Abstract
Epidemiological and experimental studies provide supportive evidence that lutein, a major carotenoid, may act as a chemopreventive agent against atherosclerosis, although the underlying molecular mechanisms are not well understood. The main aim of this study was to investigate the effects of lutein on the alleviation of atherosclerosis and its molecular mechanisms involved in oxidative stress and lipid metabolism. Male apolipoprotein E knockout mice (n = 55) were fed either a normal chow diet or a high fat diet (HFD) supplemented with or without lutein for 24 weeks. The results showed that a HFD induced atherosclerosis formation, lipid metabolism disorders and oxidative stress, but noticeable improvements were observed in the lutein treated group. Additionally, lutein supplementation reversed the decreased protein expression of aortic heme oxygenase-1 and increased the mRNA and protein expressions of aortic nicotinamide-adenine dinucleotide phosphate oxidase stimulated by a HFD. Furthermore, the decreased mRNA and protein expression levels of hepatic peroxisome proliferator-activated receptor-α, carnitine palmitoyltransferase 1A, acyl CoA oxidase 1, low density lipoprotein receptors and scavenger receptor class B type I observed in mice with atherosclerosis were markedly enhanced after treatment with lutein. Taken together, these data add new evidence supporting the anti-atherogenic properties of lutein and describing its mechanisms of action in atherosclerosis prevention, including oxidative stress and lipid metabolism improvements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis is the primary cause of cardiovascular diseases (CVD), which are the top cause of death worldwide [1, 2]. Although the precise mechanisms of atherosclerosis have not been fully elucidated, oxidative stress and dyslipidemia have been implicated in its pathogenesis [3, 4].
Nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase, a multi-subunit complex, is a primary source of reactive oxygen species (ROS) in the blood vessels, particularly in the presence of atherosclerosis. Several studies have demonstrated that the expression of its subunits, such as p22phox, p67phox, and p47phox, is significantly increased in the vessels of humans with coronary atherosclerosis [5] and that inhibition of NADPH oxidase by apocynin attenuates the progression of atherosclerosis [6]. Heme oxygenase-1 (HO-1), a crucial mediator of antioxidant and tissue-protective actions, also plays a critical role in protecting against atherogenesis [7–9] and increasing HO-1 expression in RAW 264.7 macrophages, which effectively decreases NADPH oxidase activity [10]. Additionally, the regulation of lipid metabolism, which involves cellular enzymes, membrane transport proteins and nuclear receptors, is very complicated [11]. Peroxisome proliferator-activated receptor-α (PPARα), one of the transcription factors, is a promising target for the development of novel antiatherogenic treatments due to its ability to regulate genes that are involved in lipid metabolism, such as carnitine palmitoyltransferase 1A (CPT1A) and acyl CoA oxidase (ACOX1) [12, 13]. In particular, CPT1A catalyses the transfer of long chain fatty acyl groups from CoA to carnitine for translocation across the mitochondrial inner membrane [14], whereas ACOX1 is the first enzyme of peroxisomal fatty acid β-oxidation, which catalyses the dehydrogenation of acyl-CoA thioesters to the corresponding trans-2-enoyl-CoA [15].
In recent years, accumulating evidence has shown that the low density lipoprotein receptor (LDLr) and scavenger receptor class B type I (SR-BI), the receptors of LDL and HDL, respectively, also play pivotal roles in lipid metabolic processes and atherosclerosis [16, 17]. Both oxidative damage and lipid metabolic disorders are critical and common in the pathogenesis of atherosclerosis. Therefore, studies of atherosclerosis in the fields of food and nutritional science have focused on the search for extracts of herbal plants and/or functional food ingredients that can prevent or ameliorate oxidative stress and dyslipidemia.
Lutein is one of the most prevalent carotenoids, accounting for the brilliant color pigment of many dark green leafy vegetables such as spinach and kale [18]. As an efficient quencher of singlet oxygen atoms and free radicals, lutein is a powerful antioxidant [19, 20] and is effective in preventing lipid peroxidation [21–24]. In addition, lutein reduces ROS in cultured vascular smooth muscle cells [25] and increases HO-1 mRNA expression in liver tissue, which results in the protection of mice from oxidative stress induced by d-gal [26]. Furthermore, evidence from animal studies suggests that lutein prevents cholesterol accumulation in aortic tissue in atherosclerotic guinea pigs [27]. Epidemiological data support the finding that an increase in serum lutein after supplementation reduces the serum levels of low density lipoprotein cholesterol (LDL-C) and total cholesterol (TC) [28]. Furthermore, progression of the intima-media thickness (IMT) of the common carotid arteries over 18 months was related to plasma lutein levels among a randomly sampled cohort of utility employees aged 40–60 years [29]. This result is supported by data from other studies showing an inverse association between serum levels of lutein and carotid IMT [30–33]. However, the protective effects of lutein against atherosclerosis and its association with NADPH oxidase, HO-1, PPARα, LDLr and SR-BI have not been well established.
Therefore, the objectives of the present study were to explore the effects of lutein on atherosclerosis prevention in vivo using apoE-deficient mice, a well established animal model used for studying atherosclerosis, and to better define the underlying mechanisms involved in oxidative stress related molecules (HO-1 and NADPH oxidase) and lipid metabolism-related molecules (PPARα, LDLr and SR-BI).
