Abstract
Hyperplastic growth and hypertrophic growth within adipose tissue is tightly associated with cell cycle activity. In this study, CCNG2 and CDKN2C were found to be correlated with cell cycle inhibition during fat cell differentiation, whereas CCND3, CCNA1, and ANAPC5 were positively associated with cell cycle activity during fat cell proliferation after selection based on GEO datasets available on the NCBI website. The findings were validated through comparison of expressions of these genes among different tissues/fractions in broiler chickens and time points during primary cell culture using quantitative real-time PCR. Development of broiler subcutaneous adipose tissue was investigated on embryonic days 15 and 17 and on post-hatch days 0, 5, 11, and 33 using H&E staining and PCNA immunostaining with DAPI counter stain. In addition, mRNA expressions of five cell cycle regulators as well as precursor cell and adipocyte markers were measured at those time points. The results suggest that cellular proliferation activity decreased as the fat pad grows, but a population of precursor cells seemed to be maintained until post-hatch day 5 despite increasing differentiation activity. Hypertrophic growth gradually intensified despite a slight cessation on post-hatch day 0 due to increased energy expenditure during hatching and delayed food access. From post-hatch day 5 to day 11, most of the precursor cells may become differentiated. After post-hatch day 11, hyperplastic growth seemed to slow, while hypertrophic growth may become dominant. This study provides further understanding about broiler fat tissue development which is imperative for effective control of fat deposition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding development of adipose tissue is crucial for curing obesity in humans and controlling fat deposition in meat-producing animals to yield higher-quality meat with improved feed efficiency. By now, two processes of adipose tissue development—hypertrophy (enlargement of fat cell size) and hyperplasia (increase of fat cell number)—have been generally acknowledged. These two developmental mechanisms are associated with fat cell proliferation and differentiation, and ultimately controlling of the cell cycle [1]. Most studies concerning cellularity of fat cells have suggested that hyperplastic growth occurs before hypertrophy to establish immature fat cells for lipid deposition [2]. However, it is not yet clear the ways in which the two mechanisms are orchestrated at various time points.
Studies concerning fat cell size and number at specific ages or weights have been conducted in Athens-Canadian and commercial Cobb broiler chickens [3, 4]. Proliferation status was also examined by DNA mass and proliferating cell nuclear antigen (PCNA) immunostaining during the first 7 weeks following hatch [5]. A previous study in our lab also compared the hypertrophic and hyperplastic growth from embryonic day 12 to post-hatch day 1 between Leghorn and broiler chickens [6]. These studies indicated increasing fat cell size and fat cell number as the body weight increased, as well as decreasing rate of fat cell proliferation during post-hatch development. However, none of the studies examined both embryonic and post-hatch development of adipose tissue or investigated expression of cell cycle regulators during the development of adipose tissue.
In our previous study, we found three important cell cycle inhibitors—cyclin G2 (CCNG2), cyclin-dependent-kinase inhibitor 2C (CDKN2C), and peripheral myelin protein 22 (PMP22)—in pig adipose tissue by utilizing the Gene Expression Omnibus (GEO) database and data from quantitative real-time PCR (qPCR) and western blotting [7]. All three genes showed higher expression in mature fat cells than in stromal vascular cells, as well as increasing expression during adipocyte differentiation and development of adipose tissue, indicating a role in cell cycle inhibition during adipocyte differentiation. Therefore, the same study was conducted for these inhibitors in broiler chickens to help us characterize hyperplastic growth of broiler chicken adipose tissue. In response to the previous confirmation of high expression of cell cycle inhibitors in differentiated fat cells and increasing expression of cell cycle inhibitors during fat cell differentiation and adipose tissue development [7], we hypothesized that there should be some important cell cycle activators that are highly expressed in preadipocytes and that have decreased expression during fat cell differentiation and adipose tissue development. In order to test this hypothesis, we explored the GEO datasets (GDS) and conducted qPCR in broiler chickens. To the best of our knowledge, this is the first study that combines cellularity study and cell cycle regulator expression during the development of broiler chicken adipose tissue.
Materials and Methods
Data Sources and Processing
The data mining process using two microarray datasets (GEO DataSets: GDS596 and GDS 3142) was described in our previous report [7]. Briefly, mean expression values from six tissues (heart, liver, lung, muscle, kidney and adipose) were calculated, and genes were ranked in descending order based on the ratio between expression value in adipose tissue and mean expression value in the other five tissues. Finally, three cell cycle inhibitors with high ranks (CCNG2, CDKN2C, and PMP22) and three cell cycle activators (CCND3, CCNA1, and ANAPC5) with low ranks in both datasets were selected.
The expression levels of the six genes were then further compared among different differentiation stages of preadipocytes in culture by analyzing GDS2743 and GDS2366. GDS2743 was generated to compare gene expression between undifferentiated and differentiating primary white preadipocytes from epididymal white adipose tissue of mouse. GDS2366 was generated to compare gene expression between undifferentiated and differentiated preadipocytes from subcutaneous adipose tissues in human. To determine gene expression pattern of the six genes during differentiation of adipocytes in vitro, GDS2659 was also analyzed, as it records mRNA expression at preconfluence, confluence and 1, 3, 7, and 28 days after initiation of differentiation cocktail during differentiation of 3T3-L1 preadipocytes. The gene expression values were calculated as percentages compared to the maximum values in the GEO profiles.
