Abstract
The pore size distribution and specific surface area of the attapulgite was a crucial parameter for the uptake of pigments of oil. Bleaching of the soybean oil with three attapulgites with different pore size distribution, which were assigned a, b, and c, respectively was investigated. The specific surface area and the pore size distribution of the attapulgites were characterized. The Freundlich isotherm analysis was used to evaluate the sorption capacity of the three attapulgite. Sample b gave the highest surface area and sample c the lowest. Sample b exhibited a wider pore distribution (8–65 Å) whereas samples a and c had more micropores smaller than 15 Å. Sample a, in contrast to samples b and c, was characterized by some larger pores (100–170 Å). The sorption capacity followed the sequence: attapulgite sample c > attapulgite sample a > attapulgite sample b. The sorption capacity was decided by the pore size distribution. The more pores with a distribution range 8–32 Å (i.e., close to the diameter of the pigments), the more pigments removed. The attapulgite sample c, which had most pores (8–32 Å) was the best.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vegetable oils contain numerous pigments, including chlorophyll, carotenoids, xanthophylls and their derivatives, that are removed to give the oil a color that is acceptable to the consumer [1]. Activated clays; activated carbon and silica-based products are adsorbents commonly used in the edible oil decolorization process. However, activated clay is the most popular adsorbent for decolorization of edible oil to activated carbon- and silica-based products because it is less expensive than activated carbon. Bleaching of vegetable oils by means of an activated earth is normally done by an adsorption process involving the removal of pigments originating from carotenoids, chlorophyll, and related compounds. The major pigments of the soybean oil are chlorophyll a, chlorophyll b, and β-carotene.
Natural, neutral or non-activated bleaching clays are derived from clay mineral deposits “bentonite”. The bentonite clays used in the edible oil industry range from natural neutral clays to heavily acid activated clays [2–5].
Although there are other clay minerals, such as attapulgite, with typical desirable properties, e.g., specific surface area, porosity and surface acid-base sites, they have not been much documented in the literature. Attapulgite is a special class of clay mineral under the 2:1 layer composition with commonly a lath or fibrous morphology. Attapulgites are hydrated magnesium silicates. In such materials octahedral layers of magnesium with partial substitution with aluminium and/or iron is sandwiched between (SiO)4 tetrahedral layer and Al(OH)3 octahedral unit. The tetrahedral sheet is continuous across ribbons at the apical oxygen alternately pointing up and down in adjacent ribbons. The octahedral sheet is discontinuous with a variable charge imbalance [6]. There are large reserves of attapulgite in South China (Jiang Su, Zhe Jiang and An Hui province) and in the USA (FL). This unique lath or fibrous structure with interior channels and its high surface area allows penetration of organic and inorganic ions into the structure of attapulgite. The ability of attapulgite to bleach is mainly ascribed to its high surface area because the cation-exchange capacity is lower than that of montmorillonite-type clay minerals. It is thus, an alternative material to other sorbents in processes such as wastewater treatment, in the removal of heavy metal, pesticide, colour, phenol, nicotinamide and macromolecules [6–11]. In applications of oil bleaching, comparison of rapeseed and soybean oils bleaching with synthetic adsorbents and attapulgites has been reported [12] and extensively reviewed in the latest edition of Bailey’s Industrial Oil and Fat Products [13, 14]. No work has been reported on the effect of attapulgite pore size distribution on oil bleaching.
Adsorption is a physical chemical process that involves the mass transfer of an adsorbate from the liquid phase (i.e., bulk solution) to the adsorbent surface where adsorption occurs. When the thermodynamic equilibrium concentration of the adsorbate is established between solution and adsorbent, further net adsorption will not occur. This equilibrium is defined by the concentrations of adsorbent and adsorbate in the system, and conditions of temperature, viscosity, and pH. Adsorption equilibrium is the most fundamental property of the adsorbate-adsorbent interaction. Therefore, the theoretical and empirical models that describe reversible adsorption have been developed on the basis of a thermodynamic equilibrium [15]. The Freundlich equation is an empirical model, and it is widely used to describe vegetable oil bleaching by adsorption.
The adsorption data for oil bleaching in the present study are analyzed according to Freundlich isotherm equation. This empirical equation describes heterogeneous systems and is expressed by the following equation:
where X and X e are the relative amount of pigments adsorbed and the residual relative amount of pigments at equilibrium; K and N are the Freundlich constants related to adsorption capacity and adsorption intensity. K is a constant for the system related to bonding energy, which can be defined as an adsorption or distribution coefficient, which describes the amount of adsorbate adsorbed onto attapulgite for the unit equilibrium concentration. N is related to the magnitude of the adsorption driving force and to the adsorbent site energy distribution [16]. The relative amount, X and the residual relative amount at equilibrium, X e, were obtained from the equations X = (A 0 − A e )/A 0 and X e = A e /A 0, where A 0 is the absorbance of the neutralized oil; A e is the absorbance of the oil at equilibrium [17, 18].