Materials and Methods
Animals
Male apoE knockout mice (C57/BL6 background, 8 weeks of age, 18–25 g) purchased from the Animal Center of Medical College of Peking University. The animals were maintained in constant temperature-controlled rooms (25 ± 2 °C) with controlled lighting (12 h light–dark cycles). The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and was approved by the Tongji Medical College Council on Animal Care Committee.
Experimental Procedures
The mice were randomly allocated to a control group (n = 7), a high fat diet group (HFD) (n = 12) or three lutein groups (n = 12 for each). The control group was fed normal chow, and the HFD group was given a high fat diet containing 21 % fat (5.66 % soybean oil and 15.34 % lard) and 0.15 % cholesterol by weight [27]. The three lutein groups were given the same high fat diet mixed with 0.01, 0.02 or 0.04 % lutein by weight (equivalent to 25, 50 and 100 mg/kg body weight, respectively) for 24 weeks (lutein was provided by InnoBio Co., Ltd, Dalian, China). At the end of the experiment, the animals were food deprived for 8 h, anesthetized with ketamine HCl (50 mg/kg)/xylazine (10 mg/kg), and subsequently killed by cervical dislocation. Blood was collected, centrifuged for 20 min at 5,000g at 4 °C and then stored at −80 °C. The heart, aorta and liver were excised from the mice, immediately frozen in liquid nitrogen, and stored at −80 °C until use. Three animals in each group were randomly selected for aortic sinus, aortic arch and liver Oil red O staining and hematoxylin and eosin (H&E) staining to observe any pathological changes and lipid deposition.
Assessment of Atherosclerotic Lesion Formation
Aortic arch samples fixed with 4 % paraformaldehyde, embedded in paraffin and sectioned into consecutive 8-μm thick sections. Every sixth section was stained with H&E and digitally photographed under magnification 200× for histological examination.
The heart samples were embedded in tissue freezing medium optimum cutting temperature compound (OCT) and sectioned into consecutive 8-μm thick sections at −20 °C. The distal end of the aortic sinus was recognized by the disappearance of the three aortic valve cusps as previously described [34]. Every sixth section was stained with Oil red O and digitally photographed under magnification 40×.
Atherosclerotic lesions in the aortic sinus stained with Oil red O were evaluated using IPP image analysis software. The lesion area index was calculated as the percentage of the aortic lumen area covered by atherosclerotic lesions.
Histology and Morphometry Evaluations of Lipid Deposition in Liver Tissue
Fresh samples from the same position of the liver were divided into two groups. One group of samples was fixed in 4 % paraformaldehyde and embedded in paraffin. Tissue sections (5 μm thick) were stained with H&E. The other group of samples was embedded in tissue freezing medium OCT and sectioned into consecutive 5-μm thick sections. Every sixth section was stained with Oil red O and digitally photographed under magnification 200×. Lipid deposition in the liver tissue stained with Oil red O was evaluated using IPP image analysis software.
Measurement of Lipid Parameters in Serum and Liver
The concentrations of TC, triacylglycerol (TAG), LDL-C and high density lipoprotein cholesterol (HDL-C) in serum and liver tissue were measured by enzymatic colorimetric assays using commercially available detection kits (Biosino Biotechnology Co., Ltd, Beijing, China). The atherogenic index of plasma (AIP = TAG/HDL-C) and non-high density lipoprotein cholesterol (NHDL-C = TC-HDL-C) were calculated.
Determination of Oxidative Stress Parameters in Serum and the Liver
The concentration of malondialdehyde (MDA) and the activity of superoxide dismutase (SOD) in serum and the liver were measured with enzymatic colorimetric assays using commercially available detection kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Assay of HO-1 and NADPH Oxidase in Serum
The levels of HO-1 and NADPH oxidase in serum were determined with enzyme-linked immunosorbent assays using commercially available detection kits (R&D Systems, USA).
Evaluation of ROS levels In Mouse Aortas
Dihydroethidium (Molecular Probes, Eugene, OR, USA) was used for in situ detection of ROS in mouse aortas [35, 36]. Fresh cross sections (5 μm) of unfixed but frozen aorta were immediately incubated with 5 M DHE at 37 °C for 15 min in a humidified chamber. The fluorescence level was then visualized with a fluorescence microscope. Fluorescence intensities in randomly selected areas of the images were quantified using IPP image analysis software.