Experimental Animals
Broiler eggs (Ross 708) were incubated and chickens were raised after hatching with free access to a broiler start diet (crude protein = 21.0 %, metabolizable energy = 3121 kcal/kg) provided by the Ohio Agricultural Research and Development Center (OARDC, Wooster, OH). The diet meets the NRC nutrient recommendations for chickens [8]. During this process, total subcutaneous adipose tissues on the two thighs were collected using forceps after removing the skin and feathers with scissors at embryonic days (E) 15 and 17 and post-hatch days (P) 1, 5, 11, and 33 (n = 5 for each age group) to study adipogenesis at different developmental stages. The fat pad location is shown in our previous paper [6]. At P21, subcutaneous and visceral adipose tissue at the abdomen, thigh and pectoral muscles, heart, lung, liver, and kidney were collected (n = 4 for each tissue) to investigate tissue distribution of gene expression. For post-hatch chicks, euthanasia was conducted through CO2 inhalation before tissue collection. All of the procedures were approved by The Ohio State University Institutional Animal Care and Use Committee. After tissue collection, all of the tissues were snap-frozen immediately in liquid nitrogen and stored at −80 °C for RNA isolation and quantitative real-time PCR (qPCR) [9, 10].
Histological Processing and Immunostaining
In order to quantify the change in fat cell size and number of proliferating cells in the adipose tissue, Hematoxylin and Eosin (H&E) staining, as well as immunostaining of proliferating cell nuclear antigen (PCNA) with counterstain of 4′,6-diamidino-2-phenylindole (DAPI), were conducted. Fat tissues from chickens of different age groups (n = 5 for each group) were embedded in paraffin and H&E slides were made by the Goss Histology Lab. (Histology and Immunohistochemistry Core, OSU College of Veterinary Medicine, Columbus, OH) after being fixed with 10 % neutral-buffered formalin for 24–48 h and dehydrated in 70 % ethanol. Sections (6 μm) were cut with a microtome and mounted on glass slides for PCNA and DAPI staining and were rehydrated with a series of ethanol treatments (100 %, 2 × 3 min; 95 %, 2 × 3 min; 70 %, 3 min and deionized water, 2 × 2 min) after paraffin was removed with xylene (3 × 5 min). After being equilibrated in Tris-buffered saline (TBS, pH 7.6; 3 × 10 min) and incubated in TBS containing 0.025 % Triton X-100 and 1 % nonfat dry milk for 30 min, tissue sections were then incubated overnight at 4 °C with a monoclonal PCNA antibody (PC10, Santa Cruz Biotechnology, Santa Cruz, CA) diluted in a milk solution at 1:500 ratio and rinsed 10 times by TBS with 0.025 % Triton X-100. Finally, the tissue sections were incubated for 30 min with Rhodamine conjugated goat anti-mouse secondary antibody at 1:500 dilution at room temperature and rinsed ten times with TBS containing 0.025 % Triton X-100. To visualize cell nuclei, sections were counterstained with 300nM DAPI (Invitrogen) in TBS for 10 min, and then rinsed ten times with TBS. After being dehydrated in a series of increasing alcohol concentrations (70 %, 2 min; 95 %, 2 × 2 min; 100 %, 2 × 3 min), sections were washed with xylene (2 × 3 min) and temporarily mounted with phosphate buffered saline (PBS). Stained slides were observed and imaged with a fluorescence microscope equipped with AxioCam MRc5 camera (Zeiss, Thornwood, NY). Average size of fat cells and percentage of PCNA positive nuclei were determined using ImageJ software (NIH ImageJ 1.47; http://imagej.nih.gov/ij/) from H&E stained sections and PCNA&DAPI stained sections, respectively. For H&E staining, 15 areas with 1000–2000 cells in total were randomly selected for each individual. The average cross sectional area (CSA) for each cell was calculated by dividing the total area by the total number of cells. For PCNA&DAPI staining, five sections were imaged for each individual, and the percentage of PCNA positive cells was obtained by dividing the number of cells stained by both DAPI and PCNA by the total number of cells stained by DAPI after the DAPI and PCNA images of the same section were merged.
Separation of Stromal-Vascular and Fat Cell Fraction
The stromal-vascular (SV) and adipocyte cell (FC) fractions were isolated from visceral adipose tissue at the abdomen of four 21-day-old chickens following the procedures described in our previous report [11, 12]. Briefly, the adipose tissues were minced with a razor blade after being washed with PBS and were incubated at 37 °C with 5 mL of digestion buffer per gram of tissue containing 3.2 mg/mL of collagenase II (Sigma-Aldrich, St. Louis, MO) for 1 h in a shaking water bath (180 rpm). The suspension was then passed through a nylon cell strainer (BD Falcon, Franklin Lakes, NJ) with 100-μm mesh pores to remove undigested tissue debris. The filtered cells were further separated into FC (top layer) and SV pellets after centrifugation at 200×g for 5 min. Finally, the two fractions were collected for primary cell culture, as well as RNA isolation and subsequent qPCR analysis.
