Abstract
Disturbances in peripheral and brain lipid metabolism, including the omega-3 fatty acid docosahexaenoic acid (DHA), have been reported in major depressive disorder (MDD). However, these changes have yet to be confirmed in MDD with seasonal pattern (MDD-s), a subtype of recurrent MDD. The present exploratory study quantified plasma plasmalogen and diacyl-phospholipid species, and fatty acids within total phospholipids, cholesteryl esters, triacylglycerols and free fatty acids in non-medicated MDD-s participants (n = 9) during euthymia in summer or fall, and during depression in winter in order to screen for potential high sensitivity lipid biomarkers. Triacylglycerol alpha-linolenic acid concentration was significantly decreased, and myristoleic acid concentration was significantly increased, during winter depression compared to summer-fall euthymia. 1-stearyl-2-docosahexaenoyl-sn-glycero-3-phosphoethanolamine, a diacyl-phospholipid containing stearic acid and DHA, was significantly decreased in winter depression. Concentrations of cholesteryl ester oleic acid and several polyunsaturated fatty acids between summer/fall and winter increased in proportion to the increase in depressive symptoms. The observed changes in lipid metabolic pathways in winter-type MDD-s offer new promise for lipid biomarker development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major depressive disorder with seasonal pattern (MDD-s) is a subtype of major depression characterized by recurrent episodes of depression in one season that are largely absent in other seasons. MDD-s affects 0.4–4% of the population, [1, 2] and accounts for 18% of recurrent major depressive disorder (MDD) cases in Canada [2]. It is associated with reduced quality of life and increased risk of suicide [3,4,5]. MDD-s is treated with light therapy and/or antidepressant medications, and often in combination with cognitive behavioral therapy [6, 7].
Disturbed fatty acid metabolism has been reported in non-seasonal MDD. As indicated in the literature review summary presented in Table 1, studies reported changes in the percentage composition (weight or mole percentage of total fatty acids) or concentration (amount per volume or total cell count) of several saturated, monounsaturated or polyunsaturated fatty acids in whole blood, red blood cells (RBC), plasma or serum of MDD or attempted suicide participants compared to healthy controls or controls with coronary heart disease or multiple sclerosis [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. Circulating omega-3 polyunsaturated fatty acids (n-3 PUFA) were reported to increase in two studies [9, 10], remain unchanged in two [17, 24] and decrease in fourteen [8, 11,12,13,14,15,16, 18,19,20,21,22,23, 25], consistent with a meta-analysis that showed an overall reduction in omega-3 fatty acids in MDD patients [26]. N-6 PUFA were decreased in three [10, 12, 19], increased in three [18, 20, 22] and unaltered in nine studies [8, 9, 11, 13, 15, 17, 21, 24, 25] whereas saturated and monounsaturated fatty acids when reported, were decreased in three [12, 13, 24], increased in four [11, 14, 17, 18] and unchanged in five studies [8, 10, 15, 21, 22]. Consistent with the reduction in circulating omega-3 fatty acids observed in most studies, McNamara et al. reported a 14% reduction in postmortem prefrontal cortex docosahexaenoic acid (DHA, 22:6n-3) concentration of suicide victims with MDD compared to controls who died from non-cardiovascular related causes [27].
Seasonal changes in serum fatty acid composition were also reported, but in healthy participants [28]. De Vriese et al. found reduced arachidonic acid (ARA, 20:4n-6), eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3) percent composition in serum phospholipids of healthy participants during winter compared to summer, suggesting seasonal effects on serum fatty acid profile [28]. The reductions in EPA and DHA composition during winter are consistent with some MDD studies [11,12,13, 15], suggesting that seasonal reductions in EPA and DHA may be a risk factor for altered mood.
Most of the reported changes in fatty acid composition or concentration in MDD patients occurred in RBC, plasma or serum cholesteryl esters or phospholipids (Table 2) [11,12,13, 15, 19, 20, 22, 23, 25]. In humans, the incorporation of dietary fatty acids such as DHA into plasma ethanolamine glycerophospholipids and cholesteryl esters is minimal [29, 30], which means that these lipid pools are less sensitive to fluctuations in dietary DHA intake compared to the triacylglyceride pool, where ingested DHA preferentially incorporates [29], or to the total phospholipid pool, which exhibit high within-subject variability in fatty acid levels [31]. Thus, changes in specific stable lipid pools such as ethanolamine glycerophospholipids or cholesteryl esters could reflect metabolic disturbances associated with mood progression and serve as reliable disease biomarkers. It is not known whether MDD-s is associated with seasonal changes in plasma lipid pools similar to MDD.
In the present study, we sought to address whether the symptomatic phase of MDD-s is associated with changes in plasma phospholipid molecular species and fatty acid concentrations (nmol/mL) in various lipid pools. In view of the reported changes in plasma or serum phospholipid and cholesteryl ester fatty acids in depressed patients and the potential impact of season on serum fatty acids [9, 10, 12, 13, 17, 20, 22, 23, 25, 28], we hypothesized that plasma phospholipids species and cholesteryl ester fatty acids would be altered in non-medicated MDD-s patients during winter compared to summer-fall. We focused on MDD-s because it is the most predictable subtype of MDD in which to observe prospective changes in lipid biomarkers between mood states. Moreover, unlike MDD, lipid metabolism in MDD-s remains understudied.
