Abstract
Downstream processes following aqueous enzymatic extraction (AEE) of rapeseed oil and protein hydrolysates were developed to enhance the oil and protein yields as well as to purify the protein hydrolysates. The wet precipitate (meal residue) from the AEE was washed with twofold water at 60 °C, pH 11 for 1 h. Emulsions from the AEE and the washing step were pooled and submitted to a stepwise demulsification procedure consisting of storage-centrifugation and freezing–thawing followed by centrifugation. Aqueous phases were pooled and adsorbed onto macroporous adsorption resins (MAR) to remove salts and sugars. Following extensive rinsing with deionized water (pH 4), desorption was achieved by washing with 85% ethanol (v/v) to obtain crude rapeseed peptides (CRPs). In a separate experiment, stepwise desorption was carried out with 25, 55, and 85% ethanol to separate the bitter peptides from the other peptides. Using a combination of the AEE process, washing and demulsification steps, the yields of the total free oil and protein hydrolysates were 88–90% and 94–97%, respectively. The protein recovery was 66.7% and the protein content was enriched from 47.04 to 73.51% in the CRPs. No glucosinolates and phytic acid were detected in the CRPs. From the stepwise desorption, a non-bitter fraction RP25 (containing 64–66% of total desorbed protein) had a bland color and significantly higher protein content (81.04%) and hence was the more desirable product.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aqueous enzymatic extraction (AEE) has emerged as a novel technology for coextraction of either oil or oil and protein from many oilseeds and oil-fruits [1, 2]. The main advantages of this process are the complete avoidance of organic solvents and the appropriate utilization of non-oil components (such as the protein hydrolysate) from the oilseeds. Rapeseed (Cruciferae family) is one of the most important oilseeds. In a previous study, the suitable conditions for AEE of rapeseed were optimized [3]. The combined use of several carbohydrases (pectinase, cellulase, and beta-glucanase) and a protease, Alcalase 2.4L, effectively facilitated separation of the free oil. However, a thin but distinctive layer of an emulsion between the oil and the aqueous phase remained after centrifugation. Moreover, the solid phase from the AEE consisted of an upper layer of sedimented protein hydrolysates binding oil and a lower layer of cell debris and seed hull. To recover the residual oil and protein in the emulsion and precipitate, washing and demulsification were considered as downstream processes for the AEE.
Demulsification is necessary for the purpose of further enhancing the free oil yield. Generally, boiling [4], freezing-thawing [5, 6] and phase inversion [7, 8] have been used in an attempt to break the emulsion by other researchers. Boiling and freezing–thawing are effective but may require higher energy cost than phase inversion. The idea of the latter is to reduce the moisture (the most critical variable) in the emulsion by adding oil to a threshold value followed by shearing to promote phase inversion. Above the threshold value the emulsion cannot be inverted. Hagenmaier et al. [7] and Domínguez et al. [8] pointed out the threshold values were 28 and 20%, respectively. The moisture content of the emulsion obtained in the present study was 60–70%. A lot of oil, therefore, has to be added to the emulsion for phase inversion. Since the protein molecules which formed the present emulsion are mostly peptides, the formed emulsion may be more unstable than that formed before proteolysis. Hence, a simpler storage-centrifugation demulsification operation was carried out in this work.
Another important aspect of the downstream processes is to deal with the resultant aqueous phase. Due to the complex carbohydrate hydrolysis and pH adjustment with an alkali and an acid during the AEE, the aqueous phase contained large amounts of sugars and salts besides protein hydrolysates. Additionally, native antinutritional components in the rapeseeds such as intact glucosinalates, phytic acid and tannin may have partially dissolved in the aqueous phase. Conventionally, removal of small impurities from large protein molecules is performed by membrane technologies such as dialysis and ultrafiltration. But the MW of the protein hydrolysates in the present aqueous phase is mostly (81%) lower than 600 Da, which is close to those of the sugars, glucosinalates and phytic acid and as such membrane filtration was not found to be an effective way of purifying the protein hydrolysates. Recently, macroporous adsorption resin (MAR) has been used to desalt whey protein hydrolysates [9] and wheat germ protein hydrolysates [10]. MAR has many hydrophobic zones, where the hydrophobic protein hydrolysates can be easily adsorbed onto. Conversely, the salts, sugars or other hydrophilic components can be rinsed off with deionized water. The adsorbed hydrolysates can be desorbed by disruption of the hydrophobic interactions with food-grade ethanol.
