Abstract
For those individuals who are unable to consume adequate long chain omega-3 fatty acids (LCn3) from dietary sources, fish oil supplementation is an attractive alternative Pre-emulsified fish oil supplements, an alternative to capsular triacylglycerol, may enhance the uptake of LCn3 fatty acids it contains. A randomized, Latin-square crossover design was used to compare the effects of four fish oil supplement preparations (Emulsions S, B and N) on phospholipid fatty acid (PLFA) concentrations in ten healthy volunteers compared to oil capsules over 48 h after a single dose and chylomicron fatty acid (CMFA) was evaluated over 8 h. Blood samples were collected at 0, 2, 4, 8, 24 and 48 h and fatty acid concentrations of PLFA and CMFA were determined by gas chromatography and the integrated area under the curve over 40 h (iAUC0–48) was determined. Emulsion S and Emulsion N promoted increased uptake of EPA into PLFA over 48 h when evaluating by iAUC0–48 or individual time points of assessment. No differences were observed between supplements in the CMFA concentrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The long chain n-3 (LCn3) fatty acids eicosapentaenoic acid (20:5n3, EPA) and docosahexaenoic acid (22:6n3, DHA) are provided by a diet high in fish and shellfish. Adequate levels of intake are associated with reduced risk reduction for cardiovascular and other disease states [1–6]. Current consumption levels in the US are well below recommended intake levels [7]. For those individuals who are unable to consume adequate LCn3 from dietary sources, fish oil supplementation is an attractive alternative.
The majority of fish oil supplements are available as capsular triacylglycerol but flavored emulsified lipid preparations have become available to provide an alternate approach to fish oil delivery. Pre-emulsification of fish oil supplements may enhance the uptake of the LCn3 fatty acids that they contain. Emulsification increases the lipid surface area thereby allowing enhanced digestion of the triacylglycerol by lingual and gastric lipase [8, 9]. Emulsified fish oil supplements are available as oil suspended in water (o/w) or water suspended in oil (w/o) with or without the inclusion of emulsifying agents [9]. When emulsified, the lipid droplet form and size as well as the chemical composition of the oil and water interface may directly affect the kinetics of lipid digestion and absorption [10, 11]. Supplements containing emulsified fish oil have added flavoring and may therefore have some taste advantage affecting acceptance.
The goal of the present study was to compare the absorption over 48 h of three emulsified fish oils and a capsular triacylglycerol fish oil supplement. We hypothesized that emulsified fish oils would promote enhanced uptake of LCn3 fatty acids into CMFA and PLFA after intake of one dose. The bioavailability of fish oil is generally evaluated by assessing either plasma phospholipid fatty acids (PLFA) or erythrocyte fatty acids after intake. As chylomicrons are responsible for the uptake of fatty acids directly from the gut to the periphery [12, 13] they were also examined in this investigation. A randomized, Latin-square crossover design was used to compare the effects of four fish oil supplement preparations on PLFA and chylomicron triacylglycerol fatty acid (CMFA) concentrations in ten healthy volunteers after a single dose of supplement. Reported here are the PLFA responses over 48 h and the CMFA responses over 8 h.
Methods
Experimental Protocol
This randomized controlled study was conducted at the USDA, ARS, Grand Forks Human Nutrition Research Center (GFHNRC), Grand Forks, ND. Approval for the study was obtained from the Institutional Review Board of the University of North Dakota. Informed consent was obtained from all study participants prior to initiation of the study. The study was registered at www.clinicaltrials.gov as NCT01488747.
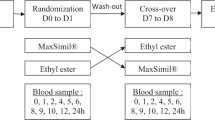
A randomized, Latin-square crossover design was used to compare the effects of four fish oil supplement preparations on PLFA and CMFA concentrations in ten healthy volunteers over 48 h after a single dose of supplement. For each supplement being tested, participants provided a fasting baseline blood sample at times zero after an overnight fast of ≥10 h after which they received a single dose of the assigned treatment with a low-fat breakfast (<10 % en). Additional blood samples were obtained at 2, 4, and 8 h. Participants were fed a low fat meal (<10 % en) at 6 h. Following the 8 h blood draw, participants were able to leave the research center and then return for both a 24 and 48 h fasting blood sample collection. During that time they were asked to consume a low fat diet devoid of any LCn3 food sources with detailed instructions provided by a dietitian. A 1–2 week wash out period was completed before the following treatment. This process was followed until all four treatments were completed.
