Abstract
Specific structured lipids (SSL), previously produced by enzymatic acidolysis of coconut oil with different levels of conjugated linoleic acid (CLA) as fatty acid (FA), using a sn-1, 3 specific immobilized lipase, were used to prepare randomized structured lipids (RSL). A fraction of each SSL was subjected to chemical interesterification with sodium methoxide catalyst in order to modify the FA positional distribution and produce the corresponding RSL. Both families of structured lipids (SL) containing CLA were physicochemically characterized. Then, analysis of variance (ANOVA) was performed to evaluate the effects of CLA content (10, 20, 30 and 40%) and FA positional distribution (specific and randomized) on physicochemical properties of SL. Free fatty acids (FFA), peroxide value (PV) and p-anisidine value (p-AV) were not significantly affected by either CLA content or distribution. As expected, the iodine value (IV) and saponification value (SV) were influenced by CLA content but not by FA positional distribution, while oxidative stability index (OSI) was affected by both factors. Dropping point (DP), cloud point (CP) and solid fat content (SFC) decreased with the increase of CLA, while viscosity increased with the level of CLA. The FA positional distribution affected practically all the evaluated physical properties of SL, except CP and DP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Structured lipids (SL) are defined, in a broad sense, as any lipids that have been restructured from natural fats to change either the position or the composition of fatty acids (FA) from the native state. They are produced by chemical or enzymatic methods in order to exhibit their maximum potency for a specific functionality or use [1]. For the purpose of this investigation, SL are those modified triacylglycerides (TAG) that contain medium- (C8–C12) or short-chain (<C8) FA and long-chain (>C12) FA attached to the same glycerol molecule. By structuring TAG in this way, the health benefits of the long-chain fatty acids (usually unsaturated, i.e., omega-3 and -6 fatty acids) are enhanced by the nutritional and metabolic advantages of medium chain FA, resulting in a combination that can provide special health properties. This kind of SL has been used in hospitals to deliver designed TAG with functional FA (i.e., essential fatty acids) to target specific diseases, metabolic conditions and for optimal nutrition, because they provide the most effective means of delivering such desired fatty acids [1, 2] since the bioavailability of fatty acids is influenced by the positional location in the TAG molecule. It is known, for example, that fatty acids esterified in position sn-2 are preferentially absorbed during digestion [3].
Also, improvements or changes in the physical and/or chemical characteristics of a TAG can also be achieved when SL are synthesized [3]. Lipid modification strategies for the production of functional fats and oils include chemically- or lipase-catalyzed interesterification and/or acidolysis reactions. Interesterification has been used to produce fats with desirable functional and physical properties for food applications [3, 4], such as trans-free margarines, cocoa butter substitutes and reduced calorie foods.
In the last 10 years, SL have been receiving an increasing interest in the food area, since they may afford a good vehicle to provide nutraceutical FA to consumers. Consequently, the application of such new designed fats in the food industry is a new area. Thus, for each new SL it is necessary to develop data on composition and distribution of FA to produce the fat or oil that best meets the target objectives. Therefore, as researchers are capable of designing and producing new SL, it is necessary to learn more about the physical, chemical and biological properties of such fats in order to find their potential applications in food systems.
Conjugated linoleic acid (CLA) is a functional FA that has been reported to show a wide range of biological effects, such as inhibition of carcinogenic tumor growth [5], reduction of atherosclerotic risk [6], and reduction of body fat [7], among others. The term CLA refers to a mixture of geometrical and positional isomers of linoleic acid (C18:2) containing conjugated double bonds. The cis-9, trans-11-CLA and the trans-10, cis-12 isomers of CLA are believed to be primarily responsible for the beneficial physiological effects of CLA. There is evidence about the relatively low intake of CLA in the diet in order to exert its therapeutic effects, for example, 3.5 g/day CLA for a 70 kg person could protect humans from certain forms of cancer [5], and doses between 0.4 and 6.8 g/day during periods from 4 weeks to 6 months showed a reduction effect on body weight [8].