Real-Time RT-PCR Analysis
Total RNA was extracted from aorta and liver tissue using TRIzol reagent. (Invitrogen, 154 Carlsbad, CA, USA). A SYBR green-based qRT-PCR kit (TaKaRa Biotechnology Co., Ltd, Dalian, China) was used according to the manufacturer’s instructions in a 7900HT instrument (Applied Biosystems, Forster, CA, USA). The specificity of the product was assessed from melting curve analysis. Gene expression levels were determined using the 2−ΔΔCt method. Gene expression levels are presented as the fold-change relative to the control. The mRNA of β-actin was quantified as an endogenous control. Quantitative real-time PCR primers were as follows:
Gene | Forward primer | Reverse primer |
|---|---|---|
HO-1 | 5′-TCACGGTCTCCAGTCGCCTCC-3′ | 5′-CGGGCTATGCTCGAGACGGC-3′ |
p22phox | 5′-GTGTGCGCAGGGTCCTCGTC-3′ | 5′-TCCAACCTGTGGCCGCTCCT-3′ |
p47phox | 5′-GGTCGACCATCCGCAACGCA-3′ | 5′-GCGGCGATAGGTGTCCTGGC-3 |
PPARα | 5′-GGAGTGCAGCCTCAGCCAAGTT-3′ | 5′-AGGCCACAGAGCGCTAAGCTGT-3 |
CPT1A | 5′-AAGAACATCGTGAGTGGCGTC-3′ | 5′-AGCACCTTCAGCGAGTAGCG-3′ |
ACOX1 | 5′-GCCTTTGTTGTCCCTATCCGT-3′ | 5′-CTTCAGGTAGCCATTATCCATCTCT-3′ |
LDLr | 5′-CACACAGCCTAGAGAAGTCGACAC-3′ | 5′-CTGTGCTTCGGTGGCCTGGTA-3′ |
SR-BI | 5′-TGGCAAGCCCCTGAGCACGTT-3′ | 5′-TAGTGTCTTCAGGACCCTGGCTGC-3′ |
β-actin | 5′-TTCGTTGCCGGTCCACACCC-3′ | 5′-GCTTTGCACATGCCGGAGCC-3′ |
Western Blot Analysis
Thoracic aorta and liver tissues were homogenized and lysed in RIPA Lysis Buffer (1 % Triton X-100, 1 % deoxycholate, 0.1 % SDS). Total protein was determined as previously described [8]. Tissue lysates with equal protein amounts were subjected to Western blot analysis and probing with specific primary antibodies overnight at 4 °C after blocking. Then, the target proteins were secondarily labeled with species-specific second antibodies conjugated to horseradish peroxidase. Immunoreactive bands were detected by means of an ECL plus Western Blotting Detection System (Amersham Biosciences, Little Chalford, UK) according to the manufacturer’s instructions. The relative density of the bands in Western blots was quantified with Quantity One 4.62 software (Bio-Rad, Hercules, CA, USA). Data were corrected for the background standardized to β-actin as the optical density (OD/mm2). The mouse monoclonal anti-β-actin antibody and rabbit anti-CPT1A antibody were purchased from Sigma-Aldrich (St. Louis, MO, USA). The rabbit polyclonal anti-HO-1 antibody, purified rabbit polyclonal anti-LDLr antibody, rabbit polyclonal anti-SR-BI antibody, rabbit polyclonal anti-PPAR alpha antibody and rabbit polyclonal anti-ACOX1 antibody were purchased from Abcam Limited (Cambridge, UK). The rabbit polyclonal anti-p47phox antibody was purchased from Merck Millipore Corporation (Billerica, MA, USA). The rabbit polyclonal anti-p22phox antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Horseradish peroxidase-conjugated secondary antibodies were purchased from Cell Signaling (Beverly, MA, USA).
Statistical Analysis
All data are expressed as means ± SD. Statistical analyses of data were performed using one-way analysis of variance with SPSS 12.0 software package (SN: 59245 46841 40655 89389 09859 21671 21957 29589 12). The results were considered statistically significant at P < 0.05.
Results
Effect of Lutein Treatment on Body Weight and Fat Accumulation
Lutein treatment significantly decreased body weight as well as abdominal, perirenal, epididymis and total adipose tissue, which were increased by HFD (Table 1).
Lutein Administration Ameliorated HFD-Induced Atherosclerosis in Mice
As indicated in Fig. 1a, representative images of the aortic arch cross section stained by H&E showed that mice fed the control diet did not exhibit appreciable atherosclerotic plaque formation. A HFD induced plaque coverage on most of the luminal surface of the aortic vessel, while smaller lesions were observed in the lutein supplement groups. Lipid deposition was assessed in cross sections of the aortic sinus using Oil red O. The results show that little lipid deposition was detected in control tissue. The extensive lipid deposits induced by a HFD were reduced by the addition of lutein in the diet (Fig. 1b). Quantitative analyses show that lutein inhibited this atherogenesis in a dose-dependent manner (Fig. 1c).
Dietary lutein reduced atherosclerosis in HFD-fed apoE-deficient mice. Representative images of cross sections taken from the aortic sinuses and aortic arch obtained from apoE-deficient mice in each group. The aortic arch sections were stained with H&E (200× magnification) (a), and the aortic sinus sections were stained with Oil red O (40× magnification) for lipid deposition (red) and counterstained with hematoxylin (blue) (b). Quantitative analysis of lipid accumulation is shown (c). Data represent the means ± SD and are normalized to % of field area. *P < 0.05, **P < 0.01 vs. control; # P < 0.05, ## P < 0.01 vs HFD. n = 3
The Administration of Lutein Alleviated Oxidative Stress in Mice
As shown in Fig. 2 and Table 2, a HFD induced ROS production in the aorta and increased concentrations of MDA and reduced activity of SOD in the serum and liver; these effects were significantly prevented by the concomitant addition of lutein. In addition, the lutein intervention apparently decreased NADPH oxidase activity in the serum and down-regulated the mRNA and protein expression levels of aortic p47phox and p22phox. Furthermore, protein expression of HO-1 in the aorta was statistically increased in lutein-treated animals compared with HFD-fed animals. However, lutein attenuated an increased mRNA expression level of aortic HO-1 in atherosclerotic mice (Fig. 3).