Study Participants
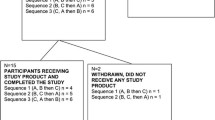
Healthy volunteers were recruited from the Greater Grand Forks area for study participation. Inclusion criteria for participation included an age of 18–60 years, a body mass index (BMI) between 22 and 32 kg/m2, and a medical history demonstrating good health. Interested volunteers applied via an online application (http://www.ars.usda.gov/pa/gfhnrc) which screened for overall eligibility (age, body weight, use of medications). Potential participants who passed the online screening were contacted to attend a screening visit at the Grand Forks Human Nutrition Center (GFHNRC) for further eligibility evaluation by the completion of a health questionnaire, assessment of medication/supplement use, and determination of dietary or supplemental intake n-3 fatty acids. Volunteers were excluded if they had an active, uncontrolled medical condition, used any omega-3 supplement (fish and flax oils or flaxseed) within the past 6 months, were talking any lipid lowering medications, or reported the consumption of >1 fish meal per week. Figure 1 illustrates the flow of participants through the trial from initial screening through study completion. Ten healthy women (n = 7) and men (n = 3) with a mean age of 34.0 ± 4.4 years and BMI of 26.1 ± 1.0 kg/m2 were included in the trial.
Supplement Treatments
The four supplements used in the trial were commercially available fish oil supplements including one capsular triacylglycerol (oil capsules; Nordic Naturals), and three emulsified products [Emulsion N (Coromega Nectar), Emulsion S (Coromega Omega-3 Squeeze), and Emulsion B (Barlean’s Omega Swirl)]. Emulsion S is an oil in water emulsion with pasteurized egg yolk as the emulsifying agent. Emulsion N and Emulsion B are gel emulsions in which oil is dispersed in a gel suspension (xanthine gum, gum Arabic and/or guar gum) in water.
The fatty acid content of the doses provided to participants is shown in Table 1. Dosing was based on our previous work [14] and supplements were matched on the content of EPA. The oil capsules and Emulsions N and S contained comparable quantities of DHA while Emulsion B contained ~50 % more.
Laboratory Methods
Plasma Fatty Acids
Blood samples were collected by venipuncture at 0, 2, 4, 8, 24 and 48 h in tubes containing EDTA. Plasma was prepared by low-speed centrifugation at 4 °C. Plasma samples were stored at −80 °C until analyzed. Immediately after sample collection, chylomicrons were isolated by centrifugal flotation through d = 1.006 saline by centrifugation [17]. Chylomicron samples were stored at −80 °C until analyzed.
Phospholipid fatty acid analysis was performed by gas chromatography (Shimadzu GC-2010, Shimadzu Medical Systems, Torrence, CA) as previously described [15]. Lipids were extracted from the plasma samples using chloroform:methanol (2:1, by vol.) according to the method of Folch et al. [16]. A known amount of internal standard (phosphatidyl choline di-heptadecanoic acid) was added to each sample prior to extraction to quantitate recovery and plasma lipid concentration. Phospholipids were separated from neutral lipids by thin-layer chromatography by developing total lipid spotted silica gel plates in petroleum ether, diethyl ether, acetic acid (80/20/1). Lipid bands were identified under ultraviolet light after being sprayed with 0.1 % dichlorofluorescein in methanol. Fatty acid methyl esters of the lipid classes were formed by transesterification with boron trifluoride (12 %) in excess methanol (Supelco, Bellefonte, PA). All results are presented as μg/mL of fatty acids.
Chylomicron fractions were directly esterified with boron trifluoride (12 %) in excess methanol (Supelco, Bellefonte, PA) and fatty acids were determined by gas chromatography as above. All results are presented as μg/mL of fatty acids.
Supplement Fatty Acid Anaylsis
The method of Folch et al. [16] was used to isolate the fish oil component of each supplement. Fish oil samples with nonadecanoic acid internal standard were esterified in 2 mL of 5 % acetylchloride solution in anhydrous methanol for 2 h at 80 °C [17]. The reaction was quenched with 0.5 mL of saturated sodium bicarbonate solution and fatty acid methyl esters were extracted into hexane for analysis. Samples were prepared and analyzed in triplicate; fatty acid content was normalized to the mass of supplement.