Primary Stromal-Vascular Cell Culture
After collection of SV cells from four chickens, they were cultured according to a method described in our previous report [13]. The cells were first washed with Dulbecco’s modified Eagle’s medium (DMEM) before centrifugation at 500×g for 10 min at room temperature. After centrifugation, the pellet was re-suspended in DMEM medium containing 10 % fetal bovine serum (FBS) to a concentration of 6 × 106 cells/mL. Cells were seeded in 12-well plates at a density of 3 × 106 cells/mL in culture medium and maintained in a humidified incubator with 5 % CO2 at 37 °C until 50 % confluence. Then, the cell medium was changed to contain 10 % FBS, 50 mM linoleic and oleic acids, and 10 μg/mL of insulin in DMEM to induce adipocyte differentiation for 3 days. Cells were collected at day −1 (before differentiation), day 0 (differentiation initiation), and day 1, 2, and 3 (after differentiation) for RNA isolation and qPCR analysis.
cDNA Synthesis and Quantitative Real-time PCR
Tissue samples were homogenized in Trizol reagent (Invitrogen, Carlsbad, CA) with a Tissuemiser homogenizer (Fisher Scientific, Pittsburgh, PA) to isolate total RNA according to the manufacturer’s protocol. For primary cell culture, cells were removed from plates using Trizol (Invitrogen) and vortexed to isolate total RNA according to the manufacturer’s instructions [14]. After being assessed via gel electrophoresis and a Nanodrop machine (NanoDrop Technologies, Wilmington, DE, USA), 1 μg of total RNA was used in reverse transcription (RT) to generate cDNA with M-MLV reverse transcriptase (Moloney murine leukemia virus RT, Invitrogen). RT conditions for cDNA amplification were 65 °C for 5 min, 37 °C for 52 min, and 70 °C for 15 min. Gene expression of the six cell cycle regulators was quantified by SYBR Green real-time PCR with primers listed in Table 1. The primers with Tm around 61 °C were designed using Primer Express Software (Applied Biosystems) to span genomic introns (>1 Kb) to avoid amplification of possible genomic DNA contamination. The qPCR was performed on an ABI 7300 real-time PCR Instrument (Applied Biosystems, Foster City, CA). The PCR reaction consisted of 1 μL of cDNA, 0.5 μL of 10 mM deoxynucleoside triphosphate mix (dNTP), 2.5 μL of GeneAmp 10× PCR buffer II containing 100 mM Tris–HCl, PH 8.3 and 500 mM KCl, 2 μL of 25 mM MgCl2, 0.5 nM of each of the forward and reverse primers, 0.385 μL of 0.2 % Rox as reference dye, 0.034 μL of 0.2 % SYBR green as detection dye, 0.125 μL of AmpliTaq Gold polymerase (Applied Biosystems) and UltraPure DNase/RNase-free distilled water (Invitrogen) up to 25 μL. Efficient amplification of real time PCR was confirmed by slope calculation of a standard curve and gel electrophoresis. The standard curve slope was determined using serial dilutions (1:1, 1:2, 1:4, and 1:8) of each template cDNA sample. The optimized thermal cycling procedure was confirmed by a normal amplification curve, one major peak in melting point analysis, and a single target band in gel electrophoresis. Correct PCR product and product size were assessed through a melting curve provided by qPCR software (Applied Biosystems) and gel electrophoresis. The conditions of qPCR were 95 °C for 10 min, 40 cycles of 94 °C for 15 s, 60 °C for 40 s, 72 °C for 30 s, and 82 °C for 32 s. The relative level of expression of a target gene was calculated from the expression value determined by the ABI software using the comparative 2−ΔΔCt method for relative quantification [15]. The geNorm program of qbasePLUS software (Biogazelle, Zwijnaarde) was used to evaluate stability of four reference genes including ACTB, RPS13, OAZ1 and RPL27. Reference genes with average expression stability values lower than 1.5 were then selected for qPCR normalization. Finally, β-actin (gACTB) was selected as an internal control (housekeeping gene) for mRNA expression in different tissues and cell fractions and at different primary cell culture time points. Ribosomal protein S13 (gRPS13) was selected as an internal control for mRNA expression in different age groups [16]. For the tissue comparison, one single relative quantity (ΔCt) was used to reflect variation in multiple tissues. However, for comparisons within the same tissue, we selected the one with lowest expression value as a calibrator. Gene expressions in different groups after normalization of the housekeeping gene were then displayed as fold increases compared to the calibrator. In order to examine separation of the stromal-vascular and fat cell fractions, differentiation of primary cells in vitro and development of adipose tissue in vivo, expressions of two adipocyte markers, adipose triglyceride lipase (ATGL) and fatty acid binding protein (FABP4), and one precursor cell marker, delta-like 1 homolog (DLK1), were also measured by qPCR. Primer sequences of chicken RPS13 (GenBank: NM_001001783), ACTB (GenBank: NM_205518), ATGL (GenBank: EU852334), FABP4 (NM_204290), and DLK1 (EU288039) have been described in our previous reports [9, 17].
Statistical Analyses
For tissue distribution of gene expression, comparisons were performed by a mixed model (MIXED) procedure using SAS software (version 9.3, SAS Institute Inc., USA) with the DIFF option to detect significant differences between pairs of least squares means. Gene expressions between two groups were compared in SAS using the Student’s t-test. Multiple comparisons of gene expression at different time points during in vitro cell culture and in vivo development were carried out as one-way ANOVA followed by Fisher’s post hoc test. Differences with P values lower than 0.05 were treated as significant differences. All of the results are presented as least squares means with standard errors of the least squares means (SEM).
Results
Data Analysis Based on the Gene Expression Omnibus (GEO) Datasets
Using the same methodology and datasets as in our previous paper [7], three cell cycle activators were selected that had relatively low expression in the adipose tissue of both human and mouse (Table S1). The ranks of CCND3, CCNA1, and ANAPC5, were 14,570, 13,622, and 9835, respectively, in GDS596 (22,215 genes in total) and 32,066, 27,701, and 40,067, respectively, in GDS3142 (45,037 genes in total). Because the genes were ranked descendingly according to the ratio between gene expression in adipose tissue and that in the other five tissues, the low ranks of these genes are due to their low expression in adipose tissue. Although there is no significant difference in expression of CCNA1 among different tissues in mouse (Table S1), we still chose CCNA1 as one target gene because of its well-known involvement in the cell cycle and its decreased expression during fat cell differentiation in other microarray studies (Fig. S1).
