (Genencor, Palo Alto, CA) cellulase was loaded at 10 mg protein/gram of biomass, β-glucosidase (Novozyme 188, Bagsvaerd, Denmark), at 10.6 mg/g of biomass and pectinase (Genencor, Palo Alto, CA, 0.57 mg/g of biomass). All samples were incubated at 50 °C with 150 rpm agitation speed. Samples were collected at 24 and 72 h for subsequent sugar analysis. All the experiments were done in duplicate to ensure consistency of results.
Sugar Analysis
Sugar analysis for the enzymatic hydrolyzate was done using a Shimadzu (LC 2010) HPLC system equipped with a Bio-Rad (Richmond, CA) Aminex HPX-87P carbohydrate analysis column (7.8 mm i.d. × 300 mm length) (equipped with deashing guard column). Degassed HPLC water at a flow rate of 0.6 mL/min was used as the mobile phase, while the temperature for the column oven was set at 60 °C. An evaporative light scattering detector (ELSD) was used to detect the eluting sugar peaks. The glucose and xylose concentrations were determined against a set of mixed sugar external standards.
Enzymatic Hydrolysis Mass Balance
AFEX treated DDGS and peanut oil cake were hydrolyzed at 15% solids loading using a suitable combination of enzymes. Spezyme CP (Genencor, Palo Alto, CA) cellulase was loaded at 10 mg protein/gram of biomass, β-glucosidase (Novozyme 188, Bagsvaerd, Denmark), at 10.6 mg/g of biomass and pectinase (0.57 mg/g of biomass). The total reaction volume was 500 mL in a 2-L conical flask. Samples were incubated in a shaking incubator at 50 °C at a 250-rpm agitation speed for a period of 72 h. The hydrolyzed slurry was transferred to a centrifuge tube and centrifuged at 8,000 rpm for 30 min to separate the solids from the liquid fraction. The supernatant was collected after centrifugation for fermentation experiments. Subsequently the solid fraction was washed with 400 mL water twice. The hydrolyzate and water washes were analyzed for determining total sugars to hydrolysis yield (total glucose/xylose produced per gram of dry biomass) (Fig. 1). The resultant solid was dried (enhanced oil cake) and analyzed for its protein, starch, free sugars and fat content.
Fermentation Inoculum and Pre-Culture Preparation
Lyophilized P. stipitis NRRL Y-7124 (CBS 5773), a natural pentose-fermenting yeast, was acquired from the ARS Culture Collection (National Center for Agricultural Utilization Research, Peoria, IL). Stock cultures were maintained in 10% glycerol at −80 °C and were used to inoculate yeast malt (YM) agar plates that were incubated 48 h at 25 °C prior to loop transfer to flask pre-cultures. Pre-cultures containing 75 mL of defined medium in 125-mL flasks were shaken at 150 rpm (1-in. stroke) for 24 h at 25 °C in a New Brunswick Psychrotherm. The flask pre-cultures were then used to inoculate a fermentor to produce the inoculums for hydrolysate fermentations. Both pre-cultures and fermentors for inoculum production were supplied a defined medium containing 150 g/L xylose and optimized nutrients. Braun Biostat ED fermentors with 2-L working volume were inoculated to an initial optical density of 0.1 at 620 nm, aerated at Kla of 0.167 min−1, controlled at 25 °C and pH 5, and then harvested after 72 h by centrifugation 15 min at 7,000 rpm, 10 °C. Enough cell paste was harvested to supply the required optical density when re-suspended in hydrolysate. The 72-h growth allowed the development of a population that was pentose-induced and readily able to consume xylose and that was also resistant to inhibitors and readily able to convert furan aldehyde inhibitors to less toxic furan alcohol forms [12, 13].