Fatty acids were measured in plasma free fatty acids, triacylglycerols, cholesteryl esters, and total phospholipids. Diacyl-phospholipid and plasmalogen molecular species, which make up the majority of the total phospholipid pool, were also quantified. Diacyl-phospholipids contain two fatty acids bound to the stereospecifically-numbered (sn)- 1 and 2 positions via an ester bond, whereas plasmalogens contain a vinyl-ether bond instead of an ester bond at the sn-1 position (reviewed in [32]). The sn-1 position typically contains a saturated fatty acid, whereas the sn-2 position contains a PUFA such as ARA or DHA [33].
We found seasonal changes in triacylglyceride myristoleic acid and n-3 fatty acid concentrations within diacyl- phospholipids and triacylglycerides, as well as positive associations between the change in depressive score and the change in cholesteryl ester oleate and several PUFA concentrations.
Experimental Procedures
Materials
Standard choline plasmalogens, (1-(1Z-octadecenyl)-2-oleoyl-sn-glycero-3-phosphocholine (STol-OLA-Gro-3-PCho), 1-(1Z-octadecenyl)-2-arachidonoyl-sn-glycero-3-phosphocholine (STol-ARA-Gro-3-PCho), 1-(1Z-octadecenyl)-docosahexaenoyl-sn-glycero-3-phosphocholine (STol-DHA-Gro-3-PCho)), ethanolamine plasmalogens (1-(1Z-octadecenyl)-oleoyl-sn-glycero-3-phosphoethanolamine (STol-OLA-Gro-3-PEtn), 1-(1Z-octadecenyl)-arachidonoyl-sn-glycero-3-phosphoethanolamine (STol-ARA-Gro-3-PEtn), 1-(1Z-octadecenyl)-docosahexaenoyl-sn-glycero-3-phosphoethanolamine (STol-DHA-Gro-3-PEtn)), diacyl-phosphatidylcholine (1-stearoyl-2-oleoyl-sn-glycero-3-phosphocholine (STA-OLA-Gro-3-PCho), 1-stearoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (STA-ARA-Gro-3-PCho), 1-stearoyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine (STA-DHA-Gro-3-PCho)) and diacyl-phosphatidylethanolamine (1-stearoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (STA-OLA-Gro-3-PEtn), 1-stearoyl-2-arachidonoyl-sn-glycero-3-phosphoethanolamine (STA-ARA-Gro-3-PEtn), 1-stearoyl-2-docosahexaenoyl-sn-glycero-3-phosphoethanolamine (STA-DHA-Gro-3-PEtn)) were obtained from Avanti Polar Lipids (Alabaster, AL, USA).
Standard free heptadecanoic acid (17:0), free palmitic acid (16:0), tripalmitin (tri-16:0), and cholesteryl-palmitate used for the thin layer chromatography (TLC) or fatty acid analysis were obtained from NuChek Prep Inc. (Elysian, MN, USA). 1,2-diheptadecanoyl-sn-glycero-3-phosphocholine (di-17:0 PC) and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine were obtained from Avanti Polar Lipids (Alabaster, AL, USA).
Participants
Ethical approval for the present study was obtained from the Sunnybrook Research Ethics Board. All participants provided informed written consent prior to beginning the study. A total of 15 medication-free participants were recruited. Participants were aged between 18 and 65 years and had a history of MDD-s based on at least two episodes of depression that presented a seasonal pattern over the past 3 years were recruited. Participants who used an antidepressant, hypnotic or antipsychotic, or had abnormal liver, kidney or lung function, anemia, hypothyroidism, neurological or neurodegenerative conditions, cancer, inflammatory disease, other acute medical conditions or infection were excluded from the study. None of the participants received light or cognitive behavioral therapy during winter.
Each participant came for a baseline visit in summer or early fall (August–October) and a follow-up visit during winter (December–February). A structured clinical interview was used to diagnose depressive episodes based on DSM-5 criteria, and the Beck Depression Inventory-II (BDI-II) was used to assess depressive symptom severity [34]. The BDI-II is a 21-item self-report scale that measures the severity of depressive symptoms. Each item is endorsed on a 4-point scale that ranges from 0 to 3, corresponding to statements of increasing severity. The individual items are summed to create a total score between 0 and 63. Although it is recommended that total scores be interpreted according to the characteristics of each individual population, a total score of 0–13 is often considered minimal, 14–19 mild, 20–28 moderate, and 29–63 severe.
During each visit, a blood sample was collected in K2-ethylenediaminetetraacetic (EDTA) tubes and centrifuged for 10 min at 1000×g at 4 °C. Samples were collected in the morning at 9:00 AM (±1:00 h), after a ≥10 h overnight fast. Plasma was isolated and stored at −80 °C until analysis. Only participants who were euthymic in summer and depressed in winter were included in the final analysis. Lipid assays described below were performed in a blinded and randomized manner.