Bitterness often accompanies protein hydrolysates. Several traditional debittering methods have been reviewed by Adler-Nissen [11]. Recently, Cheison et al. [9] have developed a novel method for simultaneous desalting and debittering of whey protein hydrolysates using MAR. In their study, the ethanol concentration was manipulated to desorb the peptides into fractions, thus separating a bitter group. The underlying principle in the use of MAR to remove the bitter peptides is that the highly hydrophobic and short bitter peptides require higher ethanol concentrations (75%) to desorb.
The present study was intended to investigate the conditions of the downstream processes following the rapeseed AEE including washing the precipitate, breaking the emulsion, purifying and debittering of the protein hydrolysates. The physicochemical and sensory properties of the hydrolysates fractions were analyzed.
Materials and Methods
Materials
Dehulled rapeseeds of Brassica napus (cv. 10# Chinese-double) were obtained from the Institute of Oil Crops Research of the Chinese Academy of Agriculture Sciences (Wuhan, China) The rapeseeds contained 6.31% moisture, 46.99% oil, 24.85% protein, 3.01% crude fiber, and 3.86% ash. On a fat-free dry solids basis, the rapeseeds contained 3.78 mg/g total glucosinolates, 3.66% phytic acid and 1.05% tannin. Rapeseed isolate (93.35% on a dry basis, N × 6.25) was prepared in our lab. Enzymes used were the same as described previously [3]. A nonpolar styrene-based macroporous adsorption resin (MAR), branded DA201-C was sourced from Jiangsu Suqing Water Treatment Engineering Group (Wuxi, China).
Aqueous Enzymatic Extraction of Rapeseed Oil and Protein Hydrolysates
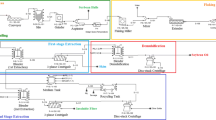
The process was carried out as summarized in Scheme 1.
Optimization of Washing Precipitate
The wet precipitate obtained from the above AEE process was sampled (50 g) and washed with a two-fold weight of water at 60 °C. The pH (8–11) and washing time (15–90 min) were varied. After washing, the emulsion, the aqueous phase and the residual meal were obtained by centrifugation (1,819g for 15 min). The freeze-dried meal was pulverized and analyzed for residual oil. The emulsified oil yield was calculated according to the relationship in Eq. 1. The aqueous phase was collected, its volume determined and sampled for determination of the protein content. The protein hydrolysates yield was expressed as the percentage of the amount of protein in 50 g of the precipitate.
Optimization of Demulsification
The emulsions from the AEE and optimized washing process under the conditions (60°C, pH 11 for 1 h), were pooled and sampled (20 g), then stored in a refrigerator (4 °C) for 0, 12, 24, and 48 h followed by warming in a water bath (30 °C) for 1 h before centrifugation (1,819 g for 15 min). To investigate the effect of the centrifugation speed on the demulsified oil yield, the emulsion was stored for 24 h, warmed in a water bath as above, sampled (20 g) and then centrifuged in the range of 1,819–10,733g at room temperature for 15 min. The free oil was drawn out using an auto-pipettor, dried at 70 °C in a vacuum oven to constant weight and taken as the demulsified oil. The demulsified oil yield was expressed as the percentage of the amount the oil in 20 g of emulsion.
Washing and Demulsification Under Optimal Conditions
After optimizing demulsification, the total wet precipitate obtained from the AEE process was washed with two times the weight of water under the following conditions: 60 °C, pH 11 for 1 h, followed by centrifugation (1,819 g for 15 min). The emulsions from the AEE and the optimized washing process were pooled and stored in a refrigerator (4 °C) for 24 h. Next, the emulsion was warmed in a water bath (30 °C) for 1 h and then centrifuged at 8,694g for 15 min. The residual emulsion was further demulsified by freezing and thawing [6]. The emulsion was frozen at −18 °C for 20 h and thawed in a water bath (35 °C) for 2 h, and then centrifuged at 8,694g for 15 min. The oils collected from both demulsification steps were pooled and dried at 70 °C in a vacuum oven to constant weight.
