Abstract
Pistacia atlantica subsp. mutica (PAM) and kurdica (PAK) kernel oils showed significantly lower unsaturated to saturated fatty acid ratios (6.39, 6.33, respectively) and calculated oxidizability (Cox) values (3.99, 4.13, respectively) than those of the P. vera L. cv. Ohadi (PVO) kernel oil (8.91, 4.41) samples. The highest peroxide value was observed for the PAK oil (4.07 mequiv kg−1) (PAM, 1.94; PVO, 0.37) samples. Iodine values for the PAM, PAK, and PVO oils were 104.26, 104.77, and 110.66, respectively. The saponification number of the PVO oil was significantly greater than the PAM and PAK oils, which were statistically not different. The unsaponifiable contents, which were composed mainly of sterols, ranged from 5.63 to 6.14%. Statistically the total tocopherols contents of the PAM (818.58 mg α-tocopherol kg−1) and PVO (815.90 mg α-tocopherol kg−1) oils were significantly higher than that of the PAK oil (499.91 mg α-tocopherol kg−1). Total phenolics contents differed significantly, the greatest concentration was for the PAM oil (81.12 mg gallic acid kg−1), followed by the PVO (62.84 mg gallic acid kg−1) and PAK (56.51 mg gallic acid kg−1) oil samples. The wax contents of the oil samples were statistically in the same range, namely 5.67–6.48%. Oxidative stability data indicated that the PAM oil is the most resistant to the formation of lipid oxidation products, followed by the PAK and PVO oil samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pistacia is a genus of the family Anacardiaceae, consisting of 11 or more species, which are shrubs or trees. Three Pistacia species occur naturally in Iran, including P. vera Linnaeus, P. khinjuk Stocks and P. atlantica Desf. P. atlantica is one of the most widely distributed wild species, and it is considered to be an Irano-Turanian species with distribution from south-west Asia to north-west Africa (Morocco) [1]. Four subspecies or varieties have been described for P. atlantica: cabulica, kurdica, mutica, and atlantica [2]. P. atlantica subsp. mutica (PAM) and P. atlantica subsp. kurdica (PAK) trees grow in large populations and cover an area more than 1,200,000 ha mainly in the western, central and eastern parts of Iran.
The fruits of P. atlantica smell mastic-like (an aromatic resin used as a spice) and are sometimes used in grape distillate to flavor the alcoholic drink called “Raki.” Fruits from the P. atlantica shrub grown on the Lesvos island are called “Tsikuda” and are occasionally chewed by the local people (because of their spicy flavor). The fruits of P. atlantica are also used for tanning and as fodder for cattle [1]. In Iran, they are called “Bene” (Fig. 1) and are used by the natives as foods after grinding and mixing with other ingredients [3]. Also, they can be extracted for their oil.
The study of the chemical composition of major and minor components of edible fats and oils and their relation with oxidative stability, which in turn affects the oil quality, is of scientific interest. The oils from P. atlantica fruits are considered important among the newer sources of edible oils but little has been published on their physicochemical characteristics [3]. Proximate analysis of kernels from whole mature fruits of the PAM and PAK showed that the moisture and oil contents were 54 and 57%, and 2.60 and 2.70%, respectively [3]. The oil content of the fruits were approximately the same, as the fruits of P. vera L. from Iran (55–58%). Compared to other vegetable seeds oils (sunflower, peanut, cotton, corn) the kernels of P. atlantica fruits appear to have a very larger percentage of oil. A combined study of the chemical composition and the oxidative stability of the kernel oil from the P. altantica fruit had not been done previously. Hence, this study was undertaken to investigate the chemical composition and oxidative stability of the kernel oils from the two subspecies of P. atlantica grown in Iran, and to compare their properties with those of the kernel oil from P. vera L. cv. Ohadi (PVO).
Materials and Methods
Materials
Ripe fruit samples (10 kg each) of the PAK, PAM, and PVO were collected from fields in Islamabad in the Ilam province, in Marvdasht in the Fars province, and in Feizabad in the Khorasan Razavi province, respectively, and were stored at −18 °C until analysis. Fatty acid methyl ester (FAME) standards, and all chemicals and solvents used in this study were of analytical reagent grade and supplied by Merck and Sigma Chemical Companies.
Oil Extraction
After drying in the shade, the pericarps of the Pistacia fruits were removed and the kernels were ground to powder in a grinder. The powders were extracted with n-hexane (1:4 w/v) by agitation in the dark at ambient temperature for 48 h. The solvent was evaporated in vacuo at 40 °C to dryness.