Fermentation
Fermentations on the thawed hydrolysate were carried out at 25 °C in fleakers (200-mL reaction volume) equipped with automatic pH control at 6.0 and aerated at 33 mL/min at 250 rpm stirring (Kla of 0.16 min−1). The P. stipitis Y-7124 was inoculated to an initial optical density of ~6 at 620 nm (or 1 g dry cell mass/L). This level of inoculum was somewhat high for several reasons: to defeat any competing contaminants; to reduce the dependence of the fermentation on cell growth, since cell growth is typically much more susceptible to inhibitors compared with ethanol production; and to prime the culture for xylose utilization, allowing it to use both xylose and glucose sequentially without diauxic lag. Sampling of fleakers was carried out to monitor sugars, ethanol, xylitol, ribitol (adonitol), acetic acid and furans by HPLC using an Aminex HPX-87H column at 65 °C and 0.0017 N H2SO4 mobile phases at 0.6 mL/min with refractive index and UV detection. Alternatively, an HPX-87P column (method described earlier) with water as the mobile phase was used for separation of individual sugars other than glucose in selected samples. Cell growth was monitored via optical density and standard plate counting of viable cells.
Results and Discussion
Feedstock Processing
Five oil seeds (canola, sunflower, sesame, soy and peanut) and dried distiller’s grain with solubles (DDGS) were selected based on their world wide availability and production volume [14]. The oilseeds were milled and extruded to remove the oil. The resulting oil cakes and DDGS were extracted with hexane using an accelerated solvent extractor to remove residual oil from the cakes. Total extracted oil was close to the theoretical maxima (90% or more), based on the crude fat content (Table 1), for most samples except DDGS (76%) and sesame (83%). The extracted cakes were air-dried to remove the residual hexane and stored at −20 °C until further use.
As expected, the crude protein, starch and total water soluble carbohydrate (WSC) content increased after hexane extraction. DDGS contained the highest glucan content (20.8%) followed by soy, peanut and canola. It was noted that sunflower and sesame oil cakes have glucan compositions below 10%, which is considered to be fairly low for an economically viable feedstock. The total hemicellulose content was between 7 and 15% for the oil cakes, except DDGS which was close to 21%. Total lignin (i.e. Klason and acid-soluble) content for canola, sunflower and sesame cakes is approximately 16, 13 and 20%, respectively. On the other hand, soy cake, peanut cake and DDGS have total lignin content under 10%. It is important to note that there is interference by protein when measuring Klason lignin and hence lignin content, for at least some seeds is not what one would consider as a “true” lignin.
Interestingly, soy cake was also found to contain significant amounts of acetic acid, unlike other oil cakes, based on the acid hydrolysis composition data that is comparable to DDGS (~2–3%). The most probable source of acetylation in cell walls is the hemicellulose backbone that provides recalcitrance to cell wall degrading microbes and fungi. Non starch polysaccharides (NSP) are subdivided in to three categories namely (1) cellulose, (2) non cellulosic polysaccharides (arabinoxylans, mixed-linked β-glucans, mannans, galactan and xyloglucans) and (3) pectic polysaccharides (polygalacturonic acids which may be substituted with arabinan, galactan and arabinogalactan). Details about the structure of these components have been reported elsewhere [15]. Specific enzyme activity is needed to break down some of these polysaccharides [16].
Feedstock Pretreatment
Lignocellulosic fibers comprise of a complex network of cellulose, hemicellulose and lignin [17] that is difficult to hydrolyze due to poor enzyme accessibility. To improve accessibility of enzymes to the interwoven polysaccharides, a thermochemical pretreatment is typically necessary. For this purpose, AFEX pretreatment was carried out to pretreat the biomass. The range of AFEX reaction parameters that were screened for optimization were 1:1 ammonia to biomass loading, 40/60/80% biomass moisture content (dwb) and 70/100 °C residence temperature for all substrates. The optimal AFEX conditions for various substrates are shown in Table 2. It was expected that the feedstocks with lower lignin content would require a lower pretreatment severity. This trend was observed in the case of peanut, showing higher glucan conversion at lower moisture (40%) and temperatures (70 °C). However, soy and sesame cake required more severe pretreatment conditions of 80% moisture and 100 °C. One possible reason for that is the higher acetyl content for soy, compared to other oilseeds that retards accessibility of xylanases to acetylated xylans [16]. The rate of deacetylation of hemicellulose by ammonia is higher at more severe AFEX conditions (unpublished data).