Effects of lutein on oxidative stress induced by HFD in mice. Lutein prevented increases in ROS production in the aortas of mice fed a HFD. ROS in the aortas of the mice was detected with DHE, which reacts with ROS and forms ETH; this compound binds to DNA and produces a red fluorescence signal, which can be visualized with a fluorescence microscope (×200) and quantified (a–b). Fluorescence intensities in randomly selected areas of the images were quantified with IPP image analysis software. Results are presented as the mean ± SD. *P < 0.05, **P < 0.01 vs control; # P < 0.05, ## P < 0.01 vs HFD. n = 3
Effects of lutein on the mRNA and protein expression levels of HO-1 (a–b), p47phox (c–d) and p22phox (e–f) in mouse aortas. Total RNA was extracted from the aortas of mice by Trizol. HO-1, p47phox and p22phox mRNA expression levels were analyzed by real-time RT-PCR. The mRNA of β-actin was quantified as an endogenous control. Aortic lysates were prepared and immunoblotted with the corresponding antibodies. Blotting with anti-β-actin was used as a protein loading control. HO-1, p47phox and p22phox are presented as the fold-change relative to the control. Results are presented as the mean ± SD. *P < 0.05, **P < 0.01 vs control; # P < 0.05, ## P < 0.01 vs HFD. n = 4
Lutein Treatment Improved Lipid Metabolism in Mice
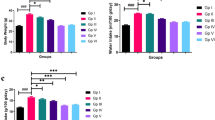
H&E and Oil red O staining of lipid deposition in liver tissues were analyzed to evaluate the effect of lutein on hepatic steatosis. As illustrated in Fig. 4, the control group had a small, single fat vacuole. A great number of vacuoles were seen in the HFD group, while the lutein groups had fewer vacuoles than that in HFD group. In addition, the lutein intervention significantly decreased the concentrations of TC and TAG in serum and the liver as well as the serum level of LDL-C. Meanwhile, API and NHDL-C were markedly decreased in the lutein groups compared with that in HFD group. However, there was no apparent difference in the serum HDL-C level among the groups (Table 3). To examine the potential molecular signaling pathways of lutein against HFD-induced dyslipidemia, the main proteins involved in lipid metabolism were evaluated. The results showed that administrations of lutein increased mRNA and protein expressions of PPARα as well as CPT1A and ACOX1 in liver (Fig. 5). In addition, the decreased mRNA and protein expression levels of hepatic LDLr and SR-BI in the mice with atherosclerosis were significantly reversed after intervention with lutein (Fig. 6).
The lutein intervention ameliorated hepatic steatosis in apoE-deficient mice induced by HFD. Representative images of liver sections from apoE-deficient mice in each group; the sections were stained with H&E (200× magnification) (a). The lipid droplets in the hepatocytes were stained red by Oil red O staining (200× magnification) (b). Quantitative analysis of hepatic fat accumulation is shown (c). The data represent the mean ± SD and are normalized to % of field area. *P < 0.05, **P < 0.01 vs control; # P < 0.05, ## P < 0.01 vs HFD. n = 3
Effects of lutein on the mRNA and protein expression levels of hepatic PPAR-α (a–b), CPT1A (c–d) and ACOX1 (e–f) in mice. After being treated with lutein for 24 weeks, total RNA was extracted from the livers of mice by Trizol. The PPAR-α, CPT1A and ACOX1 mRNA expression levels were analyzed using real-time RT-PCR. The mRNA of β-actin was quantified as an endogenous control. Hepatic lysates were prepared and immunoblotted with the corresponding antibodies. Blotting with anti-β-actin was used as a protein loading control. PPARα, CPT1A and ACOX1 are presented as the fold-change relative to the control. Results are presented as the mean ± SD. *P < 0.05, **P < 0.01 vs control; # P < 0.05, ## P < 0.01 vs HFD. n = 3
Effects of lutein on the mRNA and protein expression levels of hepatic LDLr (a–b) and SR-BI (c–d) in mice. After being treated with lutein for 24 weeks, total RNA was extracted from the livers of mice by Trizol. The LDLr and SR-BI mRNA expression levels were analyzed using real-time RT-PCR. The mRNA of β-actin was quantified as an endogenous control. Hepatic lysates were prepared and immunoblotted with the corresponding antibodies. Blotting with anti-β-actin was used as a protein loading control. LDLr and SR-BI are presented as the fold-change relative to the control. Results are presented as the means ± SD. *P < 0.05, **P < 0.01 vs control; # P < 0.05, ## P < 0.01 vs HFD. n = 3
Discussion
Lutein may act as a preventive agent in atherosclerosis, although the exact mechanism of such a protection is unclear. In the present study, lutein displayed efficacy in protecting against HFD-induced atherosclerosis in apoE-deficient mice through improving lipid metabolism and antioxidant defense. Our results provide the first evidence, to our knowledge, that the attenuated oxidative stress in response to lutein treatment was due to the increased HO-1 expression and decreased NADPH oxidase expression in the aorta. The data also demonstrated that lutein had hypotriglyceridemic and hypocholesterolemic effects that were potentially mediated by up-regulating hepatic PPARα, LDLr and SR-BI.