Both of the parameters K and N can be calculated by plotting of log(X/m) versus logX e. The values of K and N can be obtained from the intercept on the y-axis and slope of the linear line.
As to physisorption on porous materials, it’s generally accepted that adsorption mechanism and process may be significantly different as a consequence of difference in porous structure. In the IUPAC classification of pore size [19], the definitions of “macropores”, “mesopores” and “micropores” depend on the different adsorption mechanism at pores with specified range: in micropores the whole accessible volume is regarded as adsorption space and the process occurs due to micropore filling [20], as distinct from surface coverage, which takes place on the walls of macropores. On the other hand, physisorption in mesopores takes place in 1–3 stages (monolayers–multilayer adsorption and capillary condensation) depending on the specific absorbent/absorbate combination and absorption conditions. Pore size distribution is a crucial parameter for the uptake or exclusion of large molecules, such as pigments in soybean oil. In this paper, we report an investigation on the effect of the attapulgite pore size on the pigments removal of neutralized soybean oil with three attapulgites having different pore size distribution.
Materials and Methods
Materials and Characterisation
Neutralized soybean oil was supplied by Eastocean Oils and Grains Industries (Zhangjiagang) Co. Ltd. (China) and used as received.
Three attapulgites typical of major attapulgire ores in China were obtained from Oilbetter Co. (China) and surface areas and pore size were determined by the BET method of N2/77 K adsorption isotherms using a Surface Area and Pore Size Analyzer ST-2000B (Beijing Puqi institute of analysis instrument, China). In the BET method, the surface areas and the pore size distributions were calculated from adsorbed nitrogen volume by an automatic volumetric apparatus, and samples were outgassed with He for 16 h at 105°C prior to the adsorption measurement. The surface areas of the three attapulgites were determined to be 171.7, 181.28, and 135.47 m2/g, respectively.
Bleaching
The vacuum bleaching tests were accomplished in a 4-neck round-bottom flask, which was heated from the outside. The equipment was constructed such that the neutralized soybean oil and attapulgite sample could be agitated with a stirrer at approximately 250 rpm throughout the bleaching process. The temperature was monitored with a mercury thermometer. A vacuum pump was used to maintain the necessary pressure at 40 mbar. After the bleaching process and cooling of the oil to the room temperature, the oil was separated through a pressure filter. All bleaching experiments were carried out at temperatures of 110 ± 1°C and 40 min of contact time [21]. The pigments content of the oil was measured at 475 nm wavelength [22].
Results and Discussion
Color removal from neutralized sunflower oil was studied with four different kinds of commercial bleaching earths [2]. Acid activated bentonite used for cotton oil bleaching was studied [3]. The adsorption characteristics of three bleaching clays having different degrees of activation were studied in a palm oil physical refining process [4]. Acid activation of two bentonites and bleaching of rapeseed oil by the two acid bentonites was obtained [5]. The bentonite used for oil bleaching belongs to the general family of 2:1 layered Ca-silicates composed of regular stacking of 2D plate-like layers bound together with weak inter-atomic forces [23]. The chemical structures of bentonite consist of two sheets of tetrahedral silica fused to an edge-shared octahedral-based sheet of either magnesium or aluminium hydroxide. Attapulgite is a family of fibrous hydrated magnesium silicate. It has a structure similar to the 2:1 layered structure of bentonite, formed by two tetrahedral silica sheets enclosing a central sheet of octahedral magnesia except that the layers lack continuous octahedral sheets [6].
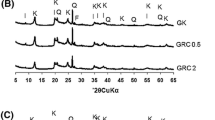
Figure 1 presents the N2 adsorption isotherm and pore size distribution of three attapulgite materials and Table 1 presents their surface area. It is seen that sample b gives the highest surface area and sample c the lowest. The pore size distributions of the three samples are also different. Sample b exhibits a wider pore distribution (8–65 Å) whereas samples a and c have more micropores smaller than 15 Å. Sample a, in contrast to samples b and c, is characterized by some larger pores (100–170 Å).
Figure 2 shows the adsorption isotherm plots of oil bleaching by three different attapulgite samples. As seen in Fig. 3, sample b has best bleaching of oil and sample c the worst.