Diacyl-Phospholipid and Plasmalogen Extraction
Phospholipids containing plasmalogens and diacyl-phospholipids were extracted from plasma by methanol precipitation and solid phase extraction as established by Otoki et al. [35]. Eighty microliters of plasma were mixed with 20 μL of 25 μM 2Na-EDTA in water and 500 μL of methanol containing 0.002% butylated hydroxytoluene. Surrogate standards were not added because deuterated standards for most of the phospholipid species analyzed (plasmalogens in particular) do not exist. The samples were centrifuged at 10,000×g for 10 min at −10 °C, and 600 μL of the supernatant was collected. The remaining precipitate was re-extracted with 500 μL of methanol and subjected to centrifugation at 10,000×g for 10 min at −10 °C. The resulting 500 μL of the supernatant was combined to the first supernatant, to yield a total of 1100 μL of methanol solution. Four hundred μL of the pooled methanol extract was loaded onto a silica Sep-Pak solid phase extraction cartridge (100 mg; Waters, Milford, MA, USA) equilibrated with methanol. The eluted methanol was collected. The cartridge was then loaded with an additional 1.6 mL of methanol to elute residual phospholipids, yielding 2 mL of methanol in total. Ten microliters of the final aliquot was injected into an Agilent liquid chromatography tandem mass spectrometry (LC–MS/MS) system operated in multiple reaction monitoring (MRM) mode to analyze plasmalogen and diacyl-phospholipid molecular species. The following phospholipid species were analyzed: STol-OLA-Gro-3-PCho, STol-ARA-Gro-3-PCho, STol-DHA-Gro-3-PCho, STol-OLA-Gro-3-PEtn, STol-ARA-Gro-3-PEtn, STol-DHA-Gro-3-PEtn, STA-OLA-Gro-3-PCho, STA-ARA-Gro-3-PCho, STA-DHA-Gro-3-PCho, STA-OLA-Gro-3-PEtn, STA-ARA-Gro-3-PEtn and STA-DHA-Gro-3-PEtn.
A standard curve for each of the compounds was established by plotting standard concentration on the y-axis and standard area on the x-axis. Concentrations were calculated based on an equation derived from each of the external standard curves as follows:
where the slope is derived from the standard curve plot, 80 µL is the volume of plasma used for the extraction, 1100 µL is the total volume of the methanol extract, 400 µL is the volume of methanol extract used for the solid phase extraction, 2000 µL is the final elution volume following solid phase extraction (i.e. 400 µL methanol phospholipid extract + 1600 µL used to elute residual phospholipids), and 10 µL is the volume injected into the LC–MS/MS.
The standard percentage recovery was determined by spiking 80 µL of a plasma sample with 200 pmol of diacyl phospholipid and plasmalogen standard mix and calculating the amount recovered with the following equation:
The observed value is the measured amount of each phospholipid (pmol) in spiked plasma minus the amount (pmol) in non-spiked plasma. The expected value is 200 pmol × (400/1100) × (10/2000) where 1100 µL is the total volume of methanol extract, 400 µL is the volume of methanol extract used for the solid phase extraction, 2000 µL is the volume of elution following solid phase extraction and 10 µL is the volume injected in the instrument. The percentage recovery was 105% for STol-OLA-Gro-3-PCho, 134% for STol-ARA-Gro-3-PCho, 118% for STol-DHA-Gro-3-PCho, 89% for STol-OLA-Gro-3-PEtn, 124% for STol-ARA-Gro-3-PEtn, 123% for STol-DHA-Gro-3-PEtn, 123% for STol-OLA-Gro-3-PEtn, 36% for STol-ARA-Gro-3-PEtn, 69% for STol-DHA-Gro-3-PEtn, 85% for STA-OLA-Gro-3-PEtn, 109% for STA-ARA-Gro-3-PEtn and 78% STA-DHA-Gro-3-PEtn. The low percentage recovery for STol-ARA-Gro-3-PEtn is likely due to the low amount of STol-ARA-Gro-3-PEtn spiked into the sample (200 pmol) relative to the total amount of STol-ARA-Gro-3-PEtn present in 80 µL of human blood (~3600 pmol).
Matrix effects reflecting the extent of ion suppression were calculated using the following equation:
A value close to 100% reflects minimal matrix effects. In this study, calculated matrix effect values were between 84 and 107%, suggesting minimal matrix interference.
LC–MS/MS analysis
Plasmalogen and diacyl-phospholipids were analyzed by LC–MS/MS consisting of an Agilent LC system 1290 (Agilent Corporation, Palo Alto, CA, USA) equipped with a vacuum degasser, a quaternary pump and an autosampler, and an Agilent 6460 Triple Quadrupole MS system (Agilent Corporation).
Samples and standards were separated on a C8 column (2.0 × 100 mm; GL Science, Tokyo, Japan) with a binary gradient consisting of solvent A (methanol: water 68:32 (v/v) containing 0.1 mM sodium acetate) and solvent B (methanol containing 0.1 mM sodium acetate) [33]. The column temperature was maintained at 40 °C. The gradient profile is shown in Supplementary Table 1. Samples were analyzed in MRM mode. Electrospray ionization was used as the ion source with the following experimental parameters: Gas temperature, 300 °C; Sheath Gas temperature, 220 °C; Sheath Gas flow, 11 ml/min; Nebulizers, 45 psi; Capillary gas, 3500/−3500 V. Other optimization parameters and MRM pairs are described in Supplementary Table 2.
Lipid Extraction and Analysis
Total lipids were extracted from plasma by the Folch method and fractionated into phospholipids, triacylglycerols, cholesteryl esters and unesterified (free) fatty acids by thin layer chromatography (TLC) [36].
