Abstract
There is increasing interest in India for suitable alternative fuels that are environment friendly. This search has led to mahua oil (MO) as one alternative for diesel fuel in India. Mahua oil methyl esters (MOME) were prepared by transesterification using potassium hydroxide (KOH) as catalyst and nuclear magnetic resonance (NMR) testing was done to determine the conversion of vegetable oil to biodiesel (MOME). The properties of MOME were close to those of diesel oil. Engine testing was conducted using a single-cylinder 4-stroke direct-injection, constant-speed compression-ignition diesel engine using MO, MOME and B20 as fuels. The engine ran smoothly with MOME and B20, but heavy smoke emissions were observed when MO was used as fuel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A large number of investigations have been carried out internationally in the area of vegetable oils as fuel. Jamieson [1] listed over 350 oil-bearing crops while Duke et al. [2] identified 70 species of oilseeds with considerable potential. Sunflower, safflower, soybean, cottonseed, winter rape and peanut oils have been reported to be suitable substitutes for petroleum-based fuels.
Mahua, Madhuca longifolia of the family Sapotaceae, is a medium to large tree with a wide round canopy. Mahua is a slow-growing species, attaining a mean height of 0.9–1.2 m at the end of the fourth year but may attain a height of up to 20 m. The variety latifolia is common throughout the Indian sub-continent, including Bangladesh. It is of deciduous nature and thrives in dry tropical and sub-tropical climates. As a plantation tree, mahua is an important plant having vital socio-economic value. This species can be planted along the roadside and canal banks on a commercial scale and in social forestry programs, particularly in tribal areas. The seed kernel contains about 50% oil. The oil yield by screw pressing is 34–37% and the fresh oil from properly stored seed is yellow in color [3].
Coupland et al. [4] determined the physical properties of liquid edible oils. Shashikant et al. [5] studied the effects of methanol quantity, acid concentration, and reaction time to reduce free fatty acid content of MO during pretreatment for making biodiesel. Sukumar et al. [6, 7] used methyl esters of mahua oil (MOEE) as the sole fuel in a diesel engine. They prepared MOEE by transesterifcation with H2SO4 as catalyst, and tested the fuel in a 4-stroke direct-injection natural-aspirated diesel engine. Tests were carried out at a constant speed of 1,500 rev/min and different brake mean effective pressures. The performance of MOEE was similar to diesel fuel.
Bhat et al. [8] tested four blends of MO with diesel and pure oil in a diesel engine at 20:1, 18:1 and 16:1 compression ratios. The fuel blends were prepared in 20, 40, 60 and 80% (vol) proportions with diesel. They did not modify the fuel supply system and reported that blends of MO and diesel resulted in lower thermal efficiency compared to operation with diesel oil alone.
GC, HPLC and NMR are methods often used to evaluate fuel quality [9]. The first report on spectroscopic determination of the yield of a transesterification reaction utilized 1H NMR [10]. The protons of the methylene group adjacent to the ester moiety in triglycerols and the protons in the alcohol moiety of the methyl ester product were used to monitor yield. B20 blends are better than other blends. Hence for the present work, a B20 blend of MOME and diesel was used.
In the present work, our objectives were to prepare MOME by transesterification using KOH as catalyst, compare the properties of raw MO, MOME and a B20 blend to diesel oil, and determine the suitabilities of MO, MOME and a B20 blend as fuels in a diesel engine.
Materials and Methods
Generally, vegetable oils contain fatty acids (palmitic, stearic, oleic, linoleic, lignoceric, eicosenoic, arachidic and behenic). Of these, MO contains the saturated fatty acids palmitic (hexadecanoic acid) and stearic (octadecanoic acid) and the unsaturated acids oleic (octadec-9-enoic acid) and linoleic (9,12-octadecadienoic acid). The mahua oil was commercially available in the local market and used as the raw material. The fatty acid composition of MO is given in Table 1.
Preparation of Biodiesel (MEMO) and NMR Testing
To prepare biodiesel, the transesterification reaction was performed in a 1,000-mL round-bottom vessel. The reactor vessel was filled with 420 mL MO. Methanolic KOH, which was prepared by dissolving 4 g KOH in 170 mL methanol, was added to the MO oil in the reactor. For refluxing, a vertical water-cooled condenser was placed on top of the vessel and the reactor was immersed in a constant-temperature water bath maintained at 70 °C. Agitation was provided with a magnetic stirrer during the reaction. The reaction was carried out for 2 h. After transesterification, the condenser was removed and the products were heated to remove excess methanol. The products were transferred to a 1-L separatory funnel to separate the phases. The top layer containing the esters was washed with warm water to remove impurities such as soap and other residues. Finally, the methyl esters were dried using 10 g anhydrous NaSO4.
The MO and MOME samples were analyzed by putting into a 5-mm (inner diameter) NMR tube. Proton NMR spectra were obtained by using a Bruker AMX 400 spectrometer operating at 300 MHz. The samples were dissolved in chloroform-d (CDCl3). 1H-NMR spectra were obtained under the following conditions; spectra width 7692.3 Hz, data points 32,768, pulse width 6.5 μs and pulse delay1.54.
Evaluation of MO, MOME, Diesel and B20 Properties
Flash point, viscosity, copper strip corrosion, cloud point and density of the oil were determined using ASTM D93, D445, D130, D2500 and D4052 procedures, respectively.