These facts make of big interest the development of nutraceutical or functional fats consisting of SL containing CLA that could provide the important physiological properties of CLA, enhanced by the metabolic benefits of medium chain FA in SL; but also with good characteristics that allow their use in ordinary foods. There are reports published on the synthesis of SL containing CLA [9–11], however, information available about the physicochemical properties of these SL is limited.
We previously reported the synthesis of SL containing CLA in a solvent-free system by using an immobilized lipase from Mucor miehei to incorporate CLA from a mixture of fatty acids (MFA) containing 73% CLA into coconut oil and tricaprylin TAG [11]. The cis-9, trans-11-CLA and the trans-10, cis-12 isomers of CLA accounted for 97.5% of the total CLA present in the MFA, and they were present in about equal amounts. Thus, we were able to produce coconut oil- and tricaprylin-based SL containing approx. 10, 20, 30 and 40% of CLA. Because CLA was mainly esterified in positions sn-1 and 3, due to the specificity of the enzyme, these SL are termed in this study as specific structured lipids or SSL. The main goal of the present work was to modify the FA positional distribution in the glycerol backbone of those SSL by chemical interesterification (i.e., randomization) in order to synthesize the corresponding RSL. Physicochemical properties of both families of SL were evaluated, as were the effects of CLA content and FA positional distribution on their physicochemical properties.
Experimental Procedures
Materials
Coconut oil-based SSL, containing approx. 10, 20, 30 and 40% w/w CLA were prepared by Rocha-Uribe and Hernandez [11], using Lipozyme IM, a sn-1, 3 specific immobilized lipase from Mucor miehei (Novo Nordisk, Franklinton, NC, USA). The cis-9, trans-11-CLA and the trans-10, cis-12 isomers of CLA accounted for about 95% of the total CLA present in such SL.
A mixture of 37 fatty acid methyl esters purchased from Sigma and CLA references of cis-9, trans-11 and trans-10, cis-12-CLA purchased from Matreya (Matreya Inc., State College, PA, USA) were used to identify the FA. All other chemicals and reagents were obtained from Sigma Chemical Company (St. Louis, MO, USA). Solvents were obtained from VWR-Scientific Products (McGaw Park, IL, USA).
Preparation of Samples
Two 400 g samples of SSL, for each CLA content, were divided in two equivalent parts. One of them was cooled, placed in plastic bottles and stored in a freezer (−20 °C) under inert atmosphere until analysis. The other part was subjected to chemical interesterification to randomize the fatty acids.
Chemical Interesterification
A half of the total amount of each SSL sample was chemically interesterified in order to modify the FA positional distribution (i.e., randomize) and to produce the corresponding RSL. About 200 g of each SSL was loaded in a 500-mL flask, heated to 70 °C in a hot plate with magnetic stirrer; 0.5% sodium methoxide was added as catalyst and the mixture was mixed at 70–75 °C. After heating for 3–5 min the color of the mixture became brownish due to the formation of the active catalyst, which is a reaction complex between the catalyst and the SSL. After the reaction had been carried out for 30 min, the mixture was cooled to 60 °C, and 0.5% citric acid solution (50% conc.) was added to neutralize the catalyst while stirring was maintained. Then, 0.3% silica gel was added to remove soap traces and to neutralize any remnant of non-neutralized catalyst, a vacuum was applied to remove excess of water, and then the mixture was filtered with the addition of 0.3% filter aid. The filtrate was bleached in a vacuum at 98 °C with 0.2% silica gel, and 0.4% bleaching clay. Filter aid (0.2%) was added to the mixture and this was filtered through a Whatman No. 41 filter paper. The final samples of RSL were cooled, placed in plastic bottles, and stored in a freezer (−20 °C) under an inert atmosphere until analysis.