Samples were analyzed by gas chromatography (Thermo Trace-1310, Thermo Fisher Scientific, Waltham, MA, USA) with a Supelco SP-2560 capillary column (75 m, 0.18 mm ID, 0.14 µm film thickness. Samples were injected into a split/splitless injector held at 250 °C with a column flow of 2 mL/min and running a 100:1 split. The oven temperature profile was adapted from Masood et al. [18]: 150 °C held for 0.25 min, ramped at 35 °C/min to 200 °C, then ramped at 8 °C/min to 225 °C, held at 225 °C for 3.20 min followed by a 80 °C/min ramp to 245 °C, where the oven was held for 4 min. Analytes were detected using a flame ionization detector held at 250 °C.
Statistical Analysis
Sample size was estimated utilizing data from a previous bioavailability study by the investigators [14]. Area under the curve (AUC) data for EPA integrated over 48 h for treatment emulsified and capsular triacylglycerol fish oils were, respectively, 26.9 ± 8.9 and 15.1 ± 5.2 % h (mean ± SD) with a within-subject SD = 5.2. Using a within-subject SD of 6 and α = 0.05, a crossover trial which includes four treatments required ten subjects to achieve >85 % power to detect a difference in EPA AUC of 10 % h between the capsular fish oil and one of the three emulsified fish oils. We therefore included ten volunteer participants in this trial.
For plasma phospholipid and chylomicron fatty acids area under the curve (AUC) from time 0 through 8, 24 or 48 h (AUC0–8 h, AUC0–24 h, AUC0–48 h) was calculated for each subject for each treatment for EPA, DHA, total n-3 and the n-6:n-3 ratio. In addition, the incremental area under the curve for 0–48 h (iAUC0–48) was calculated by correcting for baseline (time 0) values.
The mixed-models procedure (Proc MIXED) in SAS was used to test for effects of the four treatments on the changes from baseline and the AUCs. Subjects were considered random effects and treatment was the fixed effect. Tukey contrasts were used for post hoc comparisons of the four treatment groups. Data are presented as percentage, mean ± SEM, or AUC0–8 h, AUC0–24 h, and AUC0–48 h. All statistical analyses were performed using SAS Version 9.4 (SAS Institute, Inc., Cary, NC). A p value of <0.05 was considered statistically significant.
Results
Plasma Phospholipid Fatty Acids
Plasma PLFA content of participants (µg/mL; mean ± SEM) at baseline for emulsified and capsular fish oil supplement treatments are presented in Table 2. No statistically significant differences were observed in baseline PLFA levels between treatments demonstrating that participants returned to baseline levels before each treatment was administered.
Changes from baseline (µg/mL ± SEM) at each time point over 48 h for plasma PLFA content of EPA, DHA, Total n-3, and n-6:n3 is illustrated in Fig. 2. Significantly increased levels of EPA were observed with Emulsion B > oil capsules at 8 h; Emulsion B > Emulsion S and Emulsions N and S > oil capsules at 24 h; and Emulsion B > Emulsions S and N at 48 h. Interestingly, although Emulsion B contained 50 % more DHA, there is no difference in the PLFA levels at any time point. By 48 h, there were no significant differences in EPA, DHA, total n-3 or the n-6:n-3.
Change from baseline concentration (µg/mL; mean ± SEM) of plasma phospholipid EPA (a), DHA (b), Total n-3 (c) and n-6:n-3 (d) over 48 h in healthy adults (n = 10) receiving a capsular or emulsified fish oil supplement at time 0. Emulsion B contains a larger amount of DHA; treatments were matched on EPA; *p < 0.05
Table 3 includes the mean ± SEM, AUC (µg/mL h), concentration maximum [C max (µg/mL)], and time to maximum [t max (h)] for plasma phospholipid fatty acids at 8, 24 and 48 h after intake of supplements for. No differences were seen between treatments. The iAUC0–48 for EPA, DHA, Total n-3, and n-6:n3 are shown in Fig. 3. Emulsion S and Emulsion N have significantly increased iAUC0–48 for EPA compared to oil capsules and Emulsion B (p < 0.05).