Freundlich isotherm of oil bleaching (stirred at 250 rpm, vacuum pressure at 40 mbar, heated at 110 ± 1 for 40 min) by three attapulgite samples with different pore size distribution. X/m, the relative amount of pigments adsorbed on adsorbent; X e, the residual relative amount of pigments at equilibrium
Figure 3 has shown the log/log plot of Freundlich isotherm of Fig. 2. This model was based on the idea that adsorption depended on the energy of the adsorption sites. This model applies to adsorption on heterogeneous surfaces with interaction between adsorbed molecules and the application of the Freundlich equation also suggests that sorption energy exponentially decreases on completion of the sorptional centres of an adsorbent [24]. Table 2 shows the results of Freundlich isotherm analyses calculated for adsorption of pigments from oil using the three different attapulgite clays. The isotherm data and the related correlation coefficients (R 2 values) are given in the same table. Both of the parameters K and N affect the adsorption isotherm. As seen from Table 2, for all the adsorbents tested, the value of N ranged between 0.1 and 1, indicating a favorable adsorption. The N values for the attapulgite b was smaller than for the other two attapulgites, suggesting that energy distribution of the adsorption sites of attapulgite b is broader and the attapulgite b provides an relatively easier access for the pigment molecule to interact with the active sites in comparison with the attapulgite a and c [15, 25]. The term N also expresses an affinity between the adsorbate and the adsorbents. Therefore, similar values of N for attapulgite a and c indicate that their affinity for pigment is similar [26]. The order of magnitude of K was: attapulgite sample c > attapulgite sample a > attapulgite sample b. The Freundlich adsorption isotherm does not predict any saturation of the solid surface of the adsorbent by the adsorbate; thus infinite surface coverage is predicted mathematically. For an effective adsorption system, high adsorption capacity (X/m) and low equilibrium concentration (X e) of target compound after adsorption are needed. Thus, the larger the K value, the more effective the adsorbent for adsorption. The sorption capacity follows the sequence: attapulgite sample c > attapulgite sample a > attapulgite sample b [27].
The Freundlich isotherm may suggest heterogeneity of adsorption sites on solid surface. However, the adsorption of the pigments on porous materials does not depend on the host-guest chemical interaction alone, i.e., the pore size distribution of different adsorbents. Three attapulgite materials showed significant difference in pore size distribution (Fig. 2). Sample c had fewer large pores than samples a and b. Most of the pores of sample c range from 8 to 32 Å, which was close to the diameter of the pigments in the soybean oil (chlorophyll: 30 × 17 × 13 Å; β-carotene: 21 × 8 × 7 Å). The molecular sizes of chlorophyll and β-carotene were estimated using the Chemoffice 2006 (Cambridge Soft Corporation) program. Sample b has more pores larger than 32 Å than sample a. The sorption capacity follows the sequence: attapulgite sample c > attapulgite sample a > attapulgite sample b. Based on the results, it can be concluded that the more pores with a distribution range 8–32 Å (i.e., close to the diameter of the pigments), the more pigments removed. The surface area of the attapulgite samples was less important for pigments. Based on the pore size distribution of the adsorbents and their adsorption isotherms, the following adsorption mechanisms of the adsorbents for soybean oil bleaching are suggested: (1) The large pores of the attapulgite are distributed on the surfaces of the adsorbent, and the small pores in the centers of the attapulgite particles; (2) Because of the relatively large diameter of the pigment molecules would be required to line up coaxially with their long axis along the channel walls when the pigments were adsorbed by the smaller pores (8–32 Å); (3) The pigment molecules would lie flat across the larger pores on the surface of the attapulgite particles, which would then block the other pigments to from entering the narrower channels leading to the center of the attapulgite particles. Attapulgite sample c had fewer large pores (>32 Å) than the other two attapulgites, it was therefore the best one of the three for soybean oil bleaching even though its surface area was the lesser of other two. A diagram illustrating the effect of large versus small pores on the adsorption process is shown in Fig. 4. The forms of pigment molecules absorbed on the pores of the adsorbents were different according to the pore size of the adsorbents, and were decided by the pore size distribution. The pigment molecules connected with the active point in the form that made each molecular cover most surface area when it was adsorbed by the active point of adsorbents.