Oil Oxidation
The oil samples extracted (200 g) were placed in a fryer (Kenwood DF280, Havant, Hampshire, UK) and maintained at 170 °C for 8 h with no stirring. The surface-to-volume ratio of the oil samples in the fryer was about 0.7 cm−1. After intervals of 120 min of heating, samples were removed and analyzed.
Conjugated Diene Value (CDV)
The oil samples were diluted to 1:600 with hexane and the CDV was measured spectrophotometrically at 234 nm and read against HPLC grade hexane as a blank. An extinction coefficient of 29,000 mol L−1 was utilized to quantify the concentration of conjugated dienes formed during oxidation [4].
Fatty Acid Composition
The fatty acid composition of the oils was determined by gas-liquid chromatography and was reported in relative area percentages. Fatty acids were transesterified into their corresponding FAMEs by vigorous shaking of a solution of oil in hexane (0.3 g in 7 mL) with 2 mL of 7 N methanolic potassium hydroxide at 50 °C for 10 min. The FAMEs were identified using an HP-5890 chromatograph (Hewlett-Packard, CA, USA) equipped with a CP-FIL 88 (Supel Co., Inc., Bellefonte, PA) capillary column of fused silica, 60 m in length × 0.22 mm I.D., 0.2 μm film thickness, and a flame ionization detector. Nitrogen was used as carrier gas with a flow rate of 0.75 mL min−1. The oven temperature was maintained at 198 °C, and that of the injector and the detector at 250 °C.
Calculated Oxidizability (Cox) Value
The Cox value of the oils was calculated based on the percentage of unsaturated C18 fatty acids, applying the formula proposed by Fatemi and Hammond [5]:
Iodine Value (IV)
The IV was determined according to the AOAC Official Method 920.158 (Hanus method) [6].
Oil/Oxidative Stability Index (OSI)
A Metrohm Rancimat model 743 (Herisau, Switzerland) was used for the OSI analysis. The tests were done with 3-g oil samples at temperatures of 120 °C at an airflow rate of 15 L h−1 [7].
Peroxide Value (PV)
The spectrophotometric method of the International Dairy Federation as described by Shantha and Decker [8] was used to determine the PV (thiocyanate method).
Saponification Number (SN)
The SN was determined according to the AOAC Official Method 920.160 [6].
Total Carbonyl Compounds
Total carbonyl compounds (known as the carbonyl value, CV) of the oils was measured according to the method developed by Endo et al. [9] using 2-propanol and 2,4-decadienal as solvent and standard, respectively [10].
Total Phenolics (TP) Content
The TP content was determined spectrophotometrically using Folin–Ciocalteau’s reagent according to the method described by Capannesi et al. [11]. A calibration curve of gallic acid in methanol was performed in a concentration range of 0.04–0.40 mg mL−1.
Total Sterols (TS) Content
The TS content was quantified according to the Lieberman–Burchard color reaction [12]. Lieberman–Burchard reagent (sulfuric acid and acetic anhydride) reacts with sterols to produce a characteristic green color whose absorbance is determined by spectrophotometry at 640 nm.
Total Tocopherols (TT) Content
The TT content was determined according to the colorimetric method described by Wong et al. [13].
Unsaponifiable Matter (USM) Content
The USM content was determined by the method described by Lozano et al. [14].
Wax Content
The wax content was determined according to the method described by Mezouari et al. [15]. An accurately weighed quantity of oil (10 g) was put in an Erlenmeyer flask, and five times its volume of acetone was added. The solution (oil/acetone) was cooled and kept at 4 °C for 24 h to crystallize the waxes. The solid fraction was filtered on a previously weighed Whatman No. 1 filter paper, dried at 45 °C in a vacuum oven, and then weighed to obtain the acetone-insoluble matter.
Statistical Analysis
All experiments and measurements were carried out in triplicate, and data were subjected to analysis of variance (ANOVA). ANOVA and regression analyses were performed according to the MStatC and SlideWrite softwares. Significant differences between means were determined by Duncan’s multiple range tests. P values less than 0.05 were considered statistically significant.
Results and Discussion
Table 1 lists the fatty acid composition, the ratio between unsaturated and saturated fatty acids (USFA/SFA ratio) and the Cox value of the PAM, PAK and PVO kernel oils. It can be observed that the PAM and PAK kernel oils are distinguished from each other only due to the statistically significant but small difference in the percentage of oleic acid (C18:1). Consequently, the USFA/SFA ratio and Cox value were statistically the same in these oils. The PVO kernel oil was characterized by its higher polyunsaturated fatty acids (PUFA) (linoleic acid, C18:2) and lower SFA percentages (Palmitic acid, C16:0, and stearic acid, C18:0) than those of the PAM and PAK kernel oils, which makes it particularly prone to oxidation. Indeed, the PAM and PAK kernel oils show a lower USFA/SFA ratio and Cox value than that of the PVO kernel oil.