Fatty Acid Composition
Fatty acid composition was determined by gas chromatography (GC) of the methyl ester derivatives. The methylation process consisted of reacting a 10-mg sample with 0.5 mL of 5% sodium methoxide in methanol, with heating at 65 °C for 20 min. The reaction was stopped by the addition of 0.5 mL of saturated solution of NaCl. The methyl esters were extracted with 1 mL hexane (HPLC grade). 1 μL of methyl ester solution in hexane was injected into a high resolution system consisting of a Stabilwax (100 m x 0.25 mm i.d., 0.25 μm film) capillary column (Restek Corp, Bellefonte, PA, USA) using hydrogen as carrier gas. The column oven was raised from 150 to 200 °C at a rate of 10 °C/min, then raised from 200 to 250 °C at a rate of 3 °C/min and held at 250 °C for 20 min. A Varian (model 3400) GC system fitted with split injection mode (250 °C) and a flame ionization detector (300 °C) was used. All the fatty acids were identified by comparison of their retention times with retention times of authentic standards. The content of each particular FA was calculated according to the AOCS Ce 1-62 method [12].
Physicochemical Analysis
The characterization of SL was realized following the AOCS official methods as follows: free fatty acids (FFA, Ca 5a-40), peroxide value (PV, Cd 8-53), p-anisidine value (p-AV, Cd 18-90), oxidative stability index (OSI, Cd 12b-92), iodine value (IV, Cd 1b-87), saponification value (SV, Cd 3-25), dropping point (DP, Cc18-80), cloud point (CP, Cc 6-25) and solid fat content (SFC, Cd 16-81). FFA was calculated as % oleic acid. OSI was determined at 110 °C using an Oil Stability Instrument (Omnion, Rockland, MA, USA). DP was determined using a Mettler processor (Mettler FP80) and a dropping furnace (Mettler FP83, Mettler Instruments Corp., Hightstown, NJ, USA), using a heating rate of 1 °C/min. While, a Praxis pulsed nuclear magnetic resonance (NMR) instrument (Praxis Corp., San Antonio, TX, USA) was used to determine SFC. Temperatures considered to obtain the SFC curve included 10.0, 21.1, 26.1, 33.3, and 37.8 °C.
Viscosity of SL was evaluated at 30 and 40 °C with a programmable Brookfield rheometer mod. DV-III (Brookfield Co. Stoughton, MA, USA), with a cone and plate system, using spindle CP40.
All tests were performed in duplicate.
Experimental Design
The experimental design explored two main factors: the CLA content of SL (at four levels: 10, 20, 30, and 40%, w/w) and the FA positional distribution in the TAG molecules (at two levels: specific, and randomized). The 4 × 2 treatments were replicated two times.
Statistical Analysis
Analysis of Variance (ANOVA) was performed using STATISTICS software, version 6.1. Significant effects on chemical and physical properties attributable to the main factors (i.e., level of CLA and positional distribution of FA) were determined with a significance level of α = 0.05.
Results and Discussion
Fatty Acid Composition
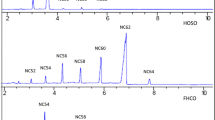
The GC system used to analyze the FA profile of the samples was adequate to resolve the cis-9, trans-11- and the trans-10, cis-12 isomers of CLA (Fig. 1), whose retention times were very close to 23.8 and 24.1 min respectively for all the analyzed samples (i.e., SSL and RSL). This means that the isomer patterns were kept constant for the whole set of samples.
The fatty acid composition of SSL and RSL with different levels of CLA is given in Table 1. The CLA content of all SL were very close to the target values of 10, 20 30 and 40%, e.g., 9.38, 18.21, 30.62 and 39.93% respectively for the SSL; and 9.22, 18.17, 30.17, and 39.45% respectively for the RSL. Because of such closeness, the concentrations of CLA were considered 10, 20, 30 and 40%, since fixed values are required for the levels of factors in the ANOVA test. For all the range of CLA content (from 10 to 40%), there was no significant difference in FA composition between SSL and RSL for the same CLA level (p > 0.05). This means that the chemical interesterification process did not affect the FA composition of SL, since there was no incorporation of new FA, but existing FA in the SSL were redistributed in a random manner among the three available positions of TAG. Thus, the only difference between both classes of SL, for each CLA content, is the positional distribution of the FA in TAG molecules. Furthermore, CLA in SSL is mainly esterified in positions sn-1 and 3 [11], while in RSL, all FA (including CLA) are randomly distributed between the three positions of the TAG backbone.