Two hundred microliters of plasma were added to 3 mL of chloroform: methanol (2:1 v/v) containing unesterified heptadecanoic acid (17:0) as an internal standard for unesterified fatty acids, followed by 0.75 mL 0.1 M NaCl. The samples were vortexed and centrifuged. The lower chloroform phase containing total lipids was pipetted into a new test-tube. Lipids were re-extracted with additional 2 mL of chloroform. The chloroform extracts were pooled, dried under nitrogen, reconstituted in 100 µL chloroform: methanol (2:1 v/v) and plated on pre-washed and heat-activated silica gel-60 TLC plates (EM Separation Technologies, Gibbstown, NJ, USA). Plates were washed in a TLC tank containing 100 mL of chloroform: methanol (2:1 v/v). The solvent front was allowed to migrate to the top of the plates, which were then kept in a vacuum oven chamber at 80 °C for at least 2 h.
Total lipids extracted from plasma were re-dissolved in 100 µL chloroform/methanol and re-plated to ensure that all lipids were transferred to the TLC plates. Lipids were resolved using heptane: diethylether: glacial acetic acid (60:40:3, v/v/v) solvent system to separate phospholipids, triacylglycerides, cholesteryl esters and free fatty acids [37]. Authentic standards (free palmitic acid, tripalmitin, cholesteryl-palmitate and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine) were run on separate lanes on the plates to identify lipid bands under ultraviolet light, after spraying with 0.02% dichlorofluorescein solution in methanol. The bands were scraped into test tubes containing di-17:0 PC as an internal standard for phospholipids, triacylglycerides and cholesteryl esters.
Each lipid fraction was transesterified in methanolic acid according to the method of Ichihara and Fukubayashi [38]. Four hundred microliters of toluene, 3 mL methanol and 600 μL HCl-methanol (8:92, v/v) were added to each lipid fraction. Samples were vortexed and heated at 90 °C for 1 h. After cooling for a few minutes, 1 mL of hexane and 1 mL of distilled water were added. Samples were vortexed and the phases were allowed to separate for a few minutes. The upper hexane layer containing fatty acid methyl esters was dried under nitrogen and reconstituted in 5 μL of hexane for the unesterified fatty acid fraction and 50 μL of hexane for the esterified fractions.
Fatty acid methyl esters were analyzed by gas-chromatography. One microliter of sample was injected at a split ratio of 10:1 into a 3800 Varian gas chromatograph equipped with a flame ionization detector (Varian Inc., Walnut Creek, CA, USA). Fatty acids were separated on a DB-23 capillary column (30 m length; 0.25 mm ID; 0.25 μm thickness; Agilent, Santa Clara, CA, USA). The detector and injector temperatures were set at 300 and 250 °C, respectively. The oven temperature program was set at 50 °C for 2 min, increased by 10 °C/min to 180 °C, held at 180 °C for 5 min, increased by 5 °C/min to 240 °C and held at 240 °C for 5 min. Helium was used as a carrier gas at a flow rate of 1.3 mL/min. A custom mix of 31 fatty acid methyl ester standards (NuChek Prep, Elysian, MN, USA) was used to identify the individual fatty acids. Results were expressed as concentration (nmol/mL; Table 2).
Statistical Analysis
Statistical analysis was performed on GraphPad Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA). Due to the small sample size (n = 9), a non-parametric Wilcoxon’s rank test was used to assess the differences between summer-fall and winter. The relation between change in fatty acid concentrations in the different lipid pools and change in BDI-II score was evaluated using a Spearman’s correlation. Corrections for multiple independent statistical tests were not performed due to the exploratory nature of the study. Statistical significance was set at p < 0.05 (two-tailed). All data are expressed as medians (interquartile range; 25th and 75th percentiles).
Results
Of 15 participants recruited, 4 dropped out before the end of the study and 2 did not meet the DSM-5 depression criteria in winter. The remaining participants (4 men, 5 women) were 46.7 ± 14 years of age and had an average of 3.3 previous episodes of depression. Median BDI-II scores significantly increased in the remaining 9 participants between summer-fall and winter (summer-fall, 5.0 (interquartile range 0.5–8.5); winter, 20.0 (interquartile range 13.5–27.0); p = 0.0039) [39], indicating minimal symptoms in summer-fall and generally mild to moderate severity in winter. BDI-II scores ranged between 0–12 in summer-fall and 9–28 in winter.
Table 2 shows fatty acid concentrations within free fatty acids, phospholipids, triacylglycerides and cholesteryl esters. There were no significant differences in free or esterified fatty acid concentrations between summer-fall and winter, except for alpha-linolenic acid (ALA) and myristoleic acid (14:1n-5) within triacylglycerides. ALA was significantly reduced by 34% and myristoleic (14:1n-5) was increased by 28% in triacylglycerides in winter as compared to summer-fall.
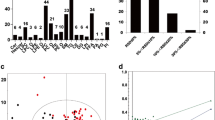
Figure 1a, b show the concentration of plasmalogen and diacyl-phospholipid species, respectively. STA-DHA-Gro-3-PEtn, a diacyl-phospholipid bearing DHA, was significantly reduced by 13.8% in winter compared to summer-fall (p = 0.0273). No significant differences were observed in plasmalogen or other diacyl-phospholipid species.