Enzymatic Hydrolysis
The AFEX pretreated oil cakes were hydrolyzed with 3 commercial enzymes that were primarily cellulases (i.e. Spezyme CP), β-glucosidases (i.e. Novo 188) and pectinases (i.e. Multifect Pectinase). These enzymes were not purified and had contaminating cross activities [16]. In this study, pectinases were included since some of the cakes are rich in homogalactans (e.g. soy cake) and arabinans (e.g. DDGS) that could be hydrolyzed by the relevant glycosyl hydrolases available in the crude pectinase mixture. The enzymes were added as milligrams of protein per gram of pretreated oil cake and the glucose yields are reported as grams of glucose per gram of pretreated oil cake (Fig. 2).
As expected, the feedstocks with higher glucan content had higher glucose yields. Of the six feedstocks analyzed, DDGS and peanut cakes produced more than 180 g of glucose/kg of oil cake (Fig. 2a). Pretreatment significantly enhances the rate of hydrolysis, which is evident in the first 24 h of hydrolysis, for almost all cases (except sesame). However, after 72 h the untreated samples show comparable yields to the AFEX treated cakes for canola, sesame, sunflower and soy. AFEX pretreatment was seen to have a considerable effect on both the overall glucose yield and rate of digestion for soy cake, DDGS and peanut oil cake. AFEX pretreatment typically promoted a 30–60% increase in the glucose yield. The xylose yields on the other hand are relatively lower (Fig. 2b). One possible reason could be that the commercial enzyme did not contain all the necessary activities to degrade the complex hemicellulosic fraction to monomeric sugars.
An interesting observation to note is that the glucose yields for most substrates were generally lower than their theoretical maxima (based on glucan composition), except for peanut cake. The maximum theoretical glucose yield can be calculated from the original glucan content by multiplying it by a correction factor of 1.11 to account for the hydrolysis of the O-glycosidic glucan linkages to yield monomeric glucose. In the case of sunflower and sesame cakes, the initial glucan composition was quite low (8.9 and 7.3%, respectively). Since the enzymes were loaded on a gm/gm of biomass basis, the quantity of enzyme per gram of glucan turned to be higher for these substrates. Consequently, more enzymes were available to hydrolyze a smaller amount of cellulose, improving the glucan conversion of sunflower (~91%) and sesame (~90%) in comparison to other substrates, like canola (~70%) and soy (~75%). In the case of AFEX DDGS the glucan conversion was approximately 80–85%. Some times glucan conversions greater than 100% is observed for lignocellulosic biomass (as seen for AFEX treated peanut cake), due to inherent inaccuracy of the acid hydrolysis based compositional analysis method. Supplementing cellulases, pectinase and β-glucosidase mixtures with other accessory enzymes like xylanases further improved both glucose and xylose yields (results not shown).
Despite marginally lower conversions compared to sesame and sunflower; peanut, soy and DDGS are potentially important feedstocks due their higher glucan content, which results in higher glucose concentrations. High sugar concentrations will help reduce the size of the reactor’s cost, the amount of feedstock processed and reduce distillation costs. Also, high glucan yields helps reduce total solids loaded for high solid loading based enzymatic hydrolysis. A minimum ethanol concentration (~4%) is required for an economical distillation process of the fermentation broth [18]. In addition, separate hydrolysis and fermentation (SHF) or simultaneous hydrolysis and fermentation (SSF) would also help achieve the minimum economical ethanol concentration.