iAUC0–48 h. change from baseline concentration (µg/mL · h; mean ± SEM) of plasma phospholipid LA (a), ALA (b), EPA (c), DHA (d), Total n-3 (e), Total n-6 (f), and n-6:n-3 (g) over 48 h in healthy adults (n = 10) receiving a capsular or emulsified fish oil supplement at time 0. Emulsion B contains a larger amount of DHA; treatments were matched on EPA. Treatments not sharing a common letter differ significantly from one another (p < 0.05)
Plasma Chylomicrons
As illustrated in Table 4, plasma chylomicron EPA and DHA of participants were significantly different between Emulsion S and Emulsion B at baseline (p < 0.05). Figure 4 shows the change from baseline concentration (µg/mL, mean ± SEM) of plasma chylomicron EPA, DHA, Total n-3, and n-6:n-3. No statistically significant differences were observed at any time point. No statistically significant differences were seen in the iAUC0–48 for CMFA (data not shown).
Discussion
In this controlled, single treatment bioavailability study, we demonstrate differences in the PLFA EPA uptake over 48 h between encapsulated and preparations of emulsified fish oil. In our previous work we demonstrated similar changes comparing one emulsified fish oil preparation to encapsulated oil [14]. Here we see significantly increased PLFA EPA in Emulsion S and Emulsion N (p < 0.05) compared to oil capsules and Emulsion B over 48 h.
The emulsification of fish oil is thought to increase both the rate and extent of absorption of the n-3 fatty acids it contains [8, 9]. We found no differences in the CMFA n-3 content over 48 h after supplement intake suggesting similar uptake of fatty acids in the encapsulated and emulsified fish oils. Contrary to our results, Garaiova et al. found both more rapid and enhanced postprandial levels of DHA and EPA in plasma triacylglycerol (TAG) [8]. It is important to note that the dose of total lipid may be responsible for the discrepant responses as the study by Garaiova provided a dose of 30 ml of lipid while our doses ranged between 4 and 17.48 g of product, depending on formulation. Others have also shown reduced CMFA uptake after n-3 fatty acid supplementation [19]. It is thought that this may be related to the total reduction in plasma TAG after fish oil intake [20, 21]. As we have no control oil treatment without n-3 fatty acids in this trial, it is impossible to determine if the single dose supplement resulted in comparatively reduced CMFA uptake.
Although both Emulsion N and Emulsion B are gel emulsions, there was significantly increased uptake of EPA into PLFA with Emulsion N. The exact formulation method of each of the emulsified products tested is proprietary and therefore unavailable; however, formulation differences may play a role in the observed outcomes. It is of interest that although the DHA content of Emulsion B was 50 % higher than the other product’s (see Table 1), there were no differences seen in the PLFA content of DHA by treatment.
After absorption, EPA and DHA are taken up in PLFA as well as triacylglycerol, cholesterol ester, and as free fatty acid with the observed response being both time and dose dependent [22, 23]. As demonstrated through stable-isotope labeling studies with oral administration of 13C-DHA, the compartmentalization of DHA in phospholipids reaches its maximum between 6 and 9 h after dosing [24]. Additionally, supplementation trials evaluating the disposition of EPA and DHA in various blood fractions and tissues demonstrate a more rapid rise of EPA concentration in all plasma lipid fractions [25–27] and tissue [26] compared to DHA. This same increased uptake of EPA into PLFA was observed in our salmon feeding trial [28]. The EPA PLFA data presented above mirrors this phenomenon likely due to the equal dosage of EPA across all study groups. In addition to enriching other lipid pools, DHA supplementation increases the amount of DHA used for β-oxidation [29], indicating that the relative insensitivity to the increased DHA dosage in Emulsion B relative to the other supplements may be due to other DHA homeostasis pathways.
In this study doses of oil from oil capsules, Emulsion S and Emulsion N had a lower content of DHA than EPA and only changes in PLFA were measured, thus it is possible that the total change in plasma DHA content was not detected. It is of interest that the DHA response of Emulsion B was not significantly different from the other supplements even though it contained more DHA, this may be partially explained by the more rapid rise in EPA concentrations after supplementation as discussed above. The chemical composition of the emulsifying agent affects the stability of the emulsion [9] which, may affect the absorbability of fatty acids; however, at 48 h there was no difference in the PLFA level of the fatty acids measured indicating any effects on differential uptake on the PLFA pool would not be significant with continued supplementation.