On the other hand, the main differences in FA composition between SL with different level of CLA (i.e., as CLA level increases) is the higher amount of longer chain and more unsaturated FA (mainly CLA), and the lower amount of medium chain FA originally present in coconut oil (i.e., C8:0, C10:0, C12:0, C14:0 and C16:0).
Free Fatty Acids, Peroxide and p-Anisidine Values
Analysis results of chemical parameters that indicate the quality (i.e., FFA, PV, and p-AV) of SSL and RSL samples are reported in Table 2. Although physicochemical changes may occur during the chemical interesterification process, there was no significant difference between FFA, PV, and p-AV among all SL (p > 0.05). This means that the final values of such parameters were not influenced by CLA level or FA distribution patterns. It can be explained if considering that, for example, during the bleaching step, to which the SSL and RSL were subjected FFA, peroxides and other secondary oxidation products are effectively removed to lower values, as observed previously by Rocha-Uribe and Hernandez [11].
Iodine and Saponification Values
Results of the parameters that describe the molecular characteristics of SL are also given in Table 2. As expected, an increase in the concentration of CLA changed both the molecular weight (indicated by a reduction in SV) (p < 0.0001) and the degree of unsaturation (indicated by an increase in IV) (p < 0.0001). But, no significant differences were found in IV and SV between SSL and RSL with the same CLA content, since such properties depend on the FA composition.
Oxidative Stability Index
Oxidative stability of fats has been reported to be affected by modifications in the molecular structure of TAG such as FA composition and positional distribution [13–15]. In the present study, the OSI values of SL were significantly influenced by FA positional distribution, the CLA level and the interaction of both factors (Fig. 2). SSL were more stable to oxidation than RLS (p < 0.001), which is similar to the reports of Wada and Koizumi [13] and Moussata and Akoh [14], as they claimed that lower concentrations of unsaturated FA in the position sn-2 of TAG, improve the oxidative stability of highly unsaturated vegetable oils. Here, unsaturated FA (i.e., CLA) are mainly esterified to positions sn-1 and 3 of SSL, while higher amounts of such FA are esterified in position sn-2 of RSL due to FA randomization, which reduces the stability of such TAG.
Among the SSL, OSI decreased with an increase in the CLA content (p < 0.0001), due to the high susceptibility of CLA to oxidation, which is even considerably higher than that of linoleic acid [16]. However, oxidative stability of RSL was not affected by CLA level (p > 0.05), which was unexpected. This finding indicated that other non-evaluated factors (i.e., oxidation products) or chemical parameters have a more determinant effect on this property for this particular group of fats (i.e., RSL). It could be the effect of thermal stress to which these fats were subjected during the additional processing steps (i.e., chemical interesterification and bleaching). The additional exposure to relatively high temperatures could explain both the lower stability with respect to their counterpart SSL and the similarity in oxidative stability found among this type of SL, since all of them were exposed to those conditions.
Dropping Point
DP measurements have traditionally been used to study the melting behavior of fats [17]. Figure 3 shows the effects of CLA content and of FA positional distribution on the DP of SL with different levels of CLA (i.e., 10, 20, 30, and 40%). Both factors and their interaction had significant effects on the DP of SL. Thus, DP decreases as the level of CLA increases (p < 0.0001). From 10 to 40% CLA in SSL there was a drop of 7.7 °C for SSL and of 8.6 °C for RSL. An increase in the content of unsaturated FA (i.e., CLA), results in a disruption of crystal structure, leading to a lower melting temperature, as reported by Norton et al. [18]. Also, for the range from 10 to 30% CLA, the DP of RSL is higher than the DP of SSL (p < 0.02), but lower for 40% CLA. Others also found that chemical interesterification increases the DP of different oils [17, 19]. This change in behavior of DP at different levels of CLA evidences the significant effect of the interaction between both factors (p < 0.04). However, as can be observed in Fig. 3, the differences in DP due to positional distribution of FA are so minor that, since a practical point of view, they are not relevant.