Plasma concentration of different plasmalogens species (a) and diacyl-phospholipids (b) in summer-fall and winter. Values are medians ± interquartile ranges (IQR; 25th and 75th percentiles) of 9 subjects. Wilcoxon’s rank test was used to assess the differences between summer-fall and winter. Asterisk significant difference between summer-fall and winter assessed by a Wilcoxon’s rank test (p < 0.05). STol-OLA-Gro-3-PCho, 1-(1Z-octadecenyl)-2-oleyl-sn-glycero-3-phosphocholine; STol-ARA-Gro-3-PCho, 1-(1Z-octadecenyl)-2-arachidonyl-sn-glycero-3-phosphocholine; STol-DHA-Gro-3-PCho, 1-(1Z-octadecenyl)-docosahexaenoyl-sn-glycero-3-phosphocholine; STol-OLA-Gro-3-PEtn, 1-(1Z-octadecenyl)-oleyl-sn-glycero-3-phosphoethanolamine; STol-ARA-Gro-3-PEtn, 1-(1Z-octadecenyl)-arachidonyl-sn-glycero-3-phosphoethanolamine; STol-DHA-Gro-3-PEtn, 1-(1Z-octadecenyl)-docosahexaenoyl-sn-glycero-3-phosphoethanolamine; STA-OLA-Gro-3-PCho, 1-stearyl-2-oleyl-sn-glycero-3-phosphocholine; STA-ARA-Gro-3-PCho, 1-stearyl-2-arachidonyl -sn-glycero-3-phosphocholine; STA-DHA-Gro-3-PCho, 1-stearyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine; STA-OLA-Gro-3-PEtn, 1-stearyl-2-oleyl-sn-glycero-3-phosphoethanolamine; STA-ARA-Gro-3-PEtn, 1-stearyl-2-arachidonyl-sn-glycero-3-phosphoethanolamine; STA-DHA-Gro-3-PEtn, 1-stearyl-2-docosahexaenoyl-sn-glycero-3-phosphoethanolamine
In an exploratory manner, we assessed correlations between seasonal changes in fatty acids within the various lipid pools and the change in BDI-II score, to test whether changes in specific lipid concentrations fluctuate with depressive severity. Significant correlations were seen in one fatty acid (16:1n-7) in phospholipids and in 8 fatty acids in cholesteryl esters (Supplementary Table 3). Figure 2a–f plots this relationship for main cholesteryl ester fatty acids, including oleic acid (OLA, 18:1n-9), linoleic acid (LNA, 18:2n-6), ARA, ALA and EPA, as well as total fatty acids. As shown, the change in OLA, LNA, ARA, ALA, EPA and total fatty acids (p < 0.05) was positively correlated to the change in BDI-II between summer-fall and winter.
Spearman’s correlation between the change in BDI-II score and change in cholesteryl-esters (CE) fatty acids between winter and summer-fall. a Oleic acid (OLA; 18:1 n-9), b linoleic acid (LNA; 18:2 n-6), c arachidonic acid (ARA; 20:4 n-6), d α-linolenic acid (ALA; 18:3 n-3), e eicosapentaenoic acid (EPA; 20:5 n-3) and f total fatty acids
Discussion
To our knowledge, this is the first study to probe seasonal changes in lipidomic markers in unmedicated MDD-s patients. We found statistically significant reductions in triacylglyceride ALA and STA-DHA-Gro-3-PEtn, and increased triacylglyceride myristoleic acid concentrations in plasma of non-medicated MDD-s patients during winter compared to summer-fall. Changes in cholesteryl ester OLA, LNA, ARA, ALA, EPA and total fatty acids between summer-fall and winter were positively correlated to the change in BDI-II scores.
The reduction in triacylglyceride ALA and increase in myristoleic acid during winter may be due to changes in their dietary intake or metabolism. We are not aware of clinical data on fatty acid intake in MDD-s patients. Two studies reported no difference in fatty acid intake between control and MDD patients, although seasonal shifts in consumption in relation to mood were not addressed [8, 16]. Energy intake, habitual food intake patterns and physical activity are known to change between seasons [40, 41]. A detailed analysis of seasonal patterns in fatty acid consumption in relation to physical activity in MDD-s patients could clarify whether the observed triacylglyceride fatty acid changes were related to diet or metabolic disturbances.
Although phospholipid DHA did not change between seasons in this study, STA-DHA-Gro-3-PEtn, an ethanolamine phospholipid containing stearic acid and DHA, decreased by 14% in winter depression compared to euthymia in summer-fall. This suggests that measuring fatty acids in total phospholipids may not yield the same level of sensitivity as measuring individual phospholipid species with LC–MS/MS. Total phospholipid DHA concentration was either unchanged [18] or reduced [13] in plasma of MDD patients compared to healthy controls. The inconsistency between studies may reflect inadequate sensitivity of measuring total phospholipid DHA concentration. Specific phospholipid species such as STA-DHA-Gro-3-PEtn may change more reliably with mood states.
The 14% reduction in STA-DHA-Gro-3-PEtn concentration in winter relative to summer-fall likely reflects metabolic disturbances in DHA or stearic acid turnover within phosphatidylethanolamine rather than seasonal shifts in DHA or stearic acid intake. This is because dietary DHA is preferentially incorporated into plasma phosphatidylcholine, but not phosphatidylethanolamine, as evidenced by one study that showed limited responsiveness of phosphatidylethanolamine to dietary DHA intake compared to phosphatidylcholine following 28 days of fish oil supplementation (3.48 g EPA and 2.28 g DHA) [30]. Instead, reduced synthesis or increased utilization may explain the STA-DHA-Gro-3-PEtn reduction. Tracer studies involving labeled STA-DHA-Gro-3-PEtn, stearic acid or DHA could be used in future studies to probe STA-DHA-Gro-3-PEtn turnover in MDD-s patients [42, 43]. The reduction in STA-DHA-Gro-3-PEtn, if confirmed in larger cohorts, might justify its development as a diagnostic biomarker of mood symptoms in MDD-s patients. It would also be worthwhile to assess whether this marker is specific to MDD-s or whether it also generalizes to other MDD subtypes.