According to previous publications, lutein dosage used in randomized clinical trial for prevent atherosclerosis or cardiovascular diseases are generally 10 or 20 mg per day, which average equivalent to 0.25 mg/kg bw [37, 38]. The doses of lutein (25, 50, 100 mg/kg bw) used in the present study were based on the surface area of the mice which calculated by the Meeh–Rubner formula, and were equivalent to 10, 20 and 40 times the recommended human daily intake of lutein in a 60 kg person [39]. Also, the lutein dosages provided are almost consistent with a previous study by Mai et al. [26].
Growing evidence indicates that the chronic and acute overproduction of ROS in pathophysiological conditions is an important etiological factor in the development of atherosclerosis [40, 41]. The modification of LDL into its oxidized form (oxLDL) by ROS is an initial step in the formation of an atheroma [42]. Researches investigated the role of dietary lutein supplementation on CVD prevention, due to their antioxidant effects [43–45]. Lutein is a beneficial antioxidant [46]. In details, lutein is not only a scavenger of reactive oxygen species (singlet molecular oxygen and peroxyl radicals), but also an effective deactivators of electronically excited sensitizer molecules. Additionally, lutein could protect cellular membranes and lipoproteins against oxidative damage, especially, improve LDL resistance to oxidation. Furthermore, lutein has a cooperative synergistic effect with other antioxidants (i.e., vitamin C and vitamin E) in scavenging reactive nitrogen species [47]. In the current study, lutein-treated mice showed lower levels of ROS and MDA as well as higher activities of SOD. These findings are consist with the results of previous studies that showed that lutein inhibited ROS production in vascular smooth muscle cells [48] and decreased MDA concentrations in the liver and eyes of guinea pigs fed a hypercholesterolemic diet [49]. In summary, the lutein-mediated prevention of atherosclerosis induced by a HFD diet is tightly linked to the lutein-mediated antioxidant defense, but the molecular mechanisms involved are not clear.
In the context of CVD, the oxidative stress that leads to increased ROS production is largely produced by NADPH oxidase, a multi-component enzyme that includes a membrane-associated heterodimer composed of two subunits (Nox2 and p22phox) and four cytosolic proteins (p47phox, p67phox, Rac, and p40phox) [50–52]. Among these subunits, p47phox and p22phox play a crucial role in increasing the activity of NADPH oxidase and closely relate to the development of atherosclerosis [5]. Previous studies showed that apoE−/− mice lacking p47phox have a marked reduction of atherosclerosis in the descending aorta [53], and increased expression of p22phox contributes to the development of atherosclerosis [54]. Considering the key role of NADPH oxidase in ROS production and atherosclerosis development, we speculate that the downregulating effects of lutein on the aortic p47phox and p22phox expression levels seen in this study may account for the low levels of ROS in lutein-treated groups. Certainly, further study is necessary to evaluate the precise molecular mechanisms.
HO-1, an antioxidant stress response protein, plays a very important role in protecting against oxidative stress [55, 56]. Epidemiological evidence has shown that the expression of HO-1 is reduced in patients with coronary atherosclerosis, which indicates that a reduced ability to induce HO-1 may be involved in the mechanism of coronary atherosclerosis [57]. A study by Wang et al. [58] showed that lycopene (a major carotenoid from tomatoes) reduces oxidative stress and is associated with increasing HO-1 protein expression in the livers of HFD-fed rats. Similarly, our data showed that lutein supplements induced HO-1 protein expression in the aorta. However, in contrast to the change in protein expression, the HO-1 mRNA level in the aorta was lower in the high dose lutein group than in the HFD group. According to previous findings that the induction of HO-1 gene expression is correlated with the production of ROS [59, 60], we postulated that the increased mRNA expression of HO-1 induced by HFD feeding acted as an adaptive response to increased oxidative stress but is not sufficient to counteract the generation of free radicals. While inhibiting oxidative stress by lutein may initially make HO-1 activation unnecessary in the absence of extra oxidant stimulus, the increased effect of lutein on the protein expression of HO-1, but not mRNA abundance, suggests that the regulation process may occur at the post-transcriptional level. However, the exact mechanism underlying this phenomenon needs further investigation. Taken together, our results suggest that lutein could mediate its protective effects against atherosclerosis by regulating inherent oxidative stress systems.
Obesity is a risk factor for cardiovascular events and mortality from all causes [61]. Though the mechanisms by which obesity increases vascular risk are unclear, excessive visceral adipose tissue is independently associated with cardiovascular events [62] and has been identified as an indicator of cardiometabolic risks [63]. A cross-sectional analysis of a nationally representative sample of American adults showed that the serum lutein level is negatively correlated with the odds ratio of obesity among both sexes [64]. However, little research has explored the effect of lutein supplementation on obesity and visceral adipose tissue. Here, our results show that lutein supplementation not only alleviated excessive body weight gain and visceral adipose tissue, but also ameliorated lipid deposition in the liver. Moreover, reductions in serum TAG, TC and LDL-C levels were observed in the lutein-treated groups. These results are consist with the data from a cross-sectional, case–control and case study by Renzi et al. [65] that showed that serum lutein was significantly related to the serum concentrations of TC and HDL-C; additionally, a recent report demonstrated that lutein supplementation reduces circulating total and LDL cholesterol levels in an early atherosclerotic population [33]. Because lipid metabolism disorder is a major risk factor for atherosclerosis [11, 12], the lipid-lowering effects of lutein are likely the main contributing factor to its antiatherogenic potential; however, the molecular mechanism by which lutein regulates lipid metabolism in the liver has not been fully investigated. Therefore, the key molecules involved in the lipid metabolism pathways that play pivotal roles in atherosclerosis formation were assessed in this study.