The PV varied significantly among the three Pistacia kernel oils and was the greatest in the PAK kernel oil (4.07 mequiv kg−1), followed by the PAM (1.94 mequiv kg−1) and PVO (0.37 mequiv kg−1) kernel oils (Table 2). This indicates that the PAK and PAM kernel oils were oxidized more than the PVO kernel oil sample. Assuming the PVs for the PAK and PAM oils are always greater, this might result in shorter storage times as compared to the PVO kernel oil samples.
The IV, which is considered as a measure of the oil unsaturation, for the PAM and PAK kernel oils was 104.26 and 104.77, respectively, compared to 110.66 for the PVO kernel oil (Table 2). These values are comparable with other seed oils such as corn oil (103–128), cottonseed oil (99–119) and mustard seed oil (92–125) [16]. The differences in the IVs between the PAM and PAK kernel oils and the PVO kernel oil were due to their different fatty acid compositions. The PAM and PAK kernel oils have a greater SFA content (C16:0 and C18:0), whereas the PVO contains a greater PUFA content (C18:2). As can be seen in Table 2, the USFA/SFA ratio is significantly greater for the PVO kernel oil than for the PAM and PAK kernel oils.
The SN varied significantly among the oils and was greater in the PVO kernel oil than the PAM and PAK kernel oils (Table 2), which were statistically the same. All the oils had very low values compared to common vegetable oils with average SN range of 175–250 [16].
The USM content of the kernel oils ranged from 5.63 to 6.14% and there was no statistically significant difference among them (Table 2). The USM fraction of vegetable oils naturally contains hydrocarbons, terpene alcohols, sterols, tocopherols and other phenolic compounds, and typically comprises 0.5–2.5% of vegetable oils, although some vegetable oils have exceptional amounts, 5–6%. The USM content is extensively used as an index of the quality of refined fat or oil or as a control index of the refining process. For example, according to Japanese Agricultural Standards, the USM, e.g. in edible safflower oils, edible soybean oils, and edible palm oils should be no more than 1.0%. The effectiveness of USMs in retarding oil deterioration has been demonstrated by many investigators.
Plant sterols, called phytosterols, are important minor components which are present in almost all vegetable oils. Oil samples can contain a complex composition of sterols with an average total sterol content of 0.3–2% in the oil but the sterol content can reach more than 10% in some plant oils. As seen in Table 2, no statistically significant differences were observed among the TS content of the three kernel oils studied (which was 5.66% on average). It was interesting to note that the USM fractions were composed mainly of sterols. Phytosterols have attracted the interest of food oil chemists because of their perceived effects on cholesterol absorption. Their relative concentrations are also indicative of the genuineness of vegetable oils [17].
The TT content of the PAK kernel oil (499.91 mg kg−1 oil) was significantly lower than the PAM (818.58 mg kg−1 oil) and the PVO (815.90 mg kg−1 oil) kernel oil samples, which were statistically the same (Table 2). The PAM and PVO kernel oils are considered to be rich sources of tocopherols since their concentrations are greater than those reported for some common vegetable oils, e.g. canola, sunflower, cottonseed, and corn oils with the TT content of about 695, 640, 630, and 605 mg kg−1 oil, respectively [18]. Tocopherols are particularly important functional constituents of the USM fraction of vegetable oils. The tocopherols have antioxidant properties and they are active as vitamin E, which makes them particularly important for human health.
The TP content among the kernel oils were significantly different with the greatest concentration for the PAM kernel oil (81.12 mg gallic acid kg−1), followed by the PVO (62.84 mg gallic acid kg−1) and PAK kernel oil samples (56.51 mg gallic acid kg−1) (Table 2). A study on canola, soybean, sunflower, corn and olive oils indicated their TP content was 48.19, 45.80, 45.27, 30.80, and 15.27 mg gallic acid kg−1, respectively [19]. Although the interest in phenolics is related primarily to their antioxidant activity, they also show important biological activity in vivo and may be beneficial in combating diseases related to excessive oxygen radical formation exceeding the antioxidant defense capacity of the human body.
Waxes are a group of insoluble high melting point compounds which are naturally found in crude vegetable oils. These compounds can be disadvantageous since they are primarily responsible for the turbidity of refined oil and increased refining losses. On the other hand, these compounds have potential applications in the cosmetic, lubricant, plastisizer, pharmaceutical, food, polymer, and leather industries. The wax content of the kernel oils studied were statistically in the same range of 5.67–6.48% (Table 2). It should be mentioned that the wax content of the Pistacia kernel oils was greater than that of rice bran oils which are characterized by their high wax contents (3–6%) among vegetable oils [15].
