Abstract
Finger or heel-pricked blood sampling for fatty acid analysis is suitable especially in newborn infants where blood sampling is difficult and phlebotomy for research can be unethical. The aim of this study was to evaluate dried blood long chain polyunsaturated fatty acids (LC-PUFA) stability during storage at −28 °C. We collected 12 blood cord samples that were analyzed immediately after blood drawing, with and without drying the blood on filter paper. Dried samples were then analyzed 7 days and 1, 3, and 6 months after collection. Butylated hydroxytoluene was added to all samples. Fatty acid composition and 13C enrichment were measured by gas chromatography and by gas chromatography-isotope ratio mass spectrometry, respectively. The fatty acid composition, expressed in mol%, of the major LC-PUFA at day 7 was not statistically different from time 0, however lower values were found by the first month of storage. The 13C enrichment of 20:4n-6 and 22:6n-3 did not differ during the whole study period. LC-PUFA analysis from dried umbilical cord blood in neonates should be performed within a week, major losses of LC-PUFA occur afterwards. However, fatty acids obtained from dried blood maintain their 13C enrichment value for up to 6 months and thus these samples are suitable for natural abundance isotopic studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the long chain-polyunsaturated fatty acids (LC-PUFA), arachidonic acid (ARA, 20:4n-6), eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3) are of major interest in humans. ARA and EPA are utilized for eicosanoid synthesis and they are involved in signal transduction [1]. DHA is an important structural component of photoreceptor and cortical neuronal membranes and is required for optimal neural development and visual function [2–5].
Plasma and red blood cell content of LC-PUFA are commonly used to evaluate certain medical conditions, such as cystic fibrosis and vitamin D deficiency [6, 7], and to estimate nutritional status or compliance in epidemiologic and interventional studies [4, 5, 8]. In an attempt to use a less invasive marker, particularly for longitudinal studies in infants, some authors have used the fatty acid pattern of cheek cell lipids. Clinical studies have shown that the PUFA profile of cheek cell phospholipids correlates significantly with that of plasma and of red blood cells [9, 10], moreover it has been recently demonstrated that cheek cell PUFA phospholipid content, except for ARA, reflected that of other tissues as well as, or nearly as well as, the contents of plasma and/or erythrocyte phospholipids [11].
Use of blood spots collected on filter paper is a well-known method for screening for genetic or metabolic diseases during the first days of life. This method is simple and not expensive, furthermore the collection is applicable even to very small and vulnerable patients. Previous investigators have developed an assay involving a finger stick and filter paper blood spotting to determine PUFA levels in blood [12] and various types of filter paper have been tested [13]. They found, that this assay may represent a fast and reliable method to measure LC-PUFA levels [12, 14, 15], moreover the fatty acid profile of whole blood provides a more balanced picture about the status of PUFA with respect to other blood specimens [16, 17]. Sample throughput can be further increased by using a one-step direct transesterification method and a fast gas chromatography data acquisition [18]. The finger stick assay was used in epidemiological studies in adults, children and infants [12, 14] and, demonstrated its ability to evaluate nutrition-induced changes in DHA status as well as to show statistically significant association between the blood level of DHA and neurodevelopment scores [19].
Data on the long-term stability of such methods is limited. In the present study we tested the stability of selected LC-PUFA in dried blood for as long as 6 months from sampling. We tested the stability of the mol% values as well as the 13C enrichment.
Materials and Methods
Study Design
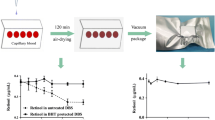
The study took place from January 2008 to December 2008. The study protocol was in accordance with the Declaration of Helsinki for research on human participants and was approved by the local ethical committee. All the included mothers were omnivores and were free from fish oil or omega 3 supplementation and provided signed informed consent before enrollment. We collected the leftover blood of 12 umbilical cord samples from the delivery suite of the “G. Salesi” Children’s Hospital, Ancona, Italy. From these whole EDTA blood samples, five 40–50-µL aliquots were then immediately spotted onto treated filter paper squares (AHLSTROM 226, Ahlstrom Corporation, Finland) and air dried for up to 10 min or until the blood samples were dried. From the same blood samples, one 50-µL aliquot was immediately processed, allowing us to determine if lipid profiles from fresh and dried blood were comparable. The samples were either immediately processed, or stored in plugged test tubes flushed for 10 s with nitrogen, at −28 °C and analyzed after 7 days, 1, 3, and 6 months.