Cloud Point
The CP test was used to measure the starting of crystallization or solidification of SL. The results are given in Fig. 4. Only the CLA level was found to have a significant effect on this property (p < 0.0001). In general, the CP of both SL decreases as the CLA level increases. As for the DP, this behavior is related with the content of unsaturated CLA which prevents SL molecules to pack and form the crystal structure, leading to a lower crystallization or melting temperature. It is important to consider that, according to Tables 1, 2 increasing the level of CLA implies not only the increase in the unsaturated degree of SL, but also an increase in the length of FA, which, in time, tends to increase the solidification and melting points. Therefore, these two molecular properties (unsaturation degree and length of FA chains) exert opposite effects on physical properties of SL molecules. However, it is evident, from these results that the degree of unsaturation is the factor that predominates since the net effect observed in this study was a decrease in both properties of CP and DP.
Solid Fat Content
The SFC of SL as a function of temperature and CLA content are reported in Fig. 5. All samples presented steep solid curves with practically no solids at 27.6 °C or higher temperatures, which was expected because DP values of all the samples were lower than 25 °C. Below 27.6 °C, both SSL and RSL have lower SFC as a function of temperature (p < 0.0001). A sharp drop in SFC occurred between 10 and 21.1 °C. Above 21.1 °C, the decrease in SFC as a function of increasing temperature was less pronounced. In general, for the range of temperatures below 27.6 (i.e., from 10 to 21.1 °C), the SFC of all RSL was consistently lower than that of the corresponding SSL (p < 0.03), indicating the effect of FA positional distribution on this physical property. Also, in the same temperature range SFC decreases as a function of increasing CLA content.
According to deMan et al. [20] plasticity occurs over a range of 15–35% SFC. Below this range the fat no longer possesses plastic properties and can be described as pourable. In this frame, only the SL with less than 40% CLA can present plastic properties at 10 °C or lower, but not at higher temperatures, which is a limited range. Out of that range, the studied SL are all pourable or liquid fats. Frying, cookie fillers, nondairy applications, and liquid products or emulsions are examples of products benefitting from fats with these kinds of physical properties.
Viscosity
Results of viscosity of SL, at 30 and 40 °C, are represented in Figs. 6, 7 respectively. In general, viscosity increases with CLA content (p < 0.0001). For the whole range of CLA level, the viscosity of SSL was higher than the viscosity of RSL with the same composition (p < 0.0001). Also, the interaction of both factors significantly affected the viscosity of SL, which is demonstrated by the change in the behavior of viscosity of RSL (p < 0.0001) between 30 and 40% CLA. As with the other physical properties evaluated previously in this study, viscosity could be affected by the length and degree of unsaturation of the esterified FA in the SL molecules. Higher molecular weight of the esterified FA increases the viscosity because there is an increment in the interactions between adjacent molecules. Also, because TAG with longer FA molecules show higher resistance to flow. However, the degree of unsaturation of the FA has the opposite effect since the higher the number of double bounds the lower the viscosity of the TAG. As a result of an increasing amount of CLA in the SL, there is an increase in both unsaturation and molecular weight. These parameters have opposite effects on the viscosity. However, from these results, it is learned that viscosity of these SL is controlled more by the length of the FA than by the degree of unsaturation. This fact contrasts with the results observed in this study for DP, CP and SFC, where the effect of saturation degree of FA predominated over the effect of chain length. The difference between both groups of physicochemical properties is that DP, CP and SFC involve solid–liquid interactions or molecular packing.