As indicated by analysis of GDS2743 and GDS2366, contrary to the lower expression of the three cell cycle inhibitors—CCNG2, CDKN2C, and PMP22—in undifferentiated preadipocytes in these datasets [7], ANAPC5 showed higher expression in undifferentiated preadipocytes than in differentiating or differentiated preadipocytes in mouse (Fig. S1A) and human adipose tissue (Fig. S1B). The other two cell cycle activators also appeared to have higher expression in undifferentiated preadipocytes (Figs. S1A and 1B).
Consistent with the findings in the previous two datasets, expression of the three cell cycle inhibitors also showed an increasing trend, whereas that of the three cell cycle activators showed a decreasing trend during differentiation of 3T3-L1 preadipocytes in GDS2659 (Fig. S1C).
Cellularity of Fat Cells During Development of Chicken Adipose Tissue
Tissue collection data indicated that, as the broiler chickens grew, both body weight and leg fat pad weight expanded at an increasing rate. The percentage of fat pad weight relative to the body weight increased from E15 to D0 but kept decreasing from D0 to D33 (Table 2).
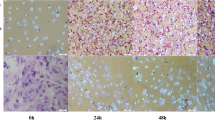
H&E staining of tissue sections of adipose tissue in different age groups of chickens showed a gradual increase in average cross-sectional area (CSA) of adipocytes from the embryonic stages to P11 with the greatest increase from E15 to E17 (121 μm2/cell/day, P < 0.001) and the smallest increase from P0 to P5 (20 μm2/cell/day). However, from P11 to P33, the average fat cell size did not change (Fig. 1).
Change of fat cell size during development of chicken adipose tissues. a Histological images of broiler leg subcutaneous adipose tissue on embryonic day 15 (E15) and day 17 (E17), post-hatch day 0 (P0), 5 (P5), 11 (P11), and 33 (P33) in 50× objective lens. Scale bar 100 μm. b Cross sectional area (CSA) of adipocytes in subcutaneous fat of broiler chickens on E15 and E17, P0, P5, P11, and P33 (n = 5 for each group). Each bar indicates mean ± SEM for one time point with 15 sections for each individual
PCNA immunostaining with counterstain of DAPI suggested that the proliferating cells gradually decreased during the growth of broiler chickens (Fig. 2a). The percentage of PCNA positive cells at E15, E17, P0, P5 and P11 were 70, 56, 40, 30, and 18 %, respectively. The decreases in the percentage of PCNA positive cells between E15 and E17, P0 and P5, and P11 and P33 were significant (P < 0.05) (Fig. 2b).
Quantification of proliferating cells during development of chicken adipose tissues. a Representative pictures of proliferating cell nuclear antigen (PCNA, red) immunostaining with nuclear counterstain of 4′,6-diamidino-2-phenylindole (DAPI, blue) on embryonic day 15 (E15) and day 17 (E17), post-hatch day 0 (P0), 5 (P5), 11 (P11), and 33 (P33) in 200× objective lens. Scale bar 50 μm. PCNA positive cells were counted as overlap of two color channels. b Percentage of PCNA positive cells in subcutaneous fat of broiler chickens on E15 and E17, P0, P5, P11, and P33 (n = 5 for each group). Each bar indicates mean ± SEM for one time point with at least five sections for each individual (color figure online)
Expression of Selected Genes in Various Tissues of Broiler Chickens
The qPCR data showed statistical differences in gene expression among various tissues including subcutaneous and visceral fat tissue, thigh and pectoral muscle, heart, lung, liver and kidney. Generally, all three cell cycle inhibitors tend to be highly expressed and all three cell cycle activators tend to be expressed at low levels, in both subcutaneous and visceral fat tissue at the abdomen. The three inhibitors showed lowest mRNA expression in the liver. However, highest expression of the three activators was not observed in the same tissues. CCND3 demonstrated the highest expression in thigh muscle and lung, and CCNA1 showed the highest expression in both thigh and pectoralis major muscle, whereas ANAPC5 exhibited the highest expression in the liver and kidney (Fig. S2).
Expression of Selected Genes in Stromal Vascular (SV) and Fat Cell (FC) Fraction in 21-Day Broiler Chickens
The DLK1 gene, a marker for adipose-derived precursor cells, showed more than fivefold greater expression in the SV fraction than in the FC fraction (P < 0.01). On the other hand, FABP4 and ATGL, which are involved in free fatty acid transportation and mobilization, were expressed 16-fold (P = 0.056) and eightfold (P < 0.05) higher, respectively, in the FC fraction than in the SV fraction. Because the SV fraction should consist mainly of precursor cells that can differentiate to fat cells under adipogenic conditions, whereas the FC fraction contains mature fat cells, the expression of the three marker genes indicates that separation of the two fractions was successful. Expression of the three cell cycle activators—CCND3, CCNA1, and ANAPC5—was 3.6 (P < 0.05), 6 (P < 0.001), and threefold (P < 0.0001) higher, respectively, in the SV fraction than in the FC fraction, whereas expression of the two cell cycle inhibitors—CCNG2 and CDKN2C—was 2.7 (P = 0.085) and 6 times (P < 0.01) higher, respectively, in the FC fraction than in the SV fraction, indicating that cell cycle activity is high in the SV fraction and low in the FC fraction. However, unlike the other two cell cycle inhibitors, PMP22 showed an inverse pattern—more than fourfold greater (P < 0.01) expression in the SV fraction than in the FC fraction, indicating that PMP22 may possibly be involved with processes other than the cell cycle (Fig. 3).