The focus of this study was the acute bioavailability of the LCn3 in the fish oil supplements tested, so we are unable to make any inferences about the effect longer term intake of these supplements would have on LCn3 concentration in various blood fractions. Chronic supplement intake differs from a single dose as circulating fatty acid levels then represent much more extensive lipid metabolism [30]. In an early study, Katan et al. demonstrated the kinetics of the incorporation of supplemental fish oil into plasma cholesteryl esters; a half-life of 4.8 days was observed for EPA on daily doses of 3–9 g of fish oil while the half-life of DHA was significantly longer than for EPA, at 10.3 days [26].
There are several potential limitations to our study. The sample size of the intervention was small, but as this was a cross-over design the power was adequate to differentiate treatment differences. As we did not supply all of the food to participants over the 48 h. test period, it is possible that directions to comply with a very low fat diet (<10 % en) were not strictly adhered to. This has the potential to confound our primary outcome of PLFA. However, as the n-3 content of all participants was increased and the n-6 content reduced, clear treatment effects were observed.
Strengths of our study include that the supplement intake was well controlled and that all participants assigned to treatment completed all aspects of the trial. Participants reported high compliance to the assigned low fat diet.
In summary, Emulsion S and Emulsion N promoted increased uptake of EPA into PLFA over 48 h when evaluating by iAUC0–48 h or individual time points of assessment. No differences were observed between supplements in the CMFA concentrations. Although there were no observable differences in CMFA uptake, n-3 fatty acids were well absorbed and incorporated into PLFA. In practice, these results indicate that although there is limited advantage of emulsified fish oil preparations in the extent of EPA uptake, it is evident that both capsular triacylglycerol and emulsified fish oil preparations are readily absorbed and incorporated into PLFA.
Abbreviations
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- PLFA:
-
Phospholipid fatty acid
- CMFA:
-
Chylomicron fatty acids
- LCn3:
-
Long chain omega-3 fatty acids
References
Raatz SK, Silverstein JT, Jahns L, Picklo MJ (2013) Issues of fish consumption for cardiovascular disease risk reduction. Nutrients 5:1081–1097
MacLean CH, Newberry SJ, Mojica WA, Khanna P, Issa AM, Suttorp MJ, Lim Y-W, Traina SB, Hilton L, Garland R (2006) Effects of omega-3 fatty acids on cancer risk: a systematic review. J Am Med Assoc 295:403–415
Ruxton C, Reed SC, Simpson M, Millington K (2004) The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J Hum Nutr Diet 17:449–459
Yurko-Mauro K (2010) Cognitive and cardiovascular benefits of docosahexaenoic acid in aging and cognitive decline. Curr Alzheimer Res 7:190–196
Nettleton JA, Katz R (2005) n-3 long-chain polyunsaturated fatty acids in type 2 diabetes: a review. J Am Diet Assoc 105:428–440
Harris WS (2004) Fish oil supplementation: evidence for health benefits. Clevel Clin J Med 71:208–210
Kris-Etherton PM, Harris WS, Appel LJ (2003) Omega-3 fatty acids and cardiovascular disease: new recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol 23:151–152
Garaiova I, Guschina IA, Plummer SF, Tang J, Wang D, Plummer NT (2007) A randomised cross-over trial in healthy adults indicating improved absorption of omega-3 fatty acids by pre-emulsification. Nutr J 6:4
Please update the Reference [9]
Michalski MC, Genot C, Gayet C, Lopez C, Fine F, Joffre F, Vendeuvre JL, Bouvier J, Chardigny JM, Raynal-Ljutovac K (2013) Multiscale structures of lipids in foods as parameters affecting fatty acid bioavailability and lipid metabolism. Prog Lipid Res 52:354–373
Fave GCT, Armand M (2004) Physicochemical properties of lipids: new strategies to manage fatty acid bioavailability. Cell Mol Biol 50:815–831