Cholesteryl ester fatty acid concentrations did not differ significantly between summer-fall and winter, yet significant positive associations between summer-fall to winter changes in BDI-II scores and cholesteryl ester OLA, LNA, ARA, ALA, EPA and total fatty acids concentrations were observed. Maes et al. reported an inverse association between depressive scores and cholesteryl ester ALA and EPA percent composition or concentrations [12], which is opposite to our findings. It is not clear whether some of these associations reflect metabolic perturbations related directly to mood symptoms, or differences in dietary fatty acid intake, either of which may have differed between the populations studied. MDD-s is known to be a good model for atypical depression, which often involves hypersomnia and overeating [44], two factors that impact fatty acid intake and potentially cholesteryl ester composition between seasons.
The observed reduction in ALA triacylglyceride concentration is in general agreement with two MDD studies which reported reduced ALA concentration in serum or plasma cholesteryl ester, phospholipid and total lipid [12, 16], and opposite to one MDD study which showed increased plasma total lipid ALA concentration [18]. Except for myristoleic acid, we did not observe changes in the concentration of other fatty acids that were previously found altered in various MDD studies. Notwithstanding dietary, activity, age or gender confounders, differences in lipid profile could be due to divergent lipid metabolic pathways that are impacted in MDD and MDD-s. Thus, the decrease in ALA could be related to reduced intake or increased utilization for β-oxidation or synthesis into EPA, possibilities that merit follow-up with detailed dietary assessments and tracers that inform on net oxidation and liver synthesis-secretion rates [45].
Limitations of this study include the small sample size, absence of dietary or physical activity information and lack of a control group to disentangle effects of season from those of depression. However, the study is strengthened by a within-subjects design, which increases power by accounting for between-subjects variability. Multiple independent comparisons used in this study increase the risk of false positives and should be confirmed in larger studies. The between-group variation reported herein, however, is valuable for designing adequately powered follow-up studies. Overall, the present findings provide a starting point for larger confirmatory studies to characterize disturbed lipid metabolic pathways in MDD-s.
In summary, this exploratory study identified seasonal disturbances in lipid metabolism in unmedicated MDD-s participants. The changes in ethanolamine phospholipid DHA concentration in particular, and associations between cholesteryl ester n-3 fatty acids and symptom severity, add to the evidence suggesting the involvement of n-3 fatty acids in MDD, extending the finding to MDD-s. The STA-DHA-Gro-3-PEtn changes identify a novel species that can be measured reliably for potential use as a biomarker of progression or response to therapy. Replication of these findings in adequately powered and controlled studies is merited.
Abbreviations
- ALA:
-
Alpha-linolenic acid
- ARA:
-
Arachidonic acid
- di-17:0 PC:
-
1,2-Diheptadecanoyl-sn-glycero-3-phosphocholine
- DHA:
-
Docosahexaenoic acid
- EDTA:
-
Ethylenediaminetetraacetic
- EPA:
-
Eicosapentaenoic acid
- LNA:
-
Linoleic acid
- LC–MS/MS:
-
Liquid chromatography tandem mass spectrometry
- MDD-s:
-
Major depressive disorder with seasonal pattern
- MRM:
-
Multiple reaction monitoring
- OLA:
-
Oleic acid
- PtdCho:
-
Phosphatidylcholine
- PUFA:
-
Polyunsaturated fatty acids
- STA-ARA-Gro-3-PCho:
-
1-Stearoyl-2-arachidonoyl -sn-glycero-3-phosphocholine
- STA-DHA-Gro-3-PCho:
-
1-Stearoyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine
- STA-OLA-Gro-3-PCho:
-
1-Stearoyl-2-oleoyl-sn-glycero-3-phosphocholine
- STol-ARA-Gro-3-PCho:
-
1-(1Z-octadecenyl)-2-arachidonoyl-sn-glycero-3-phosphocholine
- STol-DHA-Gro-3-PCho:
-
1-(1Z-octadecenyl)-docosahexaenoyl-sn-glycero-3-phosphocholine
- STol-OLA-Gro-3-PCho:
-
1-(1Z-octadecenyl)-2-oleoyl-sn-glycero-3-phosphocholine
- PE:
-
Phosphatidylethanolamine
- STA-ARA-Gro-3-PEtn:
-
1-Stearoyl-2-arachidonoyl-sn-glycero-3-phosphoethanolamine
- STA-DHA-Gro-3-PEtn:
-
1-Stearoyl-2-docosahexaenoyl-sn-glycero-3-phosphoethanolamine
- STA-OLA-Gro-3-PEtn:
-
1-Stearoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine
- STol-ARA-Gro-3-PEtn:
-
1-(1Z-octadecenyl)-arachidonoyl-sn-glycero-3-phosphoethanolamine
- STol-DHA-Gro-3-PEtn:
-
1-(1Z-octadecenyl)-docosahexaenoyl-sn-glycero-3-phosphoethanolamine
- STol-OLA-Gro-3-PEtn:
-
1-(1Z-octadecenyl)-oleoyl-sn-glycero-3-phosphoethanolamine
- RBC:
-
Red blood cells
- TLC:
-
Thin layer chromatography
References