Chemicals and Materials
n-Hexane, HCl-methanol and BHT were purchased from Sigma-Aldrich (St. Louis, MO, USA); potassium chloride was from Riedel-de Haën (Seelze, Germany). Solvents were all HPLC grade. GC reference standards GLC-461 containing 32 fatty acid methyl esters were purchased from Nu-Check Prep (Elysian, MN, USA). The BHT solution was prepared in a concentration of 0.5 mg/mL in acetone.
Filter paper was prepared according to Ichihara [20] from Ahlstrom 226 chromatography paper (Ashlstrom corporation, Finland). We chose this kind of paper because of its high absorbency and because of its use in our neonatology for bloodspot screening. Circles of 1.5 cm of diameter filter paper were sprayed with the BHT solution and air-dried for 30 min. The dried BHT-impregnated filter paper was prepared immediately prior to the deposition of the blood sample.
Fatty Acid Analysis
The aliquot of fresh blood was immediately processed as described previously [12]. The filter paper samples were transferred to test tubes with 1 mL of MeOH/HCl, 3 N and maintained in a dry bath at 90 °C for 1 h. Then, 2 mL of water and 2 mL of saturated solution of KCl were sequentially added, and fatty acid methyl esters (FAME) were finally extracted using 2 mL of n-hexane + BHT (50 mg/L). FAME were analyzed with a gas chromatography using a flame ionization detector (Agilent Santa Clara, USA model 6850) controlled by a workstation using Agilent ChemStation software. Chromatographic separation was performed on a capillary column (Omegawax 250 Supelco® 30 m × 0.25 mm × 0.25 μm). The injection was performed at 250 °C, detector was at 290 °C and the oven temperature program was from 60 to 200 °C at 20 °C/min, from 200 to 205 °C at 0.40 °C/min, from 205 to 208 °C at 0.10 °C/min and from 208 to 213 °C at 3.0 °C/min and maintained at 213 °C for 10 min. Fatty acids (FA) from C14 to C24 were detected, standard mixture of FA was used to identify individual FA and to calculate the response factor (GLC-461, Nu Check Prep, Elysian, MN, USA). The composition is reported as a mole percentage (mol%) of individual FA in total FA.
13C Enrichment of Selected Fatty Acids
The 13C enrichment of palmitic acid (C16:0), oleic acid (C18:0), ARA and, DHA methyl esters derived from dried blood spots, was analyzed by using a gas chromatographer isotope ratio-mass spectrometer (Delta Plus XL; Finningan MAT, Bremen, Germany) as previously described [4]. Separation of methyl esters was achieved on a DB225 column (30 m × 0.25 mm × 0.25 μm film thickness; Agilent J & W, Folsom, CA). Injections were performed in splitless mode at an injector temperature of 250 °C. After the chromatographic separation the fatty acids methyl esters undergo a combustion step, passing through the combustion interface, resulting in the formation of CO2. The CO2 produced is eluted by a helium stream to the IRMS source for 13C/12C analysis.
The 13C content of individual FA was expressed as δ13C value relative to Pee Dee Belemnite (PDB) carbonate [21]. The following formula was used to calculate δ13C:
where R x is the isotopic ratio of the sample and R s is the isotopic ratio of the reference standard. The defined reference standard for 13C has been PDB with 13C:12C of 0.0112372.
To determine 13C values relative to the PDB reference, we introduced PDB reference and CO2 with a known δ13C value (−61.5079) at appropriate time points during chromatography.
All samples were analyzed in duplicate.
Statistical Analysis
Data were expressed as means ± SD. The effect of storage time was evaluated with repeated measures by ANOVA and a post hoc Bonferroni test.
A p value <0.05 was considered significant. When replicate samples are described in the manuscript it means that separate spots, were analyzed independently.
All statistical analyses were performed using SPSS (version 18.0, SPSS Inc, Chicago, Illinois).
Results
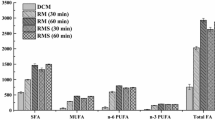
The first part of this study aimed to determine the equivalence of the FA composition of fresh and dried blood samples. We found no significant differences (p = 0.594) in FA mol% composition due to blood spotting and air drying. We also wanted to evaluate the effect of short time storage on lipid composition; dried blood spots were stored at −28 °C for 7 days, 1, 3 and 6 months. The FA composition of the cord blood samples is reported in Table 1. There were striking differences between the lipid profile of the sample processed immediately and those of the sample processed after 6 months. There were marked decreases in the LC-PUFA ARA (20:4n-6), EPA (20:5n-3), and DHA (22:6n-3). Significant differences in FA amount were evident even after 1 month of storage at −28 °C. ARA, EPA and DHA decreased by 9, 28 and 20 % after 30 days of storage, in contrast saturated fatty acids, such as C16:0, increased by 19 %. The change from baseline to 180 days of storage (6 months) was very important, the percentages of ARA, EPA and DHA decreased by 25, 62 and 45 % (Fig. 1).