Relative gene expression of DLK1, FABP4, ATGL, and six cell cycle regulators in stromal vascular (SV) and fat cell (FC) fractions (n = 4 for each group) from chicken adipose tissue. Each bar represents mean ± SEM. Statistical significance is indicated by *(P < 0.05), **(P < 0.01) and ***(P < 0.001). The expression values are normalized by gACTB gene
Expression of Selected Genes During Primary Cell Culture
During primary cell culture, the induction of differentiation brought about a 34 % decrease (P < 0.05) in expression of DLK1, but a twofold increase (P < 0.05) in expression of ATGL. From day 1 to day 3 after differentiation, DLK1 expression was at the lowest level, whereas ATGL remained at a continuously high level. Unlike ATGL, another fat cell marker—FABP4—exhibited a constant increase after 1 day of differentiation. After 1 day of differentiation, there was a rapid increase in expression with more than a ninefold increase between day 1 and day 2 (P < 0.05), as well as a twofold increase between day 2 and day 3 (P < 0.05) (Fig. 4).
Expression of DLK1, FABP4, ATGL and six cell cycle regulators during chicken primary adipocyte differentiation in vitro. mRNA expression was detected at 1 day before differentiation induction (day −1), initiation of differentiation (day 0), and 1, 2, and 3 days after differentiation induction and normalized by gACTB gene. Differences in gene expression among the four time points (n = 4 for each time point) were examined using ANOVA followed by Fisher’s post hoc test. Significant differences (P < 0.05) between different time points are indicated by different letters (a–e)
As was the case for FABP4, expression of the cell cycle inhibitors CCNG2 and CDKN2C also increased as the cells matured. Expression of CCNG2 was maintained at a low level before day 3 but displayed more than a twofold increase from day 2 to day 3 (P < 0.05), whereas expression of CDKN2C increased every day (P < 0.05) during the cell culture. However, PMP22 exhibited a unique pattern because it showed a decreasing trend from day 0 to day 2. Expression of both CCND3 and ANAPC5 increased from day 0 to day 1 (P < 0.05). However, CCNA1 seemed to be more sensitive to differentiation induction, as it decreased immediately after the induction of differentiation, and stayed at the low level until day 3 (P < 0.05) (Fig. 4).
Expression of Selected Genes During Development of Chicken Adipose Tissue
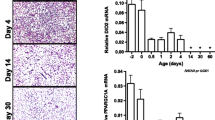
Expression of DLK1 was steady from E15 to P11 but decreased on P33 relative to P5. Expression of FABP4 and ATGL was low in the embryos and increased ninefold (P < 0.01) and 70-fold (P < 0.0001) respectively from E17 to the time of hatch (P0). From P0 to P5, expression of FABP4 decreased by 38 % (P < 0.05) and expression of ATGL decreased by 75 % (P < 0.01). From P5 to P33, expression of all three genes seemed to exhibit a gradually decreasing trend as the chickens grew (Fig. 5).
Relative expression of DLK1, FABP4, ATGL and six cell cycle regulators during in vivo adipose development of broiler chickens. mRNA expression was detected in subcutaneous adipose tissue on embryonic day 15 (E15) and day 17 (E17), post-hatch day 0 (P0), 5 (P5), 11 (P11), and 33 (P33) and normalized by chicken ribosomal protein S13 (gRPS13) gene. ANOVA followed by Fisher’s post hoc test was performed to examine the differences in gene expression among the six groups (n = 4 for each group), which are indicated by different letters (a–d), if significant (P < 0.05)
The five cell cycle regulator genes, other than CCNA1, were expressed at a low level in embryonic stages, but increased significantly on P0 or P5. For CCND3 and CDKN2C, the expression decreased significantly on P11 compared to P5 and remained at the same low level as in embryos until P33. For CCNG2 and PMP22, there was no significant change of expression from P5 to P33. Expression of ANAPC5 demonstrated a decreasing trend from P5 to P33. The expression on P33 was lower than on P5 but higher than on E15 and E17. Unlike the other genes, expression of CCNA1 was at a low level from E15 to P5 and increased to a high level on P11 and P33 (P < 0.05) (Fig. 5).
Discussion
As indicated in Table 2, age is a significant factor for increases in fat cell size and decreases in the quantity of proliferating cells. Therefore, hyperplastic growth activity is attenuated, while hypertrophic growth is promoted during development of adipose tissue. On the other hand, the close relationship between growth mechanism of fat tissues and adipocyte proliferation or differentiation makes study of fat cell proliferation and differentiation a potential route for better understanding and regulation of adipose tissue development. Due to the fact that broiler chickens have greater adipose weight than layers [18], minimizing their fat accumulation is advantageous to improve feed conversion and maximize productive efficiency of the industry. In this study, subcutaneous fat tissue from the broiler legs showed continuous accumulation with increasing speed as body weights increased; the same as for abdominal fat tissue in a previous report [5]. Moreover, the proliferation rate on P11 and P33 indicated by PCNA immunostaining was also similar to that of abdominal fat tissue on P10 and P28 [5]. However, unlike the continuous increase of fat cell size in abdominal adipose tissue, the increase of fat cell size in subcutaneous adipose seems to stop after P11, indicating adipose development and function varies depending upon the specific location. Therefore, investigation of the subcutaneous fat tissue growth in this study is useful for a comprehensive understanding of adipose tissue development.