References
Park EY, Ming H (2004) Oxidation of rapeseed oil in waste activated bleaching earth and its effect on riboflavin production in culture of ashbya gossypii. J Biosci Bioeng 97(1):59–64
Kaynak G, Ersoz M, Kara H (2004) Investigation of the properties of oil at the bleaching unit of an oil refinery. J Colloid Interface Sci 280:131–138
Gülsah KE, Laçin O (2006) Statistical modelling of acid activation on cotton oil bleaching by Turkish bentonite. J Food Eng 75:137–141
Rossi M, Gianazza M, Alamprese C (2003) The role of bleaching clays and synthetic silica in palm oil physical refining. Food Chem 82:291–296
Christidis GE, Scott PW, Dunham AC (1997) Acid activation and bleaching capacity of bentonites from the islands of Milos and Chios, Aegean, Greece. Appl Clay Sci 12:329–347
Akyuz S, Akyuz T (2005) Study on the interaction of nicotinamide with sepiolite, loughlinite and palygorskite by IR spectroscopy. J Mol Struct 744–747:47–52
Potgieter JH, Potgieter VSS, Kalibantong PD (2006) Heavy metals removal from solution by palygorskite clay. Miner Eng 19:463–470
Huang J, Wang X, Jin Q (2007) Removal of phenol from aqueous solution by adsorption onto OTMAC-modified attapulgite. J Environ Manage 84:229–236
Perderiset M, Baillif P, Jaurand MC (1988) Chemical analysis and photoelectron spectroscopy of the adsorption of macromolecules on the surface of attapulgite. J Colloid Interface Sci 121:381–391
Liu P, Guo J (2006) Polyacrylamide grafted attapulgite (PAM-ATP) via surface-initiated atom transfer radical polymerization (SI-ATRP) for removal of Hg(II) ion and dyes. Colloids Surf A 282–283:498–503
Sanchez MMJ, Rodriguez CMS, Andrades MS (2006) Efficiency of different clay minerals modified with a cationic surfactant in the adsorption of pesticides: influence of clay type and pesticide hydrophobicity. Appl Clay Sci 31:216–228
Boki K, Hidehito M, Naohito K (1994) Bleaching rapeseed and soybean oils with synthetic adsorbents and attapulgites. J Am Oil Chem Soc 71:595–601
Taylor DR (2005) Adsorptive Separation of Oils. In: Fereidoon S (ed) Bailey’s industrial oil and fat products, vol.5: edible oil and fat products: processing technologies, 6th edn. Wiley, New York, pp 267–284
Greyt WD, Kellens M (2005) Bleaching. In: Fereidoon S (ed). Bailey’s industrial oil and fat products, vol.5: edible oil and fat products: processing technologies, 6th edn. Wiley, New York, pp 285–339
Proctor A, Toro-Vazquez JF (1996) The freundlich isotherm in studying adsorption in oil processing. J Am Oil Chem Soc 73:1627–1633
Grégorio C, Harmel N P, Frédéric G (2007) Removal of C.I. basic green 4 (malachite green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: kinetic and equilibrium studies. Sep Purif Technol 5:97–110
Zhang WM, Chen JL, Pan BC (2006) Synergistic adsorption of phenol from aqueous solution onto polymeric adsorbents. J Hazard Mater 128:123–129
Bayrak Y (2003) Adsorption isotherms in bleaching hazelnut oil. J Am Oil Chem Soc 80(11):1143–1146
Sing KSW, Everett DH, Haul RAW (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 57:603–619
Dubinin MM, Stoeckli HF (1980) Homogeneous and heterogeneous micropore structure in carbonaceous adsorption. J Colloid Interf Sci 75:34–42
Taylor DR, Jenkins DB, Ungermann CB (1989) Bleaching with alternative layered minerals: a comparison with acid-activated montmorillonite for bleaching soybean oil. J Am Oil Chem Soc 66:334–341
Stout LE, Chamberlain DF, Mcklvey JM (1949) Factors influencing vegetable oil bleaching by adsorption. J Am Oil Chem Soc 26:120–126
Xie S, Zhang S, Wang F (2007) Preparation, structure and thermomechanical properties of nylon-6 nanocomposites with lamella-type and fiber-type sepiolite. Compos Sci Technol. doi: 10.1016/j.compscitech.2007.01.012
Boki K, Moriaki K, Naohito K (1992) Adsorption isotherms of pigments from alkali-refined vegetable oils with clays minerals. J Am Oil Chem Soc 69(4):372–378
Franck D, Crini G, Vebrel J (2006) Removal of organic pollutants from aqueous solutions by adsorbents prepared from an agroalimentary by-product. Bioresour Technol 97:2173–2181
Zhang F, Itoh H (2003) Adsorbents made from waste ashes and post-consumer PET and their potential utilization in wastewater treatment. J Hazard Mater 101:323–337
Sukdeb P, Lee KH, Kim JU (2006) Adsorption of cyanuric acid on activated carbon from aqueous solution: effect of carbon surface modification and thermodynamic characteristics. J Colloid Interface Sci 303:39–48
Acknowledgments
The authors express their gratitude to the NSFC (National Natural Science Foundation of China), for its financial support (Contract NO: 20376028). We also thank the Testing and Analysis Center of South Yangtze University.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Huang, J., Liu, Y., Liu, Y. et al. Effect of Attapulgite Pore Size Distribution on Soybean Oil Bleaching. J Amer Oil Chem Soc 84, 687–692 (2007). https://doi.org/10.1007/s11746-007-1094-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-007-1094-9