Enzymatic Hydrolysis at High Solids Loading
For this study we used hexane extracted peanut oil cake and DDGS cake since both substrates showed promising results during preliminary hydrolysis experiments (Fig. 2). For an accurate mass balance, the experiments were performed using 100 g of AFEX treated feedstock in a 15% solids loading experiment. After completing the enzymatic hydrolysis for 168 h, the liquid fraction was separated from the undigested solid residue by centrifugation and stored at −20 °C for fermentation experiments. Subsequently, the solid residue was washed twice with deionized water to remove residual sugar in the solid stream. The liquid extracts were combined to measure the total glucose produced during the hydrolysis process. Under these conditions, 158 g glucose per kg of DDGS cake and 171 g glucose per kg of peanut cake were obtained, generating glucan conversions of approximately 70 and 108%, respectively.
Past research had evaluated the effect of cell wall degradation of polysaccharides in soybean meal to that of animal nutrition and its performance. The combination of cellulases + pectinase + xylanase + mannanase gave the most efficacious (P < 0.05) among the enzyme combinations evaluated using canola meal and soybean meal. It was shown that by improving effective combinations of enzymes, the NSP digestibility went from 11 to 30% in canola meal alone. In our experiments, the pretreated cakes were digested with a combination of enzymes (cellulase + β-glucosidase + pectinase) and the resultant residual solids were analyzed for their feed value. The composition of the resulting undigested solid residues (or enhanced cakes) was determined by Dairy One (Ithaca, NY) (shown in Table 3). With the available data one can estimate the feed value based on the reports available in the literature [19].
In a modern-day edible oil extraction plant, the oilseeds are typically extracted in two stages: (1) Mechanical expeller/press extraction for reducing oil content to 20–25% (w/w), followed by (2) Hexane extraction to remove residual oil. In one of the recent approaches, enzymes have been used to extract oil from oil seeds [20]. One of the major advantages of the AFEX process is that unlike other thermochemical treatments (e.g. dilute acid, organosolv) the temperature severity of pretreatment is fairy low (i.e. 70–100 °C for AFEX vs. 150–200 °C for acidic treatments). Lower temperatures help reduce protein degradation and improves digestibility of important amino acids, like lysine. Ammoniation based treatments are currently employed in the detoxification of oilseeds like groundnuts to remove toxic aflatoxins. Some of the primary drawbacks of this process are the associated capital costs for handling ammonia and poor feed characteristics (i.e. protein solubility) of the treated substrate. However, the ammonia treated feedstock would be more favorable for ethanol production and recovery of the undigested protein residue as animal feed (for both ruminant and non-ruminant animals due to lower fiber content).
The left over solid residue after fermentation could be used as an animal feed (Fig. 3). However, in this work, we have determined the feed value and mineral content of the oil cake residue (or enhanced cake) for peanut and DDGS left after enzymatic hydrolysis, prior to fermentation (Table 3). In general, the peanut oil cake residue had a higher available protein content compared to the enhanced DDGS cake. The protein content of the enhanced cakes increased from 35 to 66% for DDGS cake and from 59 to 89% for peanut cake (on a dry weight basis). Note that protein measurements were performed using nitrogen content analysis (i.e. Kjeldahl analysis) and, in this study, the feedstocks were pretreated in the presence of ammonia which partially reacts with the biomass thereby increasing its nitrogen content. However we believe that these reactions should not increase the nitrogen content of the biomass by more than 2–5% (on a dry weight basis), based on on-going studies for AFEX treated corn stover (unpublished data). More detailed studies are underway to check the amino acid content of these enhanced cakes compared to untreated substrates. The two oil cake residues had similar fat, water soluble carbohydrate and ash content. The enhanced DDGS oil cake has a much higher hemicellulose content (where, hemicellulose = NDF − ADF) than the enhanced peanut cake. This is not unexpected since most of the hemicellulose in AFEX DDGS is not hydrolyzed (less than 20–30% conversion) by the conventional cellulase mixtures that lack suitable hemicellulase activity [16]. Nevertheless, the enhanced cake is rich in protein content, minerals and has a lower ash content which should allow it to be used as an animal feed and/or nutrient supplement.