PPARα, which belongs to the super family of ligand-activated nuclear hormone receptors is thought to be the principal regulator in the fatty acid oxidation through modulation of CPT1A and ACOX1, the rate-limiting enzymes in mitochondrial and peroxisomal fatty acid oxidation respectively [66]. In addition, PPARα was reported to play a very important role in inhibiting atherosclerosis in LDL receptor-deficient mice [67]. It has been published that lipopolysaccharide injections decrease PPARα mRNA in the liver and thymus tissues of chickens during an inflammatory response, and this decrease is partially reversed by increasing the dietary lutein content to 50 mg/kg feed [68]. In addition, lutein also induces PPAR expression in bovine adipose tissue [69]. Recently, β-cryptoxanthin, a carotenoid, was discovered to ameliorate HFD-induced obesity and abdominal adipose tissue weight gain via inducing PPARα expression in rats [70]. This evidence made us speculate that increased β-oxidation of fatty acids may be responsible for the TAG-lowering effect of lutein displayed in this study based on the significantly higher mRNA and protein expression levels of PPARα as well as CPT1A and ACOX1.in the livers of lutein-treated animals.
LDLr, a transmembrane glycoprotein, mediates the binding and endocytosis of lipoproteins containing apolipoprotein B and E, particularly LDL [16]. SR-BI, which is considered the receptor of HDL, is predominantly expressed in the liver and steroidogenic tissues, where it mediates the selective uptake of cholesteryl ester from HDL [17]. Evidence suggests that decreases in LDLr result in fatty acid metabolism disequilibrium and lipidosis in the liver tissue [71]. Mice deficient in both SR-BI and apolipoprotein E develop early, occlusive, atherosclerotic coronary artery diseases and die prematurely at 6–8 weeks of age [72, 73]. A previous study conducted with apoE-KO knockout mice determined that astaxanthin, which is similar in structure to lutein, might improve cholesterol metabolism through increasing mRNA abundance of hepatic LDLr, which in turn enhances the uptake of LDL from the circulation, and up-regulating intestinal SR-BI mRNA, thereby reducing cholesterol absorption [74]. Similarly, the present study showed for the first time that lutein up-regulated the mRNA and protein expression levels of LDLr and SR-BI in the liver. Because LDLr and SR-BI play key roles in mediating cholesterol, the favorable impact of lutein treatment on TC and LDL-C exhibited in this study may result partly from the modulation of LDLr and SR-BI.
In conclusion, this study demonstrated that lutein plays a role as a regulator of the expression of genes involved in oxidative stress (NADPH oxidase, HO-1) and lipid metabolism (PPARα, CPT1A, ACOX1, LDLr and SR-BI), thereby mitigating the progression of atherosclerosis. The present findings regarding the beneficial effects of lutein illustrate that in the prevention of CVD, lutein administration could represent an adjunct or alternative therapeutic strategy.
Abbreviations
- ACOX1:
-
Acyl CoA oxidase 1
- AIP:
-
Atherogenic index of plasma
- CPT1A:
-
Carnitine palmitoyltransferase 1A
- HDL-C:
-
High density lipoprotein cholesterol
- HO-1:
-
Heme oxygenase-1
- LDL-C:
-
Low density lipoprotein cholesterol
- LDLr:
-
Low density lipoprotein receptor
- MDA:
-
Malondialdehyde
- NADPH:
-
Nicotinamide-adenine dinucleotide phosphate
- NHDL-C:
-
Non-high density lipoprotein cholesterol
- PPAR:
-
Peroxisome proliferator-activated receptors
- ROS:
-
Reactive oxygen species
- SR-BI:
-
Scavenger receptor class B type I
- SOD:
-
Superoxide dismutase
- TC:
-
Total cholesterol
- TAG:
-
Triacylglycerol
References
American Heart Association Statistics Committee and Stroke Statistics Subcommittee (2013) Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 129:e28–e292
Yang ZJ, Liu J, Ge JP, Chen L, Zhao ZG, Yang WY (2013) Prevalence of cardiovascular disease risk factor in the Chinese population: the 2007–2008 China National Diabetes and Metabolic Disorders Study. Eur Heart 33:213–220
Victor VM, Rocha M, Solá E, Bañuls C, Garcia-Malpartida K, Hernández-Mijares A (2009) Oxidative stress, endothelial dysfunction and atherosclerosis. Curr Pharm Des 15:2988–3002
Koba S, Hirano T (2011) Dyslipidemia and atherosclerosis. Nihon Rinsho 69:138–143
Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, Wierzbicki K, Korbut R, Harrison DG, Channon KM (2006) Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol 26:333–339
Kinkade K, Streeter J, Miller FJ (2013) Inhibition of NADPH oxidase by apocynin attenuates progression of atherosclerosis. Int J Mol Sci. doi:10.3390/ijms140817017
Araujo JA, Zhang M, Yin F (2012) Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol. doi:10.3389/fphar
Ishikawa K, Sugawara D, Wang Xp, Suzuki K, Itabe H, Maruyama Y, Lusis AJ (2001) Heme oxygenase-1 inhibits atherosclerotic lesion formation in ldl-receptor knockout mice. Circ Res 88:506–512
Wu BJ, Kathir K, Witting PK, Beck K, Choy K, Li C, Croft KD, Mori TA, Tanous D, Adams MR, Lau AK, Stocker R (2006) Antioxidants protect from atherosclerosis by a heme oxygenase-1 pathway that is independent of free radical scavenging. J Exp Med 203:1117–1127
Taillé C, El-Benna J, Lanone S, Dang MC, Ogier-Denis E, Aubier M, Boczkowski J (2004) Induction of heme oxygenase-1 inhibits NAD(P)H oxidase activity by down-regulating cytochrome b558 expression via the reduction of heme availability. J Biol Chem 279:28681–28688
Wierzbicki AS, Viljoen A, Hardman TC, Mikhailidis DP (2013) New therapies to reduce low-density lipoprotein cholesterol. Curr Opin Cardiol 28:452–457
Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB (1986) Incidence of coronary heart disease and lipoprotein cholesterol levels: the Framingham Study. JAMA 256:2835–2838
Musso G, Gambino R, Cassader M (2009) Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog Lipid Res 48:1–26
Bonnefont JP, Djouadi F, Prip-Buus C, Gobin S, Munnich A, Bastin J (2004) Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol Aspects Med 25:495–520
Reddy JK, Mannaerts GP (1994) Peroxisomal lipid metabolism. Annu Rev Nutr 14:343–370
Spady DK (1992) Hepatic clearance of plasma low density lipoproteins. Semin Liver Dis 12:373–385
Krieger M (2001) Scavenger receptor class B type I is a multiligand HDL receptor that influences diverse physiologic systems. J Clin Invest 108:793–797
Khoo HE, Prasad KN, Kong KW, Jiang Y, Ismail A (2011) Carotenoids and their isomers: color pigments in fruits and vegetables. Molecules 16:1710–1738
Ribaya-Mercado JD, Blumberg JB (2004) Lutein and zeaxanthin and their potential roles in disease prevention. J Am Coll Nutr 23:S567–S587
Sindhu ER, Preethi KC, Kuttan R (2010) Antioxidant activity of carotenoid lutein in vitro and in vivo. Indian J Exp Biol 48:843–848
Leung EY, Crozier JE, Talwar D, O’Reilly DS, McKee RF, Horgan PG, McMillan DC (2008) Vitamin antioxidants, lipid peroxidation, tumour stage, the systemic inflammatory response and survival in patients with colorectal cancer. Int J Cancer 123:2460–2464
Karppi J, Nurmi T, Kurl S, Rissanen TH, Nyyssönen K (2010) Lycopene, lutein and beta-carotene as determinants of LDL conjugated dienes in serum. Atherosclerosis 209:565–572
Lusis AJ (2000) Atherosclerosis. Nature 407:233–241
Wang MX, Jiao JH, Li ZY, Liu RR, Shi Q, Ma L (2013) Lutein supplementation healthy nonsmokers. Atherosclerosis 22:380–385
Lo HM, Tsai YJ, Du WY, Tsou CJ, Wu WB (2012) A naturally occurring carotenoid, lutein, reduces PDGF and H2O2 signaling and compromised migration in cultured vascular smooth muscle cells. J Biomed Sci. doi:10.1186/1423-0127-19-18
Mai J, Shen X, Shi D, Wei Y, Shen H, Wu M (2010) Effect of lutein on relieving oxidative stress in mice induced by D-galactose. Wei Sheng Yan Jiu 39:430–432
Kim JE, Leite JO, DeOgburn R, Smyth JA, Clark RM, Fernandez ML (2011) A lutein-enriched diet prevents cholesterol accumulation and decreases oxidized LDL and inflammatory cytokines in the aorta of guinea pigs. J Nutr 141:1458–1463
Xu XR, Zou ZY, Xiao X, Huang YM, Wang X, Lin XM (2011) Effects of lutein supplement on serum inflammatory cytokines, ApoE and lipid profiles in early atherosclerosis population. J Atheroscler Thromb 20:170–177
Dwyer JH, Navab M, Dwyer KM, Hassan K, Sun P, Shircore A, Hama-Levy S, Hough G, Wang X, Drake T, Merz CN, Fogelman AM (2001) Oxygenated carotenoid lutein and progression of early atherosclerosis: the Los Angeles atherosclerosis study. Circulation 103:2922–2927
Dwyer JH, Paul-Labrador MJ, Fan J, Shircore AM, Merz CN, Dwyer KM (2004) Progression of carotid intima-media thickness and plasma antioxidants: the Los Angeles Atherosclerosis Study. Arterioscler Thromb Vasc Biol 24:313–319