References
Akoh CC (1995) Structured lipids-enzymatic approach. Inform 6:1055–1061
Lee K, Akoh CC (1998) Structured lipids: synthesis and applications. Food Rev Int 14:17–34
Osborn HT, Akoh CC (2002) Structured lipids-novel fats with medical, nutraceutical, and food applications. Comp Rev Food Sci Food Saf 1:93–103
Høy CE, Xu X (2001) Structured triacylglycerols. In: Gunstone FD (ed) Structured and modified lipids. Marcel Dekker, New York, pp 209–240
Ip C, Scimeca JA, Thompson HJ (1994) Conjugated linoleic acid. A powerful anticarcinogen from animal fat sources. Cancer 74:1050–1054
Lee KN, Kritchevsky D, Pariza MW (1994) Conjugated linoleic acid and atherosclerosis in rabbits. Atherosclerosis 108:19–25
Park Y, Albright KJ, Liu W, Storkson JM, Cook ME, Pariza MW (1997) Effect of conjugated linoleic acid on body composition in mice. Lipids 32:853–858
Li Y, Watkins BA (2006) CLA in human nutrition and health: human studies. In: Akoh CC (ed) Handbook of functional lipids. Taylor and Francis Group, New York, pp 363–388
Kim IH, Yoon CS, Cho SH, Lee KW, Chung SH, Tae BS (2001) Lipase incorporation of conjugated linoleic acid into tricaprylin. J Am Oil Chem Soc 78:547–551
Kawashima A, Nagao T, Watanabe Y, Kobayashi T, Ikeda I, Tominaga Y, Shimada Y (2004) Preparation of regioisomers of structured TAG consisting of one mole of CLA and two moles of caprylic acid. J Am Oil Chem Soc 81:1013–1019
Rocha-Uribe A, Hernandez E (2004) Solvent-free enzymatic synthesis of structured lipids containing CLA from coconut oil and tricaprylin. J Am Oil Chem Soc 81:685–689
AOCS (1998) Official methods and recommended practices of the American Oil Chemists’ Society. AOCS Press, Champaign IL
Wada S, Koizumi C (1983) Influence of position of unsaturated fatty acids esterified glycerol on the oxidation rate of triglyceride. Lipids 60:1105–1109
Moussata CO, Akoh CC (1998) Influence of lipase-catalyzed interesterification on the oxidative stability of melon oil triacylglycerols. J Am Oil Chem Soc 75:1155–1159
Akoh CC, Moussata CO (2001) Characterization and oxidative stability of enzymatically produced fish and canola oil-based structured lipids. J Am Oil Chem Soc 78:25–30
Zhang A, Chen ZY (1997) Oxidative stability of conjugated linoleic acids relative to other polyunsaturated fatty acids. J Am Oil Chem Soc 74:1611–1613
Marangoni AG, Rousseau D (1998) The influence of chemical interesterification on physicochemical properties of complex fat systems 1. Melting and crystallization. J Am Oil Chem Soc 75:1265–1271
Norton IT, Lee-Tuffnell CD, Ablett S, Bociek SM (1985) A calorimetric, NMR and X-Ray diffraction study of the melting behavior of tripalmitin and triestearin and their mixing behavior with triolein. J Am Oil Chem Soc 62:1237–1244
Laning SJ (1985) Chemical interesterification of palm, palm kernel and coconut oils. J Am Oil Chem Soc 62:400–405
deMan L, deMan JM, Blackman B (1995) Effect of tempering on the texture and polymorphic behavior of margarine fats. Fats Sci Technol 97:55–60
Acknowledgments
This work was supported in part by a research grant from PROMEP-SEP (to CA36).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Rocha-Uribe, A., Hernandez, E. Effect of Conjugated Linoleic Acid and Fatty Acid Positional Distribution on Physicochemical Properties of Structured Lipids. J Am Oil Chem Soc 85, 997–1004 (2008). https://doi.org/10.1007/s11746-008-1295-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-008-1295-x