Results of PCNA&DAPI staining and H&E staining indicate that the proliferation of fat cells decreased as fat cell size increased from E15 to P11, indicating that hypertrophic growth gradually became dominant, whereas hyperplastic growth slowed. Although the proliferation activity of fat cells showed a decreasing trend from P11 to P33, the average fat cell became smaller. This result seems to contradict the previous reports that fat cell size in abdominal fat tissue keeps increasing during this period [5, 19], indicating developmental differences between subcutaneous and abdominal fat tissues.
Unlike previous reports that only studied cellularity in fat tissue [5, 6, 19], we also detected expression of some precursor cell and adipocyte markers, as well as selected cell cycle markers, for a better understanding of the developmental mechanisms of broiler subcutaneous fat tissue. Higher expression of the DLK1 gene in the SV fraction agrees with our previous paper [20], and supports previous reports that claim DLK1 as a precursor cell marker [21] and an inhibitory factor in adipose development [22–24]. Greater expression of FABP4 and ATGL in the FC fraction also agrees with our previous reports [9, 20], validating their reliability as adipocyte markers.
Compared to the three cell cycle inhibitors selected in the previous paper [7], all three activators showed contrary expression patterns in GDS596, GDS3142, GDS2743, GDS2366, and GDS2659, suggesting their different association with fat cell differentiation compared to the three cell cycle inhibitors. The different association is possibly related to their different functions during the cell cycle. The function of these genes within the cell cycle is well known. Cyclin A associates with cyclin-dependent kinase (cdk) 2 in the S phase and later with cdk1 in the G2 phase. The cyclin A-cdk1/2 complex is required for entry into and completion of the S phase, as well as entry into the M phase of the cell cycle [25]. CCNA1, as one member of A-type cyclins, is reported to contribute to G1/S cell cycle progression in somatic cells [26]. D-type cyclins are essential for G1 progression in the cell cycle. As a member of D-type cyclins, CCND3 is a subunit of the cyclinD-cdk4/6 complex. CyclinD-cdk4/6 phosphorylates the retinoblastoma protein and thus releases E2F transcriptional factors, which in turn regulate many genes involved in DNA synthesis, as well as other cyclins downstream [27]. ANAPC5 is a subunit of anaphase-promoting complex/cyclosome (APC/C), which mediates timely degradation of cell cycle regulators in mitosis and in the G1 phase to promote cell cycle progression [28]. In general, high expression of CCNA1, CCND3, and ANAPC5 may promote DNA synthesis and completion of mitosis during cell proliferation.
On the other hand, the three cell cycle inhibitors exhibit adverse functions during the cell cycle. CCNG2 is an unconventional cyclin that blocks G1/S transition [29]; CDKN2C is a member of the INK4 family of cyclin-dependent kinase inhibitors and can block G1 progression by inhibiting cyclin-dependent kinase 4 or 6 (CDK4/6) [30]; PMP22 is a homologue of murine growth arrest-specific 3 (gas3), which is involved in G0 growth arrest [31]. Therefore, high expression of CCNG2, CDKN2C, and PMP22 may promote cell cycle inhibition during cell differentiation.
Because the cell cycle becomes inhibited at the G0 phase after terminal differentiation of fat cells from preadipocytes [32], CCNA1, CCND3, and ANAPC5 showed higher expression in the SV section which mainly contains precursor cells, whereas CCNG2 and CDKN2C showed higher expression in the FC section. In primary cell culture, CCNA1 and ANAPC5 exhibited decreased expression after initiation of differentiation, whereas CCNG2 and CDKN2C exhibited increasing expression during adipocyte differentiation. Moreover, high expression of ATGL and FABP4 after 3 days of differentiation suggests that many stromal vascular cells have differentiated into fat cells, because ATGL and FABP4 are respectively involved in triglyceride hydrolysis and fatty acid trafficking in maturing fat cells [33, 34].
The validation of involvement of CCNA1, CCND3, ANAPC5, CCNG2, and CDKN2C in adipocyte differentiation based on the GEO database and comparison of mRNA expression between different tissues and fractions, as well as different time points in primary cell culture, together with the marker genes for precursor cells and adipocytes, provide a possible way to analyze the early-stage development of broiler adipose tissue at the molecular level. Although all three cell cycle inhibitors showed lowest expression in liver as in pigs [7], PMP22 exhibited a reverse pattern of expression from CCNG2 and CDKN2C. Therefore, its regulation in broilers may be different from that in pigs [7]. Due to limitations of the current studies regarding mRNA expression and the lack of literature concerning PMP22, we cannot make inferences about the regulation of PMP22 in broiler adipose tissue. Further study of the function of PMP22 is needed.
Although PCNA&DAPI staining indicated that the proliferation rate continued declining from the embryonic stage onward, expression of precursor cell marker, DLK1, reached its highest point at P5, which may indicate that a large proportion of precursor cells were maintained until P5. This conclusion is also supported by the increasing expression of two of the cell cycle activators—CCND3 and ANAPC5—during this period. However, the increasing expression of the three cell cycle inhibitors also suggests that increasing differentiation activity may also occur in a group of preadipocytes to promote increase of adipocyte number when the other group is proliferating. Meanwhile, change in fat cell size shown by H&E staining also indicates a dramatic increase in cell size of the differentiated fat cells. Therefore, the period before P5 is an important stage for both hypertrophic and hyperplastic growth with hyperplastic growth occurring before hypertrophic growth. In addition, P0 also seems to be an important time point because hypertrophic growth is undermined by dramatic hydrolysis of triglycerides as an energy supply during hatching [6, 9]. In addition, the starved condition immediately after hatch but before access to food may also aggravate the loss of fat [6, 9]. Therefore, the expression of ATGL dramatically increased in terms of both mRNA and at the protein level [9]. Meanwhile, FABP4 also reached peak expression in both mRNA and protein [6], perhaps due to its function in transportation of cellular fatty acids during lipolysis as reported by Coe et al. [35] and Verhoef et al. [36].