Pichia Fermentation
Fermentation of glucose (C6 sugar) to ethanol is a well established process, with Saccharomyces cerevisiae (or Baker’s yeast) being the dominant fermentative microbe used in the US corn ethanol industry [5]. However, native strains of S. cerevisiae are unable to utilize xylose (and other C5 sugars). On the other hand, yeasts such as P. stipitis, Candida shehatae, and Pachysolen tannophilus are able to ferment both C6 and C5 sugars to ethanol. Among the prevalent xylose fermenting yeasts, P. stipitis has shown the most promise for industrial application because it ferments xylose to ethanol at a high metabolic yield [12]. Furthermore, P. stipitis has no absolute vitamin requirements for xylose fermentation and is able to ferment a wide range of sugars, including cellobiose [12, 13]. In Fig. 3 we have mentioned SHF and simultaneous saccharification and fermentation (SSF). The SSF process is preferred to SHF. The SSF process requires enzymes and microbes that can hydrolyze the biomass and co-ferment the resulting monomeric C6 and C5 sugars to ethanol [13].
Peanut Oil Cake Hydrolysate Fermentation
As shown in Fig. 4, P. stipitis (strain Y-7124) produced 15.2 g/L ethanol in less than 24 h, with near complete consumption of 26 g/L glucose and ~13 g/L of other sugars, including (but not graphed individually) xylose (~0.2 g/L), arabinose (~5.9 g/L), galactose (0.7 g/L), and potentially mannose. At peak ethanol, residual sugars included 0.4 g/L glucose, 0.08 g/L galactose, and 4.0 g/L arabinose. A small amount of glycerol (0.4 g/L) initially present (not shown) was also consumed in less than 6 h. This accumulation of ethanol corresponded to a yield of approximately, 0.4 g ethanol per gram of sugar supplied. The furfural and HMF levels in this fermentation were not a factor since they were very low, <0.1 mM and 0, respectively (not shown). The yeast gradually consumed the available furfural. Prolonged operation of the fermentor beyond the 24-h peak in ethanol production led to gradual decline of the ethanol and production of acetic acid at 0.6 g/L (not shown). Viable cells increased from 2.65 to 3.8 × 108 cells/mL as ethanol was produced and then remained stable thereafter (not shown).
DDGS Hydrolysate Fermentation
As shown in Fig. 4, ethanol accumulation reached 14.8 g/L in less than 24 h when P. stipitis was provided DDGS hydrolysate containing 27 g/L glucose and ~8 g/L of other sugars, including (but not graphed individually) xylose (2.9 g/L), arabinose (4.1 g/L), and galactose (1.2 g/L). This was a yield of approximately 0.4 g ethanol per g of sugar supplied. Prolonging the fermentation beyond 24 h led to a decline in ethanol concentration. At peak ethanol, residual sugars included 0.06 g/L glucose, 0.5 g/L galactose, 0.5 g/L xylose, and 2.9 g/L arabinose. Significant glycerol was initially measured at 14.8 g/L, with negligible use after 24 h and only 3.2 g/L being utilized after 72 h. Low levels of inhibitors (not shown) were observed in the DDGS hydrolysate–only 0.5 mM furfural, 0.1 mM HMF, and 0.4–0.7 g/L acetic acid—though these levels were not high enough to cause an initial lag in fermentation. Viable cells (not shown) increased from 2.6 to 3.7 × 108 cells/mL as ethanol was produced and then stabilized after peak ethanol was reached.
In conclusion, we demonstrated edible oil cakes have tremendous potential as lignocellulosic feedstocks for an integrated, in-house biodiesel process and deserve further detailed studies for commercial implementation in the near-future. Soy oil cake is another promising candidate that needs to be further explored due to its potentially high glucan and hemicellulose content (~30%) compared to peanut (~20%) and DDGS (~40%).