Purification of the Aqueous Phase by Macroporous Adsorption Resin (MAR)
The aqueous phases from the AEE and optimized washing processes were pooled and sampled (50 mL). The sample with a protein concentration of about 40 mg/mL was adjusted to different pH values (3, 4, 5, 6, and 7) with acetate acid. At each pH, the solution was pumped through a glass column (180 mL) packed with MAR at a flow rate of 1 mL/min. Then, the column was washed with 1 L deionized water previously adjusted to the same pH as the sample solution at a flow rate of 1.5 mL/min. The water-eluent was collected, its volume determined and sampled for determination of the protein content. Next, 1 L of 85% (v/v) ethanol was used to desorb the peptides without changing the flow rate. The protein content of the ethanol-eluent was determined. The complete course was monitored using a UV detector at 220 nm. The ethanol-eluent was freeze-dried to obtain the crude rapeseed peptides (CRPs). The dynamic adsorption capacity and protein hydrolysates recovery were calculated according to Eqs. 2 and 3, respectively.
Separation of the Bitter Peptides
The pH of a sample of aqueous phase from AEE and washing and the deionized water for rinsing were both adjusted to 4 with acetic acid. Next, the sample was pumped through a column with MAR and the column was rinsed with 1 L deionized water as described above. After rinsing, step-wise desorption with ethanol (25, 55, and 85%) rather than one-step desorption with 85% ethanol was adopted. First, the column was washed with excess 25% ethanol. The eluent was monitored using a UV detector at 220 nm. The ethanol was pumped until the absorbance nearly returned to the baseline. Then, the ethanol concentration was increased to 55% and the column was washed until the absorbance nearly returned to the baseline. Finally, the column was washed with 85% ethanol. Three fractions (RP25, RP55, and RP85, respectively) were obtained and freeze-dried.
Relative Bitterness
The relative bitterness of fractions (CRPs, RP25, RP55, and RP85) was evaluated according to Cheison et al. [9] with some modifications. The relative bitterness was determined by making a 2% (protein concentration) hydrolysates solution with potable water at 25 °C. The tests were done in a uniformly illuminated room and the samples were randomized for the test. A five-member panel was asked to score the fractions for bitterness. The fractions were compared to a reference sample (rapeseed protein isolate) assigned a zero score and regarded as not being bitter. The fractions with scores of 0–2, 3–6 and 7–10 were regarded as not being bitter, somewhat bitter and very bitter, respectively.
Color Measurement
The protein hydrolysates (100 mg) were dissolved in 50 mL deionized water and then measured for reflectance using a WSC-S Color-difference Meter Hunter a, b and L parameters were recorded. The instrument was standardized with a white ceramic plate (L = 99.99, a = −0.70, b = 1.25). The browning index (BI) was calculated according to the method of Palou et al. [12]. The BI represents the purity of the brown color and is reported as an important parameter in processes where enzymatic or non-enzymatic browning takes place [12].
Free Amino Acid Content
The hydrolysates were dissolved in 5% trichloroacetic acid (TCA) for 2 h followed by centrifugation at 3,000 g for 10 min. Then, the supernatants were submitted to online derivatization by O-phthaldialdehyde (OPA) and 9-fluorenylmethyl chloroformate (FMOC-Cl, for proline analysis). RP-HPLC analysis in an Agilent 1100 assembly system with a Zorbax 80A C18 4.6-mm id × 180-mm column was carried out using the conditions prescribed by the equipment manufacturer.
Total Amino Acids Analysis
Seventeen amino acids were analyzed following the conventional 6N HCl hydrolysis of the fractions at 110 °C for 24 h. For tryptophan, the samples were hydrolyzed in 5N NaOH containing 5% SnCl2, followed by RP-HPLC analysis with online derivation using an Agilent 1100 Assembly as described in preceding sections.