The 13C enrichment of blood ARA, DHA, C16:0 and C18:0 is depicted in Fig. 2. The 13C enrichment of these FA did not differ significantly during the study period.
Discussion
LC-PUFA analysis from dried umbilical blood stored at −28 °C should be performed within a week, major losses occur afterwards. However, fatty acids obtained from dried blood maintain their 13C enrichment value up to 6 months and thus these samples are suitable for tracer studies even in the tiniest infants.
Comparison of dried blood spot results with those from fresh blood is an important tool to assess suitability of dried blood for analysis of FA composition. Our study shows no differences in FA composition between dried blood and fresh whole blood, these data confirm the findings reported previously by Gupta et al. [22]. We decided to use dried blood spots because degradation processes are reduced once the spots are fully dried, while in whole blood samples the enzymatic and chemical processes can occur even once the sample is defrosted due to the higher water content [23].
FA analysis from dried blood has been previously reported [7, 24] but these studies did not look at the long time storage stability. Our study provides new information on the rate of FA degradation, especially of LC-PUFA, in dried blood samples stored at −28 °C. Our results indicate that a decrease in major LC-PUFA was evident even after 1 week of storage and became statistically significant after 1 month. Decreases in individual fatty acids from baseline to month 1 were 9 % for ARA, 29 % for EPA and 20 % for DHA (Fig. 1).
Several studies have been conducted to assess the impact of the storage temperature on fingertip whole blood PUFA degradation. Marangoni et al. found that PUFA seem to be stable over a 3 week storage period at 4 °C. These results are not in agreement with those found in this paper and this is mainly due to the difference in the storage temperature (4 vs −28 °C) [12]. This was consistent with a recent report [25], in which degradation of long chain unsaturated fatty acid was observed when whole blood on chromatographic paper was stored at −20 °C for up to 180 days.
Moreover Marangoni et al. used a Schleicher Schull chromatography paper whereas we used an Ahlstrom product. Unfortunately we cannot compare the FA profile obtained from Schleicher Schull from that obtained from Ahlstrom paper because the first one is no longer available on the market. Schleicher Schull was merged by Whatman Ltd. In Europe filter papers used in most newborn screening are Whatman 903 and Ahlstrom 226. The UK uses Ahlstrom 226 in newborn bloodspot screening [23]. In 2008 the center for Disease Control and Prevention (CDC) of Atlanta compared two filter papers (Ahlstrom grade 226 and Whatman grade 903 Whatman Ltd., Sanford, ME, USA) by analyzing a large array of newborn screening analytes. Data supported that the performances of filter paper grades (226 and 903) were essentially equivalent [26].
Metherel et al. [27] evaluated the effect of increasing the BHT amount on FA degradation. They found that EPA + DHA decreased significantly after 14 days with 2.5 mg/mL BHT, and after 21 days with 5.0 mg/mL BHT. The storage of the dried blood spots in sealed tubes provided additional protection against PUFA degradation and extended the storage stability to at least 8 weeks. The BHT concentration used in our protocol was 0.5 mg/mL (0.05 %) as described by Ichihara [20]. Furthermore, we stored our samples in sealed test tubes at −28 °C. Our results are quite disappointing in terms of PUFA stability and can lead us to conclude that storage of blood samples at −28 °C is the most detrimental to PUFA stability and we wonder if higher concentration of BHT could improve the stability providing better protection against PUFA degradation.
It is interesting to note the high intra-individual variability in PUFA degradation during the storage period (Fig. 1). This difference could be due to the levels of antioxidant, such as α-tocopherol and antioxidative enzymes, present in red blood cells [28]. Blood PUFA degradation observed at temperature below −80 °C appears to be due to iron-mediated peroxidation pathways triggered by the release of iron during hemolysis. Attempts have been made to prevent PUFA from degradation. Storage of blood spot sample at room temperature with a combination of an antioxidant (BHT), a chelating agent (EDTA) and a support that decreases the water activity has been shown to prevent PUFA loss for up to 4 weeks of storage [13]. Furthermore in a recent article Metherel et al. demonstrated that PUFA degradation during storage of dried blood spots at −20 °C may be prevented by using a cryoprotectants, such as glycerol, and by pretreating the chromatographic paper with an iron-chelating agent [29].