Blazer DG, Kessler RC, Swartz MS (1998) Epidemiology of recurrent major and minor depression with a seasonal pattern. The National Comorbidity Survey. Br J Psychiatry 172:164–167
Levitt AJ, Boyle MH, Joffe RT, Baumal Z (2000) Estimated prevalence of the seasonal subtype of major depression in a Canadian community sample. Can J Psychiatry 45:650–654
Sun J, Guo X, Ma J, Zhang J, Jia C, Xu A (2011) Seasonality of suicide in Shandong China, 1991–2009: associations with gender, age, area and methods of suicide. J Affect Disord 135:258–266
Postolache TT, Mortensen PB, Tonelli LH, Jiao X, Frangakis C, Soriano JJ, Qin P (2010) Seasonal spring peaks of suicide in victims with and without prior history of hospitalization for mood disorders. J Affect Disord 121:88–93
Michalak EE, Tam EM, Manjunath CV, Levitt AJ, Levitan RD, Lam RW (2005) Quality of life in patients with seasonal affective disorder: summer vs winter scores. Can J Psychiatry 50:292–295
Westrin A, Lam RW (2007) Seasonal affective disorder: a clinical update. Ann Clin Psychiatry 19:239–246
Lam RW, Levitt AJ, Levitan RD, Enns MW, Morehouse R, Michalak EE, Tam EM (2006) The Can-SAD study: a randomized controlled trial of the effectiveness of light therapy and fluoxetine in patients with winter seasonal affective disorder. Am J Psychiatry 163:805–812
Edwards R, Peet M, Shay J, Horrobin D (1998) Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord 48:149–155
Ellis FR, Sanders TA (1977) Long chain polyunsaturated fatty acids in endogenous depression. J Neurol Neurosurg Psychiatry 40:168–169
Fehily AM, Bowey OA, Ellis FR, Meade BW, Dickerson JW (1981) Plasma and erythrocyte membrane long chain polyunsaturated fatty acids in endogenous depression. Neurochem Int 3:37–42
Huan M, Hamazaki K, Sun Y, Itomura M, Liu H, Kang W, Watanabe S, Terasawa K, Hamazaki T (2004) Suicide attempt and n-3 fatty acid levels in red blood cells: a case control study in China. Biol Psychiatry 56:490–496
Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Meltzer HY (1999) Lowered omega3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res 85:275–291
Maes M, Smith R, Christophe A, Cosyns P, Desnyder R, Meltzer H (1996) Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20: 4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord 38:35–46
Peet M, Murphy B, Shay J, Horrobin D (1998) Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry 43:315–319
Rizzo AM, Corsetto PA, Montorfano G, Opizzi A, Faliva M, Giacosa A, Ricevuti G, Pelucchi C, Berra B, Rondanelli M (2012) Comparison between the AA/EPA ratio in depressed and non depressed elderly females: omega-3 fatty acid supplementation correlates with improved symptoms but does not change immunological parameters. Nutr J 11:82
Sublette ME, Segal-Isaacson CJ, Cooper TB, Fekri S, Vanegas N, Galfalvy HC, Oquendo MA, Mann JJ (2011) Validation of a food frequency questionnaire to assess intake of n-3 polyunsaturated fatty acids in subjects with and without major depressive disorder. J Am Diet Assoc 111(117–123):e111–e112
Vareka T, Vecka M, Jirak R, Tvrzicka E, Macasek J, Zak A, Zeman M (2012) Plasma fatty acid profile in depressive disorder resembles insulin resistance state. Neuro Endocrinol Lett 33(Suppl 2):83–86
Assies J, Pouwer F, Lok A, Mocking RJ, Bockting CL, Visser I, Abeling NG, Duran M, Schene AH (2010) Plasma and erythrocyte fatty acid patterns in patients with recurrent depression: a matched case-control study. PLoS One 5:e10635
Amin AA, Menon RA, Reid KJ, Harris WS, Spertus JA (2008) Acute coronary syndrome patients with depression have low blood cell membrane omega-3 fatty acid levels. Psychosom Med 70:856–862
Frasure-Smith N, Lesperance F, Julien P (2004) Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol Psychiatry 55:891–896
Feart C, Peuchant E, Letenneur L, Samieri C, Montagnier D, Fourrier-Reglat A, Barberger-Gateau P (2008) Plasma eicosapentaenoic acid is inversely associated with severity of depressive symptomatology in the elderly: data from the Bordeaux sample of the Three-City Study. Am J Clin Nutr 87:1156–1162
Tiemeier H, van Tuijl HR, Hofman A, Kiliaan AJ, Breteler MM (2003) Plasma fatty acid composition and depression are associated in the elderly: the Rotterdam Study. Am J Clin Nutr 78:40–46
Riemer S, Maes M, Christophe A, Rief W (2010) Lowered omega-3 PUFAs are related to major depression, but not to somatization syndrome. J Affect Disord 123:173–180
Aupperle RL, Denney DR, Lynch SG, Carlson SE, Sullivan DK (2008) Omega-3 fatty acids and multiple sclerosis: relationship to depression. J Behav Med 31:127–135
Rees AM, Austin MP, Owen C, Parker G (2009) Omega-3 deficiency associated with perinatal depression: case control study. Psychiatry Res 166:254–259
Lin PY, Huang SY, Su KP (2010) A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry 68:140–147