Q Value (Average Hydrophobicity) Calculation
The Q values of protein hydrolysates were calculated according to the method described by Adler-Nissen [13]. Hydrophobicity values of amino acid side chains were adopted from Tanford [14].
Molecular Weight Determination
The molecular weight of the protein hydrolysates was determined using HP-SEC as described in our earlier work [3].
Proximate Analysis
The oil (Soxhlet extraction method with anhydrous ether), protein (Kjeldahl N × 6.25), moisture, ash and crude fiber contents were determined according to the AOAC official methods [15]. The soluble sugars content was determined as described by Dubois et al. [16]. Some glucosinolates were inevitably hydrolyzed during the AEE, therefore, the total concentrations of the intact glucosinolates and the hydrolyzed glucosinolates products (isothiocyanates and oxazolidine-2-thione) were measured by the method of Wetter and Youngs [17]. Phytate was determined according to the method of Makower [18]. The phytate phosphorus was determined colorimetrically according to the AOCS Official Method Ca 12-55 [19]. The phytic acid content of the sample was calculated from the measured phytate phosphorus value using 3.55 as the conversion factor. The tannin content was analyzed by the vanillin-HCl, 1% HCl in methanol extraction method, which is specific to determination of condensed tannin [20], using catechin as the standard.
Statistical Analysis
Analysis of variance (ANOVA) was performed using SPSS ver. 13.0 for Windows (SPSS Institute, Cary, NC). Significance of differences was defined at p < 0.05.
Results and Discussion
Our previous study [3] showed that 73–76% free oil and 80–83% protein hydrolysates were obtainable from the optimized AEE with 100 g dry rapeseeds as the starting materials. In present work based on an eight-fold scale-up of the optimized AEE, the free oil yield rose to 78–80% while the protein hydrolysates yield was not significantly different from the previous values (Table 1). The reason for the oil yield increment is probably due to the fact that the relative oil loss (the residual oil on the surfaces of the reactor and centrifuge tubes relative to the total oil) was reduced to lower amounts on the bigger eightfold scale. As shown in Table 1, the solid phase contained appreciable oil and protein amounts and washing was, therefore, considered to improve the oil and protein hydrolysates yields.
Figure 1 shows that increasing the washing pH significantly increased the yields of both the emulsified oil and protein hydrolysates. At high pH, the protein in the precipitate dissolved more easily into the aqueous phase and the oil bound with the protein was extracted as well at the same time. The correlation between the pH and the solubility of rapeseed protein was also reported by Xu et al. [21]. But pH above 11 may introduce possible detrimental effects (like saponification) to the oil quality, thus further increases were not attempted. The maximum recoveries of the emulsified oil and protein hydrolysates (91.2% and 88.8%, respectively) were achieved from a washing pH of 11 for 1 h. Increasing time did not increase the recoveries significantly. Under the optimal conditions (60 °C, pH 11 for 1 h) the total wet precipitate (800 g dry rapeseeds as the starting materials) was washed, thereby ca. 9% of the total oil and ca. 14% of the total proteins were obtained. Therefore, the total protein hydrolysates yield from the combination of the AEE and the following washing rose to 94–97%, which indicates that most rapeseed proteins were recovered. On the other hand, the oil yield from the washing process was also desirable but the oil was obtained only in an emulsified state and needed further treatment to release the free oil.
The total emulsions pooled from the AEE process and the subsequent washing steps contained ca. 14% of the total oil and were submitted to a demulsification step to separate the oil. A maximum demulsified oil yield of 28% was obtained when the emulsion was stored at 4 °C for 24 h followed by centrifugation at 1,819g for 15 min. The emulsion is thermodynamically unstable in nature. During storage, the small oil droplets may aggregate and coalesce spontaneously to reduce the total free energy of the system, thereby facilitating the oil separation. Once the storage time was selected, the centrifugation speed was optimized. Figure 2 shows that the demulsified oil yield increased remarkably with the centrifugation speed until a maximum of 55% was obtained at 8,694g for 15 min. The effective centrifugal demulsification may be mainly attributed to the cleavage of the protein molecules, which emulsified the oil due to the proteolysis in the AEE process. Undoubtedly, the above storage-centrifugation demulsification operation saves more energy than other methods such as boiling and freezing-thawing followed by centrifugation. To improve the yield of the demulsified oil, the residual emulsion obtained after the centrifugation was further demulsified using the freezing and thawing method. By this method another demulsified oil yield of ca. 20% was obtained. Therefore, the total demulsified oil yield was ca. 75% with the two-stage demulsification.