The aim of the second part of this study was to monitor the effect of long time storage on the isotope ratio of individual FA at natural abundance level. No significant changes in 13C enrichment were seen with prolonged storage (Fig. 2), as no isotope fractionation occurs during the degradative process. This result is very reassuring as we have obtained important kinetic data on LC-PUFA metabolism from isotopic analysis at the natural abundance level [4]. This preliminary study supports the feasibility of studying lipid metabolism using the small amount of capillary blood already gathered from fingertips or from the heel, for example during the newborn blood-spot test.
We decided to gain experience on the FA analysis from dried blood as this is a simple approach analysis and it is easy to perform. We believe that this method is non-invasive, cost saving and it can be applied to the FA analysis of larger numbers of infants. However, the quantitative analysis must be performed within 1 week. In our view, measuring the samples after 7 days under the experimental conditions described above associates with an unacceptable loss of PUFA. An improved approach to preserve long chain PUFA from oxidation should be developed. We were reassured by the very good stability of the stable isotope content of the major fatty acids including the LC-PUFA ones. The information obtained in this project will allow us to use dried blood collected for epidemiological studies on long chain fatty acid endogenous biosynthesis base on the natural abundance approach.
Abbreviations
- ARA:
-
Arachidonic acid
- BHT:
-
Butylated hydroxytoluene
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- FA:
-
Fatty acid
- PUFA:
-
Polyunsaturated fatty acid
References
Spector AA (1999) Essentiality of fatty acids. Lipids 34(Suppl):S1–3
Koletzko B (1992) Fats for brains. Eur J Clin Nutr 46 (Suppl 1):S51–62
Innis SM (2007) Dietary (n-3) fatty acids and brain development. J Nutr 137:855–859
Carnielli VP, Simonato M, Verlato G, Luijendijk I, De Curtis M, Sauer PJ, Cogo PE (2007) Synthesis of long-chain polyunsaturated fatty acids in preterm newborns fed formula with long-chain polyunsaturated fatty acids. Am J Clin Nutr 86:1323–1330
Lapillonne A, Picaud JC, Chirouze V, Goudable J, Reygrobellet B, Claris O, Salle BL (2000) The use of low-EPA fish oil for long-chain polyunsaturated fatty acid supplementation of preterm infants. Pediatr Res 48:835–841
Eyles DW, Morley R, Anderson C, Ko P, Burne T, Permezel M, Mortensen PB, Norgaard-Pedersen B, Hougaard DM, McGrath JJ (2010) The utility of neonatal dried blood spots for the assessment of neonatal vitamin D status. Paediatr Perinat Epidemiol 24:303–308
Rise P, Volpi S, Colombo C, Padoan RF, D’Orazio C, Ghezzi S, Melotti P, Bennato V, Agostoni C, Assael BM, Galli C (2010) Whole blood fatty acid analysis with micromethod in cystic fibrosis and pulmonary disease. J Cystic Fibrosis: Official J Eur Cystic Fibrosis Soc 9:228–233
Makrides M, Gibson RA, McPhee AJ, Collins CT, Davis PG, Doyle LW, Simmer K, Colditz PB, Morris S, Smithers LG, Willson K, Ryan P (2009) Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: a randomized controlled trial. JAMA 301:175–182
Hoffman DR, Birch EE, Birch DG, Uauy R (1999) Fatty acid profile of buccal cheek cell phospholipids as an index for dietary intake of docosahexaenoic acid in preterm infants. Lipids 34:337–342
Koletzko B, Knoppke B, von Schenck U, Demmelmair H, Damli A (1999) Noninvasive assessment of essential fatty acid status in preterm infants by buccal mucosal cell phospholipid analysis. J Pediatr Gastroenterol Nutr 29:467–474
Lapillonne A, DeMar JC, Nannegari V, Heird WC (2002) The fatty acid profile of buccal cheek cell phospholipids is a noninvasive marker of long-chain polyunsaturated fatty acid status in piglets. J Nutr 132:2319–2323
Marangoni F, Colombo C, Galli C (2004) A method for the direct evaluation of the fatty acid status in a drop of blood from a fingertip in humans: applicability to nutritional and epidemiological studies. Anal Biochem 326:267–272