McNamara RK, Jandacek R, Tso P, Dwivedi Y, Ren X, Pandey GN (2013) Lower docosahexaenoic acid concentrations in the postmortem prefrontal cortex of adult depressed suicide victims compared with controls without cardiovascular disease. J Psychiatr Res 47:1187–1191
De Vriese SR, Christophe AB, Maes M (2004) In humans, the seasonal variation in poly-unsaturated fatty acids is related to the seasonal variation in violent suicide and serotonergic markers of violent suicide. Prostaglandins Leukot Essent Fatty Acids 71:13–18
Zuijdgeest-van Leeuwen SD, Dagnelie PC, Rietveld T, van den Berg JW, Wilson JH (1999) Incorporation and washout of orally administered n-3 fatty acid ethyl esters in different plasma lipid fractions. Br J Nutr 82:481–488
Subbaiah PV, Kaufman D, Bagdade JD (1993) Incorporation of dietary n-3 fatty acids into molecular species of phosphatidyl choline and cholesteryl ester in normal human plasma. Am J Clin Nutr 58:360–368
Harris WS, Thomas RM (2010) Biological variability of blood omega-3 biomarkers. Clin Biochem 43:338–340
Brites P, Waterham HR, Wanders RJ (2004) Functions and biosynthesis of plasmalogens in health and disease. Biochim Biophys Acta 1636:219–231
Otoki Y, Nakagawa K, Kato S, Miyazawa T (2015) MS/MS and LC-MS/MS analysis of choline/ethanolamine plasmalogens via promotion of alkali metal adduct formation. J Chromatogr B Analyt Technol Biomed Life Sci 1004:85–92
Beck AT, Steer RA, Brown GK (1996) Manual for the Beck Depression Inventory-II. Psychological Corporation, San Antonio
Otoki Y, Kato S, Kimura F, Furukawa K, Yamashita S, Arai H, Miyazawa T, Nakagawa K (2017) Accurate quantitation of choline and ethanolamine plasmalogen molecular species in human plasma by liquid chromatography–tandem mass spectrometry. J Pharm Biomed Anal 134:77–85
Skipski VP, Good JJ, Barclay M, Reggio RB (1968) Quantitative analysis of simple lipid classes by thin-layer chromatography. Biochim Biophys Acta 152:10–19
DeMar JC Jr, Ma K, Bell JM, Rapoport SI (2004) Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J Neurochem 91:1125–1137
Ichihara K, Fukubayashi Y (2010) Preparation of fatty acid methyl esters for gas-liquid chromatography. J Lipid Res 51:635–640
Hennebelle M, Otoki Y, Yang J, Hammock BD, Levitt AJ, Taha AY, Swardfager W (2017) Altered soluble epoxide hydrolase-derived oxylipins in patients with seasonal major depression: an exploratory study. Psychiatry Res 252:94–101
Stelmach-Mardas M, Kleiser C, Uzhova I, Penalvo JL, La Torre G, Palys W, Lojko D, Nimptsch K, Suwalska A, Linseisen J, Saulle R, Colamesta V, Boeing H (2016) Seasonality of food groups and total energy intake: a systematic review and meta-analysis. Eur J Clin Nutr 70:700–708
Brychta RJ, Arnardottir NY, Johannsson E, Wright EC, Eiriksdottir G, Gudnason V, Marinac CR, Davis M, Koster A, Caserotti P, Sveinsson T, Harris T, Chen KY (2016) Influence of day length and physical activity on sleep patterns in older icelandic men and women. J Clin Sleep Med 12:203–213
Thompson GR, Jadhav A, Nava M, Gotto AM Jr (1976) Effects of intravenous phospholipid on low density lipoprotein turnover in man. Eur J Clin Invest 6:241–248
Galli C, Sirtori CR, Mosconi C, Medini L, Gianfranceschi G, Vaccarino V, Scolastico C (1992) Prolonged retention of doubly labeled phosphatidylcholine in human plasma and erythrocytes after oral administration. Lipids 27:1005–1012
Stewart JW, Quitkin FM, Terman M, Terman JS (1990) Is seasonal affective disorder a variant of atypical depression? Differential response to light therapy. Psychiatry Res 33:121–128
Metherel AH, Domenichiello AF, Kitson AP, Hopperton KE, Bazinet RP (2016) Whole-body DHA synthesis-secretion kinetics from plasma eicosapentaenoic acid and alpha-linolenic acid in the free-living rat. Biochim Biophys Acta 1861:997–1004
Acknowledgements
WS and AYT conceived the idea of probing lipidomics in seasonal affective disorder patients. WS and AJL conceived the study design and implementation. KN and YO contributed the plasmalogen standards and method development. YO and MH performed the analysis. AYT and WS contributed to drafting the manuscript. All authors edited and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Ethical approval
The study was approved by the Sunnybrook Research Ethics Board and complied with ethical standards.
Funding
The authors acknowledge financial support from the UC Davis Department of Food Science and Technology and College of Agriculture and Environmental Sciences, Sunnybrook Health Sciences Centre Department of Psychiatry and Sunnybrook Research Institute Hurvitz Brain Sciences Program.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Otoki, Y., Hennebelle, M., Levitt, A.J. et al. Plasma Phosphatidylethanolamine and Triacylglycerol Fatty Acid Concentrations are Altered in Major Depressive Disorder Patients with Seasonal Pattern. Lipids 52, 559–571 (2017). https://doi.org/10.1007/s11745-017-4254-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-017-4254-1