The oxidative stability of the Pistacia kernel oils was studied by following the formation of primary, secondary and tertiary lipid oxidation products in the oil during 8 h of heating at 170 °C. Primary, secondary and tertiary products that included conjugated dienes (the CDV), carbonyl compounds (the CV), and volatile acids (mainly short chain acids, C1–C3) (the OSI) formed in the oil indicating extensive lipid oxidation occurred.
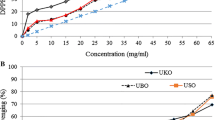
The CDV of the Pistacia kernel oils during heating are shown in Fig. 2. Measurement of the CDV is a good indication of the oxidative stability of the oil samples. During the oxidation of PUFAs containing methylene interrupted dienes and polyenes, there is a shift in the double bond positions due to isomerization and conjugation. This is accompanied by an increase in the UV absorption at 234 nm due to dienes. The increase in absorbance at 234 nm is an indicator of oxidation and is reported to increase with uptake of oxygen and formation of peroxides during the early stages of oxidation [20]. A good correlation has been reported between the CDV and PV [21]. The greater the levels of CDV, the lower will be the oxidative stability of the oils. The results of this study showed that the initial CDV for the kernel oils differs significantly and the lowest values were found for the PVO kernel oil (6.57); while those of the PAM and PAK kernel oil samples were 10.28 and 14.01, respectively. The sample order for the initial CDV for the three kernel oils was similar to that found for their PV (Table 2). As can be seen in Fig. 2, the CDVs linearly increased as the heating time increased with a significantly greater rate for the PAK kernel oil (a = 2.99), followed by the PAM (a = 2.34) and PVO (a = 1.45) kernel oils, respectively. This indicates that the kernel oils undergoing the heating process were more prone to the production of conjugated diene hydroperoxides in the order of PAK > PAM > PVO. Furthermore, the initial quality of the oils (the PVs in Table 2) plays an important role regarding the oxidative stability during heating.
The Rancimat test is an accelerated technique most commonly used for the assessment of the oxidative stability of edible fats and oils. The initial OSI of the PAM kernel oil was significantly greater (14.13 h) than that of the PAK kernel oil (11.39 h), which in turn was significantly greater than that of the PVO kernel oil (8.70 h). Our previous findings showed that the OSI of a set of oil samples decreased exponentially during the frying process [22]. The measurement of the OSI during the heating of the Pistacia kernel oils confirmed the existence of such trend as well. The logarithms of OSIs versus heating times were plotted, and the lines were fitted to the data (Fig. 3). The slope of the linear equations (a values), which were considered to be a measure of the rate of OSI decrease during the heating process, was significantly different for the kernel oils. The highest a value belonged to the PAK kernel oil, followed by the PAM and PVO kernel oils. This indicates that the PAK kernel oil was more stable during heating than the PAM and PVO kernel oils.
Table 3 shows the changes in the CV of the kernel oils during heating. The CV measures secondary decomposition products of lipid oxidation such as aldehydes and ketones. Peroxides are transformed into secondary products that contain carbonyl groups. These compounds are more stable than peroxides and the CV was a good index of the oxidative changes that occurred in the kernel oils during heating. The determination of carbonyl compounds in heated and frying oils is very important for evaluating their quality because these compounds often contribute to rancid and unpleasant flavors, and the can reduce the nutritional value of fried foods [9]. Farhoosh and Moosavi [23] indicated that the CV determination, which is a quick, simple, and cheap test, can be considered a useful measure for indicating the safety and sensory deterioration of edible fats and oils during the frying process. Our results in the present study showed that the PAM and PAK kernel oils had statistically the same CVs, but the CVs were significantly greater than that of the PVO kernel oil (Table 3). The CVs of the Pistacia kernel oils increased as the heating time increased. At the end of heating, the CV of the PAM kernel oil was approximately 2.7 times that of its initial amount (17.50 μmol g−1), whereas the values for the PAK and PVO kernel oils was approximately 4 (24.04 μmol g−1) and 4.8 (17.49 μmol g−1) times their initial values, respectively. This indicates that the PVO kernel oil is more prone to oxidation and sensory deterioration than the PAK and PAM kernel oils, respectively, although the initial quality of the PVO kernel oil was better than those of the PAK and PAM kernel oils. Therefore, regarding the fatty acid composition (Table 1) and the TT and TP content (Table 2), and assuming the same initial quality for the kernel oils, it is expected that the PAM kernel oil is the most resistant to lipid oxidation, followed by the PAK and PVO kernel oils.