The total emulsions from the AEE process and the following washing step were demulsified with the two-stage demulsification under the optimal conditions, from which a free oil yield of ca. 10% was obtained. Therefore, the total free oil yield, therefore, rose to 88–90% by the combination of the AEE process, following washing and demulsification steps. The desirable free oil yield was obtained though it is still obviously lower than that obtained from solvent extraction (generally above 95%).
As we mentioned previously, the aqueous phase from the AEE and washing process contained undesirable components and hence needed to be purified by MAR. As shown in Table 2, the pH of the sample and the deionized water for rinsing had a significant effect on the dynamic adsorption capacity and the protein hydrolysates recovery, as well as the protein content in the crude rapeseed peptides (CRPs). At pH 4 and 5, which are near the pI of the rapeseed protein [22], the dynamic adsorption capacity was significantly higher than that at other pH values and reasonably higher dynamic adsorption capacity is accompanied by higher protein hydrolysates recovery and protein content in the CRPs (Table 2). At the pH near the pI, the peptide molecules carry less net charge, which led to a weakening of the peptide-peptide intermolecular repulsion. The enhanced interactions between peptides, therefore, probably helped prevent the peptides from being rinsed out by the deionized water. Though a little precipitate appeared in the column certainly due to the slightly poor solubility of the peptides at pH 4 or 5, it was counterbalanced by the limited peptide loss during the water rinsing. A little higher protein recovery was obtained at pH 4 than pH 5 and pH 4 was, therefore, chosen for rinsing in the present study. The volume of the rinsing water was fixed at 1 L according to a preliminary experimental result that more sugars could not be eluted by use of additional water. The protein hydrolysates recovery could be slightly enhanced (2–3%) if an additional 500 mL 85% ethanol was added for desorption, which implies that some peptides were difficult to desorb due to the highly hydrophobic interaction. For reasons of economics and time, the ethanol volume for desorption was fixed at 1 L.
The proximate analysis data for the rapeseed protein hydrolysates (RPHs, a lyophilized product of the aqueous phase from the AEE for the proximate analysis) and the CRPs are summarized in Table 3. The protein content in the CRPs was enriched from a low of 47.04% in the RPHs to 73.51% certainly due to the effective removal of much of the salts and sugars from the aqueous phase. Salts were easy to rinse off the column while some sugars may be covalently bound to proteins and hence impossible to desorb with water, resulting in still high sugar content (8.72%) in the CRPs. With respect to the total glucosinates and phytic acid, their contents were greatly reduced (by more than 80%) in the RPHs compared with those in the original materials. Several reasons may explain this reduction: (a) the glucosinates and phytic acid may be partially insoluble and hence remained in the residual meal and (b) some highly unstable glucosinates like 4-hydroxyglucobrassicin were likely discomposed during the AEE [23]. After the resin treatment, the total glucosinates and phytic acid were not detectable in the CRPs, which means that CRPs have improved safety if used for food. Another antinutrient, tannin, may be highly hydrophobic and was not easy to separate from the protein hydrolysates. On the other hand, the free amino acid (FAA) contents were reduced from 3.48 to 1.63% (Table 3) following MAR adsorption and the peptides MW significantly decreased (Table 4), which means some FAAs and long peptides were less hydrophobic than the short peptides, therefore eluted by the deionized water. Moreover, the hydrophobic amino acid mole fraction increased from 29.4 to 38.6% (Table 5), which is certainly due to the easier loss of some peptides composed mainly hydrophilic amino acids during elution by the deionized water. As a result, the average hydrophobicity, i.e., the Q value increased markedly from 1,118 (RPHs) to 1,281 cal/mol (CRPs) (Table 5).