Kindel T, Lee DM, Tso P (2010) The mechanism of the formation and secretion of chylomicrons. Atheroscler Suppl 11:11–16
Schuchardt JP, Hahn A (2013) Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot Essent Fat Acids 89:1–8
Raatz SK, Redmon JB, Wimmergren N, Donadio JV, Bibus DM (2009) Enhanced absorption of n-3 fatty acids from emulsified compared with encapsulated fish oil. J Am Diet Assoc 109:1076–1081
Raatz SK, Bibus D, Thomas W, Kris-Etherton P (2001) Total fat intake modifies plasma fatty acid composition in humans. J Nutr 131:231–234
Folch J, Lees M, Sloane-Stanley G (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Lepage G, Roy CC (1986) Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 27:114–120
Masood A, Stark KD, Salem N (2005) A simplified and efficient method for the analysis of fatty acid methyl esters suitable for large clinical studies. J Lipid Res 46:2299–2305
Harris W, Windsor S (1991) N-3 fatty acid supplements reduce chylomicron levels in healthy volunteers. J Appl Nutr 43:5–15
Desager J-P, Dricot J, Harvengt C (1989) Hypotriglyceridemic action of omega-3 fatty acids in healthy subjects does not occur by enhanced lipoprotein lipase and hepatic lipase activities. Res Commun Chem Pathol Pharmacol 65:269–272
Harris WS, Hustvedt B-E, Hagen E, Green MH, Lu G, Drevon C (1997) N-3 fatty acids and chylomicron metabolism in the rat. J Lipid Res 38:503–515
Fekete K, Marosvölgyi T, Jakobik V, Decsi T (2009) Methods of assessment of n-3 long-chain polyunsaturated fatty acid status in humans: a systematic review. Am J Clin Nutr 89:2070S–2084S
Browning LM, Walker CG, Mander AP, West AL, Madden J, Gambell JM, Young S, Wang L, Jebb SA, Calder PC (2012) Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am J Clin Nutr 96:748–758
Lemaitre-Delaunay D, Pachiaudi C, Laville M, Pousin J, Armstrong M, Lagarde M (1999) Blood compartmental metabolism of docosahexaenoic acid (DHA) in humans after ingestion of a single dose of [13C] DHA in phosphatidylcholine. J Lipid Res 40:1867–1874
Hodge J, Sanders K, Sinclair AJ (1993) Differential utilization of eicosapentaenoic acid and docosahexaenoic acid in human plasma. Lipids 28:525–531
Katan M, Deslypere J, van Birgelen A, Penders M, Zegwaard M (1997) Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res 38:2012–2022
Metherel A, Armstrong J, Patterson A, Stark K (2009) Assessment of blood measures of n-3 polyunsaturated fatty acids with acute fish oil supplementation and washout in men and women. Prostaglandins Leukot Essent Fat Acids 81:23–29
Raatz SK, Rosenberger TA, Johnson LK, Wolters WW, Burr GS, Picklo MJ Sr (2013) Dose-dependent consumption of farmed Atlantic Salmon (Salmo salar) increases Plasma Phospholipid n-3 fatty acids differentially. J Acad Nutr Diet 113:282–287
Plourde M, Chouinard-Watkins R, Rioux-Perreault C, Fortier M, Dang MTM, Allard M-J, Tremblay-Mercier J, Zhang Y, Lawrence P, Vohl M-C (2014) Kinetics of 13C-DHA before and during fish-oil supplementation in healthy older individuals. Am J Clin Nutr 100:105–112
Von Schacky C, Fischer S, Weber PC (1985) Long-term effects of dietary marine omega-3 fatty acids upon plasma and cellular lipids, platelet function, and eicosanoid formation in humans. J Clin Invest 76:1626
Acknowledgments
We thank the study volunteers and the nursing and dietetics staff of the Grand Forks Human Nutrition Research Center. We thank Coromega Co., Inc. for their kind donation of supplements. Funding for this work was provided by ARS, USDA 5450-51000-049-00D and a Grant from the Brian G. Dyson Foundation, Atlanta, GA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SKR, LKJ and MRB declare no conflicts of interest.
About this article
Cite this article
Raatz, S.K., Johnson, L.K. & Bukowski, M.R. Enhanced Bioavailability of EPA From Emulsified Fish Oil Preparations Versus Capsular Triacylglycerol. Lipids 51, 643–651 (2016). https://doi.org/10.1007/s11745-015-4100-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-015-4100-2