References
Walter A (2000) In industrial uses of biomass energy. In: Rosillo-Calle F, Bajay SV, Rothman H (eds) Taylor & Francis, London, pp 200–253
Jeong GT, Park DH (2006) Batch (one- and two-stage) production of biodiesel fuel from rapeseed oil. Appl Biochem Biotechnol 129–132:668–679
Silva Nde L, Maciel MR, Batistella CB, Maciel Filho R (2006) Optimization of biodiesel production from castor oil. Appl Biochem Biotechnol 129–132:405–414
Fukuda H, Kondo A, Noda H (2001) Biodiesel fuel production by transesterification of oils. J Biosci Bioeng 92:405–416
Bothast RJ, Schlicher MA (2005) Biotechnological processes for conversion of corn in to ethanol. Appl Microbiol Biotechnol 67:19–25
Chundawat PS, Venkatesh B, Dale BE (2007) Effect of particle size based separation of milled corn stover on AFEX pretreatment and enzymatic digestibility. Biotechnol Bioeng 96:219–231
Kim S, Dale BE (2005) Ethanol fuels: E10 or E85—life cycle perspectives. Int J LCA 11:117–121
Eggeman T, Elander RT (2005) Process and economic analysis of pretreatment technologies. Bioresour Technol 96:2019–2025
Ramachandran S, Singh SK, Larroche C, Soccol CR, Pandey A (2007) Oil cakes and their biotechnological applications—a review. Bioresour Technol 98:2000–2009
LAP protocol available at National Renewable Energy Lab. http://www.nrel.gov/biomass/analytical_procedures.html
Protein estimation and sugar analysis protocol. http://www.dairyone.com/Forage/Procedures/default.htm
Slininger PJ, Dien BS, Gorsich SW, Liu ZL (2006) Nitrogen source and mineral optimization enhance d-xylose conversion to ethanol by the yeast Pichia stipitis NRRL Y-7124. Appl Microbiol Biotechnol 72:1285–1296
Slininger PJ, Liu ZL, Gorsich SW (2006) Impact of culture nutrition on the tolerance of furan inhibitors and the conversion of high xylose concentrations to ethanol by Pichia stipitis NRRL Y-7124. In proceedings of the AICHE annual meeting, 12–17 November 2006, San Francisco (paper 531d)
World statistics on World vegetable oil consumption (2007) http://www.soystats.com/2008/page_35.htm
Choct M (1997) Feed non-starch polysaccharides: chemical structures and nutritional significance. Feed milling international, pp 13–26
Dien BS, Ximenes EA, O’Bryan PJ, Moniruzzaman M, Li XL, Balan V, Dale B, Cotta MA (2008) Enzyme characterization for hydrolysis of AFEX and liquid hot-water pretreated distillers’ grains and their conversion to ethanol. Bioresour Technol 99:5216–5225
Somerville C, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, Osborne E, Paredez A, Persson S, Raab T, Vorwerk S, Youngs H (2004) Toward a systems approach to understanding plant cell walls. Science 306:2206–2211
Fan ZL, South C, Lyford K, Munsie J, van Walsum P, Lynd LR (2003) Conversion of paper sludge to ethanol in a semicontinuous solids-fed reactor. Bioprocess Biosyst Eng 26:93–101
Noblet J, van Milgen J (2004) Energy value of pig feeds: effect of pig body weight and energy evaluation system. J Anim Sci 82(E. Suppl.):E229–E238
Rosenthal A, Pyle DL, Niranjan K (1996) Aqueous and enzymatic processes for edible oil extraction. Enzyme Microb Technol 19:402–420
Acknowledgments
We thank Genencor International, Inc. for a gift of enzymes and Big River Resources, LLC for supplying distiller’s grains. This work was supported by the US Department of Energy (Contract: DE-FG36-04GO14220) in cooperation with the Midwest Consortium for Biobased Products and Bioenergy. Additional funding was provided by Michigan Research Foundation, through SPG grants.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Balan, V., Rogers, C.A., Chundawat, S.P.S. et al. Conversion of Extracted Oil Cake Fibers into Bioethanol Including DDGS, Canola, Sunflower, Sesame, Soy, and Peanut for Integrated Biodiesel Processing. J Am Oil Chem Soc 86, 157–165 (2009). https://doi.org/10.1007/s11746-008-1329-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-008-1329-4