Though a desirable purification of the protein hydrolysates was achieved, some bitterness associated with the CRPs (rank sum is 4) from one-step desorption with 85% ethanol can not be circumvented for the purpose of the protein hydrolysate use in food. To solve this problem, stepwise desorption with various ethanol concentrations rather than one-step desorption with 85% ethanol was adopted. The desorbed fractions RP25, RP55 and RP85 contained 64–66%, 29–32% and 5–7% of the total desorbed protein, respectively. The total protein recovery was not significantly different from the one-step desorption with 85% ethanol. The analysis for the properties data for the three fractions are shown in Table 6. It can be concluded that RP25 is the most desirable product because it has a bland color, significantly higher protein content (81.04%) and no bitterness relative to the native rapeseed isolates. The bitterness degree of RP55 is close to that of the CRPs, as are the color and the protein content. Fraction RP85 was the bitterest and had lower protein content (50.52%).
The relationship between the bitterness and the desorption concentration agreed with the results of Cheison et al. [9] regarding whey protein hydrolysates. The highest content of the brown color substances in RP55 were probably derived from two aspects: phenolics oxidation and the Maillard reaction during the AEE. The pronounced difference of the color of the fractions is due to the likelihood that the majority of highly hydrophobic color substances are not desorbed by a low ethanol concentration (25%) but desorbed by a higher concentration (55%).
Cheison et al. [9] concluded that the longer peptides are less hydrophobic and hence have relatively weaker hydrophobic interactions with the MAR and are easily displaced by lower ethanol concentrations. Therefore, their experimental results show that there was a remarkable migration of the short peptides with the increasing ethanol concentration and also, the bitterness of the peptide fractions increased with a concomitant increase in the short peptides number. As shown in Table 4, the most bitter RP85 had the lowest MW while somewhat bitter RP55 had higher peptides MW than non-bitter RP25. The different result was obtained due to the possible existence of higher content of peptide-sugar complexes in RP55. This proposition is supported by the significantly higher sugar content in RP55. On the other hand, the significantly different hydrophobic amino acid mole fraction and the Q value (average hydrophobicity) among the peptide fractions (Table 5) may explain the bitterness difference. RP85 has significantly more hydrophobic amino acids and a higher Q value (1,673 cal/mol). Earlier studies have shown that peptide bitterness is mainly derived from the exposure of hydrophobic amino acid side chains [24, 25]. In fact, the significantly higher tannin content (4.68%) in RP85 may have also contributed to the objectionable bitterness. The relationship between the bitterness and the Q values of the three stepwise desorption fractions complied well with the Q rule proposed by Ney and expounded by Adler-Nissen [11], i.e., all bitter peptides found had Q values above 1,400 cal/mol, whereas all the non-bitter peptides had a Q value below 1,300 cal/mol. This rule was later extrapolated to assess intact proteins by Ney. In fact, Adler-Nissen pointed out that the Q rule was only valid for individual peptides rather than the protein hydrolysates mixture and concluded that the bitterness level of a protein hydrolysate could not be predicted only by its calculated Q value, since the bitterness level was determined to a large extent by the peptide hydrophobicity distribution, too. This conclusion was also supported by our experimental results that the CRPs had a Q value below 1,300 cal/mol but showed some bitterness. The CRPs had a low Q value but it contained fractions RP55 and RP85 with higher Q values (1,466 and 1,673 cal/mol, respectively), which determined their bitterness.
Downstream processes were, therefore, successfully developed to improve the yields of the free oil and protein hydrolysates based on the AEE, as well as to obtain salt-free and non-bitter protein hydrolysates with a bland color.