After P5, the precursor cells were fewer in number as indicated by DLK1 expression, leading to less proliferation activity demonstrated by PCNA staining. Instead, many precursor cells may have become differentiated. However, as indicated by the decreased expression of CCNG2 and CDKN2C at P11, the differentiation activity of preadipocytes seems less intensive at this time because both genes have been shown to be promoted by fat cell differentiation [7]. Therefore, dramatic differentiation activity may occur sometime between P5 and P11. On the other hand, H&E staining showed that the size of fat cells did not increase from P11 to P33, whereas fat pad weight increased more than four times, indicating dramatic increase of mature fat cell number. With fewer precursor cells and consequently reduced potential for differentiation in the whole adipose tissue, one possible reason for the increase of mature fat cell number is that a population of immature differentiated cells becomes filled with lipid during this period. Therefore, hypertrophic growth should still be dominant during this period. Moreover, these immature fat cells may be able to proliferate as reported by other researchers who defined these cells as small proliferative adipocytes (SPA) [37, 38]. Upregulation of CCNA1 may be related to this process. However, due to the complicated conditions in vivo, such as communication between different types of cells and interaction between different groups of regulators, we can only speculate tentatively from a cellular study and cell cycle point of view. To verify this speculation, further research needs to be done.
In summary, our research suggests that the period from embryonic stages to P5 may be an important period for development of broiler leg adipose tissue with both dramatic proliferation and differentiation of fat cell precursors as indicated by peak expression of both cell cycle activators and inhibitors. However, the proliferation activity declines, whereas the differentiation activity increases from E15 to P5 as evidenced by the fact that the number of precursor cells decreased. Therefore, by the period between P5 and P11, a large proportion of preadipocytes may have become differentiated. Meanwhile, the average cell size also exhibits a continuous increase. Therefore, during the period before P11, both hypertrophic and hyperplastic growth is active. However, during the period from P11 to P33, hypertrophic growth seems to contribute more to the development of subcutaneous fat tissue, because both proliferation and differentiation activities decline as indicated by a decrease of PCNA positive cells and expression of cell cycle regulators. Therefore, the conclusion of our study is consistent with most reports involving animal adipose tissue in that hyperplastic growth mainly occurs in the early developmental stage. In general, this study provides further understanding of fat development in broiler chickens and thus will be helpful for regulation of fat deposition in the broiler industry.
Abbreviations
- ACTB :
-
β-Actin
- ANAPC5 :
-
Anaphase promoting complex subunit 5
- ATGL :
-
Adipose triglyceride lipase
- CCNA1 :
-
Cyclin A1
- CCND3 :
-
Cyclin D3
- CCNG2 :
-
Cyclin G2
- CDKN2C :
-
Cyclin dependent kinase inhibitor 2C
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DLK-1 :
-
Delta-like 1 homolog
- FABP4 :
-
Fatty acid binding protein 4
- GEO:
-
Gene Expression Omnibus
- H&E:
-
Hematoxylin and Eosin
- PCNA:
-
Proliferating cell nuclear antigen
- PMP 22 :
-
Peripheral myelin protein 22
- RPS13 :
-
Ribosomal protein S13
References
Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ (2001) The biology of white adipocyte proliferation. Obes Rev 2:239–254
Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, Cushman SW, Periwal V (2009) Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput Biol 5:e1000324
Cartwright AL, Marks HL, Campion DR (1986) Adipose tissue cellularity and growth characteristics of unselected and selected broilers: implications for the development of body fat. Poult Sci 65:1021–1027
Cartwright AL, Marks HL, Campion DR (1988) Adipose cellularity in nonselected and selected broiler stocks: measurements at equal weights and ages. Poult Sci 67:1338–1344
Guo L, Sun B, Shang Z, Leng L, Wang Y, Wang N, Li H (2011) Comparison of adipose tissue cellularity in chicken lines divergently selected for fatness. Poult Sci 90:2024–2034
Chen P, Suh Y, Choi YM, Shin S, Lee K (2014) Developmental regulation of adipose tissue growth through hyperplasia and hypertrophy in the embryonic Leghorn and broiler. Poult Sci 93:1809–1817
Zhang J, Suh Y, Choi YM, Ahn J, Davis ME, Lee K (2014) Differential expression of cyclin G2, cyclin dependent kinase inhibitor 2C and peripheral myelin protein 22 genes during adipogenesis. Animal 8:800–809
NRC (1994) Nutrient requirements of poultry. National Academies Press, Washington, DC
Lee K, Shin J, Latshaw JD, Suh Y, Serr J (2009) Cloning of adipose triglyceride lipase complementary deoxyribonucleic acid in poultry and expression of adipose triglyceride lipase during development of adipose in chickens. Poult Sci 88:620–630
Lee A, Suh Y, Wick MP, Lee K (2012) Temporal myosin heavy chain isoform expression transitions faster in broiler chickens compared with Single Comb White Leghorns. Poult Sci 91:2872–2876