Liu G, Muhlhausler BS, Gibson RA (2014) A method for long term stabilisation of long chain polyunsaturated fatty acids in dried blood spots and its clinical application. Prostaglandins Leukot Essent Fatty Acids 91:251–260
Bailey-Hall E, Nelson EB, Ryan AS (2008) Validation of a rapid measure of blood PUFA levels in humans. Lipids 43:181–186
Bell JG, Mackinlay EE, Dick JR, Younger I, Lands B, Gilhooly T (2011) Using a fingertip whole blood sample for rapid fatty acid measurement: method validation and correlation with erythrocyte polar lipid compositions in UK subjects. Brit J Nutr 106:1408–1415
Min Y, Ghebremeskel K, Geppert J, Khalil F (2011) Effect of storage temperature and length on fatty acid composition of fingertip blood collected on filter paper. Prostaglandins Leukot Essent Fatty Acids 84:13–18
Rise P, Eligini S, Ghezzi S, Colli S, Galli C (2007) Fatty acid composition of plasma, blood cells and whole blood: relevance for the assessment of the fatty acid status in humans. Prostaglandins Leukot Essent Fatty Acids 76:363–369
Lin YH, Hanson JA, Strandjord SE, Salem NM, Dretsch MN, Haub MD, Hibbeln JR (2014) Fast transmethylation of total lipids in dried blood by microwave irradiation and its application to a population study. Lipids 49:839–851
Ryan AS, Nelson EB (2008) Assessing the effect of docosahexaenoic acid on cognitive functions in healthy, preschool children: a randomized, placebo-controlled, double-blind study. Clin Pediatr 47:355–362
Ichihara K, Waku K, Yamaguchi C, Saito K, Shibahara A, Miyatani S, Yamamoto K (2002) A convenient method for determination of the C20-22 PUFA composition of glycerolipids in blood and breast milk. Lipids 37:523–526
Craig H (1957) Isotopic standards for carbon and oxygen and correction factors for mass-spectrometric analysis of carbon dioxide. Geochim Cosmochim Acta 12:133–149
Gupta R, Abraham RA, Dhatwalia S, Ramakrishnan L, Prabhakaran D, Reddy KS (2009) Use of dried blood for measurement of trans fatty acids. Nutr J 8:35
Koulman A, Prentice P, Wong MCY, Matthews L, Bond NJ, Eiden M, Griffin JL, Dunger DB (2014) The development and validation of a fast and robust dried blood spot based lipid profiling method to study infant metabolism. Metabolomics 10:1018–1025
Metherel AH, Armstrong JM, Patterson AC, Stark KD (2009) Assessment of blood measures of n-3 polyunsaturated fatty acids with acute fish oil supplementation and washout in men and women. Prostaglandins Leukot Essent Fatty Acids 81:23–29
Metherel AH, Aristizabal Henao JJ, Stark KD (2013) EPA and DHA levels in whole blood decrease more rapidly when stored at −20 °C as compared with room temperature, 4 and −75 °C. Lipids 48:1079–1091
Centre for Disease Control and Prevention (2009) Filter paper comparison study. http://www.cdc.gov/labstandards/pdf/nsqap/nsqap_FilterPaperStudy51809.pdf. Accessed May 2009
Metherel AH, Hogg RC, Buzikievich LM, Stark KD (2013) Butylated hydroxytoluene can protect polyunsaturated fatty acids in dried blood spots from degradation for up to 8 weeks at room temperature. Lipids Health Dis 12:22
Magnusardottir AR, Skuladottir GV (2006) Effects of storage time and added antioxidant on fatty acid composition of red blood cells at −20 °C. Lipids 41:401–404
Metherel AH, Stark KD (2015) Cryopreservation prevents iron-initiated highly unsaturated fatty acid loss during storage of human blood on chromatography paper at −20 °C. J Nutr 145:654–660
Acknowledgments
The authors are grateful to the infants’ parents and to the NICU staff.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
There are no conflicts of interest to declare.
About this article
Cite this article
Pupillo, D., Simonato, M., Cogo, P.E. et al. Short-Term Stability of Whole Blood Polyunsaturated Fatty Acid Content on Filter Paper During Storage at −28 °C. Lipids 51, 193–198 (2016). https://doi.org/10.1007/s11745-015-4111-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-015-4111-z