References
Domínguez H, Núñez MJ, Lema JM (1994) Enzymatic pretreatment to enhance oil extraction from fruits and oilseeds: a review. Food Chem 49:271–286
Rosenthal A, Pyle DL, Niranjan K (1996) Aqueous and enzymatic processes for edible oil extraction. Enzyme Microb Technol 19:402–420
Zhang SB, Wang Z, Xu SY (2007) Optimization of aqueous enzymatic of rapeseed oil and protein hydrolysates. J Am Oil Chem Soc 84:97–105
Tano-Debrah K, Ohta Y (1995) Enzyme-assisted aqueous extraction of shea fat: a rural approach. J Am Oil Chem Soc 72:251–256
Hanmoungjai P, Pyle L, Niranjan K (2000) Extraction of rice bran oil using aqueous media. J Chem Technol Biotechnol 75:348–352
Wang YY (2005) Studies on aqueous enzymatic extraction of oil and protein hydrolysates from peanut. Ph.D. dissertation, Southern Yangtze University, Wuxi
Hagenmaier R, Cater CM, Mattil KF (1972) Critical unit operations of the aqueous processing of fresh coconuts. J Am Oil Chem Soc 49:178–181
Domínguez H, Núñez MJ, Lema JM (1995) Aqueous processing of sunflower kernels with enzymatic technology. Food Chem 53:427–434
Cheison SC, Wang Z, Xu SY (2006) Use of Macroporous adsorption resin for simultaneous desalting and debittering of whey protein hydrolysates. Int J Food Sci Technol doi:10.1111/j.1365-2621.2006.01461.x
Chen YH (2006) Study on the preparation of wheat germ protein hydrolysates and its structure and biological activities. Ph.D. Dissertation, Southern Yangtze University, Wuxi
Adler-Nissen J (1986) A review of food protein hydrolysis-specific areas, In: Enzymic hydrolysis of food proteins. Elsevier, London, pp 57–109
Palou E, López-Malo A, Barbosa-Cänovas GV, Welti-Chanes J, Swanson BG (1999) Poly- phenoloxidase activity and color of blanched and high hydrostatic pressure treated banana puree. J Food Sci 64:42–45
Adler-Nissen J (1986) Appendix II: Calculation of the Q value, In: Enzymic hydrolysis of food proteins. Elsevier applied science, London, pp 336–339
Tanford C (1962) Contribution of hydrophobic interactions to the stability of the globular conformation of proteins. J Am Chem Soc 84: 4240–4247
Association of official analytical chemists (AOAS) (1995) Official methods of analysis, 16th edn. AOAC International, Gaithersburg
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Wetter LR, Youngs CG (1976) A thiourea-UV Assay for total glucosinolate content in rapeseed Meals. J Am Oil Chem Soc 53:162–164
Makower RU (1970) Extraction and determination of phytic acid in beans. Cereal Chem 47:288–292
Association Oil Chemists Society (AOCS) (1998) Official Methods and Recommended Practices of the AOCS, 5th edn. Champaign
Price ML, Scoyoc SV, Butler LG (1978) A Critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J Agric Food Chem 26:1214–1218
Xu L, Diosady LL (1994) The production of Chinese rapeseed protein isolates by membrane processing. J Am Oil Chem Soc 71:935–939
Zhou B, He ZQ, Yu HM, Mukherjee KD (1990) Proteins from double-zero rapeseed. J Agric Food Chem 38:690–694
Jensen SK, Olsen HS, Sørensen H (199) Aqueous enzymatic processing of rapeseed for production of high quality products, In: Shahidi F (ed) Canola and rapeseed: production, chemistry, nutrition and processing technology. Van Nostrand Reinhold, New York, pp 331–344
Matoba T, Hata T (1972) Relationship between bitterness of peptides and their chemical structures. Agric Biol Chem 36:1423–1431
Adler-Nissen J, Olsen HS (1979) The influence of peptide chain length on taste and functional properties of enzymatically modified soy protein. ACS Symp Ser 92:125–146
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Zhang, S.B., Wang, Z. & Xu, S.Y. Downstream Processes for Aqueous Enzymatic Extraction of Rapeseed Oil and Protein Hydrolysates. J Amer Oil Chem Soc 84, 693–700 (2007). https://doi.org/10.1007/s11746-007-1080-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-007-1080-2