Deiuliis JA, Li B, Lyvers-Peffer PA, Moeller SJ, Lee K (2006) Alternative splicing of delta-like 1 homolog (DLK1) in the pig and human. Comp Biochem Physiol B Biochem Mol Biol 145:50–59
Deiuliis JA, Shin J, Bae D, Azain MJ, Barb R, Lee K (2008) Developmental, hormonal, and nutritional regulation of porcine adipose triglyceride lipase (ATGL). Lipids 43:215–225
Oh SA, Suh Y, Pang MG, Lee K (2011) Cloning of avian G(0)/G(1) switch gene 2 genes and developmental and nutritional regulation of G(0)/G(1) switch gene 2 in chicken adipose tissue. J Anim Sci 89:367–375
Hassan A, Ahn J, Suh Y, Choi YM, Chen P, Lee K (2014) Selenium promotes adipogenic determination and differentiation of chicken embryonic fibroblasts with regulation of genes involved in fatty acid uptake, triacylglycerol synthesis and lipolysis. J Nutr Biochem 25:858–867
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the \(2^{ - \varDelta \varDelta C}_{T}\) method. Methods 25:402–408
Serr J, Suh Y, Lee K (2011) Cloning of comparative gene identification-58 gene in avian species and investigation of its development and nutritional regulation in chicken adipose tissue. J Anim Sci 89:3490–3500
Yang S, Suh Y, Choi YM, Shin S, Han JY, Lee K (2013) Loss of fat with increased adipose triglyceride lipase-mediated lipolysis in adipose tissue during laying stages in quail. Lipids 48:13–21
Emmerson DA (1997) Commercial approaches to genetic selection for growth and feed conversion in domestic poultry. Poult Sci 76:1121–1125
Hood RL (1982) The cellularity basis for growth of the abdominal fat pad in broiler-type chickens. Poult Sci 61:117–121
Shin J, Lim S, Latshaw JD, Lee K (2008) Cloning and expression of delta-like protein 1 messenger ribonucleic acid during development of adipose and muscle tissues in chickens. Poult Sci 87:2636–2646
Sul HS (2009) Minireview: Pref-1: role in adipogenesis and mesenchymal cell fate. Mol Endocrinol 23:1717–1725
Smas CM, Sul HK (1993) Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell 73:725–734
Lee K, Villena JA, Moon YS, Kim KH, Lee S, Kang C, Sul HS (2003) Inhibition of adipogenesis and development of glucose intolerance by soluble preadipocyte factor-1 (Pref-1). J Clin Invest 111:453–461
Wang Y, Kim KA, Kim JH, Sul HS (2006) Pref-1, a preadipocyte secreted factor that inhibits adipogenesis. J Nutr 136:2953–2956
Malumbres M, Barbacid M (2005) Mammalian cyclin-dependent kinases. Trends Biochem Sci 30:630–641
Ji P, Agrawal S, Diederichs S, Baumer N, Becker A, Cauvet T, Kowski S, Beger C, Welte K, Berdel WE, Serve H, Muller-Tidow C (2005) Cyclin A, the alternative A-type cyclin, contributes to G1/S cell cycle progression in somatic cells. Oncogene 24:2739–2744
Schafer KA (1998) The cell cycle: a review. Vet Pathol 35:461–478
Harper JW, Burton JL, Solomon MJ (2002) The anaphase-promoting complex: it’s not just for mitosis any more. Genes Dev 16:2179–2206
Aguilar V, Annicotte JS, Escote X, Vendrell J, Langin D, Fajas L (2010) Cyclin G2 regulates adipogenesis through PPAR coactivation. Endocrinology 151:5247–5254
Hirai H, Roussel MF, Kato JY, Ashmun RA, Sherr CJ (1995) Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol Cell Biol 15:2672–2681
Welcher AA, Suter U, De-Leon M, Snipes GJ, Shooter EM (1991) A myelin protein is encoded by the homologue of a growth arrest-specific gene. Proc Natl Acad Sci U S A 88:7195–7199
Tang QQ, Lane MD (2012) Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem 81:715–736
Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R (2004) Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306:1383–1386
Serr J, Suh Y, Lee K (2009) Regulation of adipose triglyceride lipase by fasting and refeeding in avian species. Poult Sci 88:2585–2591
Coe NR, Simpson MA, Bernlohr DA (1999) Targeted disruption of the adipocyte lipid-binding protein (aP2 protein) gene impairs fat cell lipolysis and increases cellular fatty acid levels. J Lipid Res 40:967–972
Verhoef SP, Camps SG, Bouwman FG, Mariman EC, Westerterp KR (2013) Physiological response of adipocytes to weight loss and maintenance. PLoS One 8:e58011
Kajita K, Mori I, Hanamoto T, Ikeda T, Fujioka K, Yamauchi M, Okada H, Usui T, Takahashi N, Kitada Y, Taguchi K, Kajita T, Uno Y, Morita H, Ishizuka T (2012) Pioglitazone enhances small-sized adipocyte proliferation in subcutaneous adipose tissue. Endocr J 59:1107–1114
Hanamoto T, Kajita K, Mori I, Ikeda T, Fujioka K, Yamauchi M, Okada H, Usui T, Takahashi N, Kitada Y, Taguchi K, Kajita T, Uno Y, Morita H, Ishizuka T (2013) The role of small proliferative adipocytes in the development of obesity: comparison between Otsuka Long-Evans Tokushima Fatty (OLETF) rats and non-obese Lon-Evans Tokushima Otsuka (LETO) rats. Endocr J 60:1001–1011
Acknowledgments
This work was supported by an Ohio Agricultural Research and Development Center (K. Lee) SEEDS grant and Agriculture and Food Research Initiative Competitive Grant no. 2010-65206-20716 from the USDA National Institute of Food and Agriculture.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Zhang, J., Suh, Y., Choi, Y.M. et al. Differential Expression of Cell Cycle Regulators During Hyperplastic and Hypertrophic Growth of Broiler Subcutaneous Adipose Tissue. Lipids 50, 965–976 (2015). https://doi.org/10.1007/s11745-015-4032-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-015-4032-x