Engine Performance and Emissions
Engine tests were carried out using a 4-stroke, direct-injection, naturally aspirated, water-cooled, single-cylinder, 7-HP, Kirloskar (Mysore, India) diesel engine. The diesel engine was started with MO as fuel and, under steady-state conditions, fuel flow rate, air flow rate, load and exhaust emissions were recorded. The same procedure was followed for MOME, diesel and a B20 blend.
Results and Discussion
The properties of MO, MOME, diesel and B20 blend are shown in Table 2. Except for calorific value, the properties of MOME were lower than MO and close to diesel oil.
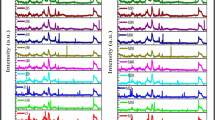
Figure 1 depicts the NMR spectrum of MO. For triglycerides (TG), protons on acyl groups resonate at 0.8–2.9 ppm, while H-1, H-2 and H-3, which are protons attached to the glycerol carbons, appear at a downfield of 4.0–5.6 ppm. When one or two acyl groups migrate from TGs, giving sn-DGs or sn-MGs as transesterfication proceeds, H-1, H-2 and H-3 shift toward a higher field due to the loss of the high electron density of the acyl group, while the protons on the acyl group could not shift. Figure 2 shows the NMR spectrum of MOME.
Compared to the spectrum of MO, a large signal at 3.6 pm was observed, which was assigned to the methyl protons of esters. In addition, some new peaks also appeared. This was attributed to the methanolysis products of sn-DGs and sn-MGs. Assignment of these signals was made based on the literature [11, 12]. The conversion of MO to MOME was calculated based on references [9, 12] and was 92.4%.
Engine Performance and Emissions
When MO was used as the sole fuel, heavy smoke emissions were observed at full load. This was attributed to higher viscosity of MO, which results in poor atomization, spray formation and combustion of the fuel. The engine ran smoothly with MOME and B20. Brake thermal efficiency is defined as the ratio of brake power to the heat supplied. Figure 3 shows the variation of brake thermal efficiency with load. The B20 blend (20% MOME and 80% diesel) gave higher efficiency than diesel fuel at higher loads. The MOME results in thermal efficiency were close to diesel fuel at higher loads, but MO gave lower thermal efficiency.
Figure 4 shows the variation of smoke opacity with load. Smoke is formed due to incomplete combustion of the fuel. Mahua oil methyl esters gave lower smoke opacity compared to MO, diesel and B20. This may be due to the presence of oxygen molecules in the structure of MOME, which resulted in better combustion of the fuel; but MO resulted in higher smoke emission.
The values for viscosity, density and flash point of MOME were lower than for MO while calorific value was greater. The transesterfication yield was 92.4%. Mahua oil gave lower thermal efficiency and higher smoke emissions than MOME, B20 and diesel; but MOME gave an efficiency close to diesel fuel at higher loads.
References
Jamieson GS (1943) Vegetable fats and oils. Reinhold, New York, pp 134–141
Duke JA, Bagby MO (1982) Comparison of oilseed yields: a preliminary review in vegetable oil fuels. ASAE Publication, ASAE, St Joseph
Bringi NV (1987) Non traditional oil seed and oils of India. Oxford and IBH, New Delhi, p 57
Coupland JN, McClements DJ (1997) Physical properties of liquid edible oils. J Am Oil Chem Soc 74:1559–1564
Shashikant VG, Hifjur Raheman (2005) Biodiesel production from mahua oil having high free fatty acids. J Biomass Bioenergy 28:601–605
Sukumar P, Vedaraman N, Boppana VB, Sankarnarayanan G, Jeychandran K (2005) Mahua oil methyl ester as biodiesel preparation and emission characteristics. J Biomass Bioenergy 28:87–93
Sukumar P, Vedaraman N, Sankaranarayanan G, Boppana V, Bharat R (2005) Performance and emission study of mahua oil ethyl ester in a 4-stroke natural aspirated direct injection diesel engine. J Renew Energy 30:1269–1278
Bhatt YC, Murthy NS, Datta RK (2004) Use of mahua oil (Madhuca Indica) as a diesel fuel extender. J Inst Eng (India): Agri Eng 85:10–14
Knothe G (2001) Analytical methods used in the production and fuel quality assessment of biodiesel. Trans ASAE 44:193–200
Fangming J, Kohei K, Hisanori K, Kazuyuki T, Takehoko M, Heiji E (2007) NMR Spectroscopic study on methanolysis reaction of vegetable oil. J Fuel 89:1201–207
Gerhard K (2000) Monitoring a progressing transesterification reaction by fibre–optic near infrared spectroscopy with correlation to 1H NMR spectroscopy. J Am Oil Chem Soc 77:489–493
Gelbard G, Brès O, Vargas RM, Vielfaure F, Schuchardt UF (1995) 1H NMR determination of the yield of the transesterification of rapeseed oil with methanol. J Am Oil Chem Soc 72:1239–1241
Acknowledgments
The authors acknowledge the help provided by the NMR Research Centre, IISc, Bangalore, India, for obtaining NMR spectra and technical support provided by Mr. Micheal, Foreman, Fuel Laboratory and Engines Laboratory, NITK, Surathkal, India.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kapilan, N., Reddy, R.P. Evaluation of Methyl Esters of Mahua Oil (Madhuca Indica) as Diesel Fuel. J Am Oil Chem Soc 85, 185–188 (2008). https://doi.org/10.1007/s11746-007-1179-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-007-1179-5