References
Browiczb K (1988) Chorology of trees and shrubs in south-west Asia and adjacent regions. Polish Scientific, Warszawa-Poznan
Saffarzadeh A, Vincze L, Csapo J (1999) Determination of the chemical composition of acron (Quercus branti), Pistacia atlantica and Pistacia khinjuk seeds as non-conventional feedstuffs. Acta Agr Kapos 3:59–69
Daneshrad A, Aynehchi Y (1980) Chemical studies of the oil from pistacia nuts growing wild in Iran. J Am Oil Chem Soc 57:248–249
Saguy IS, Shani A, Weinberg P, Garti N (1996) Utilization of jojoba oil for deep-fat frying of foods. Lebensm Wiss u-Technol 29:573–577
Fatemi SH, Hammond EG (1980) Analysis of oleate, linoleate and linolenate hydroperoxides in oxidized ester mixtures. Lipids 15:379–385
AOAC (2005) Official methods of analysis. Association of Official Analytical Chemists, Washington, DC
Farhoosh R (2007) The effect of operational parameters of the Rancimat method on the determination of the oxidative stability measures and shelf-life prediction of soybean oil. J Am Oil Chem Soc 84:205–209
Shantha NC, Decker EA (1994) Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J AOAC Int 77:21–424
Endo Y, Li CM, Tagiri-Endo M, Fugimoto K (2001) A modified method for the estimation of total carbonyl compounds in heated and frying oils using 2-propanol as a solvent. J Am Oil Chem Soc 10:1021–1024
Farhoosh R, Moosavi SMR (2006) Determination of carbonyl value in rancid oils: a critical reconsideration. J Food Lipids 13:298–305
Capannesi C, Palchetti I, Mascini M, Parenti A (2000) Electrochemical sensor and biosensor for polyphenols detection in olive oils. Food Chem 71:553–562
Sabir SM, Hayat I, Gardezi SDA (2003) Estimation of sterols in edible fats and oils. Pak J Nutr 2:178–181
Wong ML, Timms RE, Goh EM (1988) Colorimetric determination of total tocopherols in palm oil, olein and stearin. J Am Oil Chem Soc 65:258–261
Lozano YF, Mayer CD, Bannon C, Gaydou EM (1993) Unsaponifiable matter, total sterol and tocopherol contents of avocado oil varieties. J Am Oil Chem Soc 70:561–565
Mezouari S, Parkash kochhar S, Schwarz K, Eichner K (2006) Effect of dewaxing pretreatment on composition and stability of rice bran oil: potential antioxidant activity of wax fraction. Eur J Lipid Sci Technol 108:679–686
Gunstone FD, John LH, Fred BP (1994) The lipid handbook. Chapman & Hall Chemical Database, United States
Crane S, Aurore G, Joseph H, Mouloungui Z, Bourgeois P (2005) Composition of fatty acids triacylglycerols and unsaponifiable matter in Calophyllum calaba L. oil from Guadeloupe. Phytochem 66:1825–1831
Eskin NAM, McDonald BE, Przybylski R, Malcolmson LJ, Scarth R, Mag T, Ward K, Adolph D (1996) Canola oil. In: Hui YH (ed) Bailey’s industrial oil and fat products, Wiley, New York, pp 1–95
Farhoosh R, Niazmand R, Rezaei M, Sarabi M (2008) Kinetic parameter determination of vegetable oil oxidation under Rancimat test conditions. Eur J Lipid Sci Technol 110:587–592
Farmer EH, Sutton DA (1946) Peroxidation in relation to olefinic structure. Trans Faraday Soc 42:228–232
Shahidi F, Wanasundara UN, Brunet N (1994) Oxidative stability of oil from blubber of harp seal (Phoca groenlandica) as assessed by NMR and standard procedures. Food Res Int 27:555–562
Farhoosh R, Moosavi SMR (2007) Rancimat test for the assessment of used frying oils quality. J Food Lipids 14:263–271
Farhoosh R, Moosavi SMR (2008) Carbonyl value in monitoring of the quality of used frying oils. Anal Chim Acta 617:18–21
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Farhoosh, R., Tavakoli, J. & Khodaparast, M.H.H. Chemical Composition and Oxidative Stability of Kernel Oils from Two Current Subspecies of Pistacia atlantica in Iran. J Am Oil Chem Soc 85, 723–729 (2008). https://doi.org/10.1007/s11746-008-1258-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-008-1258-2