Zou Z, Xu X, Huang Y, Xiao X, Ma L, Sun T, Dong P, Wang X, Lin X (2011) High serum level of lutein may be protective against early atherosclerosis: the Beijing atherosclerosis study. Atherosclerosis 219:789–793
Xu XR, Zou ZY, Huang YM, Xiao X, Ma L, Lin XM (2012) Serum carotenoids in relation to risk factors for development of atherosclerosis. Clin Biochem 45:1357–1361
Karppi J, Kurl S, Ronkainen K, Kauhanen J, Laukkanen JA (2013) Serum carotenoids reduce progression of early atherosclerosis in the carotid artery wall among Eastern Finnish men. PLoS One 8:e64107
Daugherty A, Whitman SC (2003) Quantification of atherosclerosis in mice. Methods Mol Biol 209:293–309
Sharma P, Chakraborty R, Wang L, Min B, Tremblay ML, Kawahara T, Lambeth JD, Haque SJ (2008) Redox regulation of interleukin-4 signaling. Immunity 29:551–564
Araujo JA, Zhang M, Yin F (2012) Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol 3:119
Zou ZY, Xu XR, Lin XM, Zhang HB, Xiao X, Ouyang L, Huang YM, Wang X, Liu YQ (2014) Effects of lutein and lycopene on carotid intima-media thickness in Chinese subjects with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Br J Nutr 111:474–480
Bonds DE, Harrington M, Worrall BB, Bertoni AG, Eaton CB, Hsia J, Robinson J, Clemons TE, Fine LJ, Chew EY (2014) Effect of long-chain ω-3 fatty acids and lutein+zeaxanthin supplements on cardiovascular outcomes: results of the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA Intern Med 174:763–771
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. Faseb J 22:659–661
Haendeler J, Eckers A, Lukosz M, Unfried K, Altschmied J (2012) Endothelial NADPH oxidase 2: when does it matter in atherosclerosis? Cardiovasc Res 94:1–2
Griendling KK, Sorescu D, Ushio-Fukai M (2000) NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86:494–501
Mitra S, Deshmukh A, Sachdeva R, Lu J, Mehta JL (2011) Oxidized low-density lipoprotein and atherosclerosis implications in antioxidant therapy. Am J Med Sci 342:135–142
Voutilainen S, Nurmi T, Mursu J, Rissanen TH (2006) Carotenoids and cardiovascular health. Am J Clin Nutr 83:1265–1271
El-Agamey A, Lowe GM, McGarvey DJ, Mortensen A, Phillip DM, Truscott TG, Young AJ (2004) Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch Biochem Biophys 430:37–48
Riccioni G, Speranza L, Pesce M, Cusenza S, D’Orazio N, Glade MJ (2012) Novel phytonutrient contributors to antioxidant protection against cardiovascular disease. Nutrition 28:605–610
Young AJ, Lowe GM (2001) Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys 385:20–27
Ciccone MM, Cortese F, Gesualdo M, Carbonara S, Zito A, Ricci G, De Pascalis F, Scicchitano P, Riccioni G (2013) Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediators Inflamm. doi:10.1155/2013/782137
Lo HM, Tsai YJ, Du WY, Tsou CJ, Wu WB (2012) A naturally occurring carotenoid, lutein, reduces PDGF and H2O2 signaling and compromised migration in cultured vascular smooth muscle cells. J Biomed Sci 19:18
Kim JE, Clark RM, Park Y, Lee J, Fernandez ML (2012) Lutein decreases oxidative stress and inflammation in liver and eyes of guinea pigs fed a hypercholesterolemic diet. Nutr Res Pract 6:113–119
Sheehan AL, Carrell S, Johnson B, Stanic B, Banfi B, Miller FJ Jr (2011) Role for Nox1 NADPH oxidase in atherosclerosis. Atherosclerosis 216:321–326
Cave A (2009) Selective targeting of NADPH oxidase for cardiovascular protection. Curr Opin Pharmacol 9:208–213
Mizrahi A, Berdichevsky Y, Casey PJ, Pick E (2010) A prenylated p47phox-p67phox-Rac1 chimera is a quintessential NADPH oxidase activator: membrane association and functional capacity. J Biol Chem 285:25485–25499
Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS (2001) p47phox is required for atherosclerotic lesion progression in ApoE −/− mice. J Clin Invest 108:1513–1522
Kaneto H, Katakami N, Matsuhisa M, Matsuoka TA (2010) Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm. doi:10.1155/2010/453892
Le WD, Xie WJ, Appel SH (1999) Protective role of heme oxygenase-1 in oxidative stress-induced neuronal injury. J Neurosci Res 56:652–658
Takahashi T, Morita K, Akagi R, Sassa S (2004) Heme oxygenase-1: a novel therapeutic target in oxidative tissue injuries. Curr Med Chem 11:1545–1561
Brydun A, Watari Y, Yamamoto Y, Okuhara K, Teragawa H, Kono F, Chayama K, Oshima T, Ozono R (2007) Reduced expression of heme oxygenase-1 in patients with coronary atherosclerosis. Hypertens Res 30:341–348
Wang Y, Ausman LM, Greenberg AS, Russell RM, Wang XD (2010) Dietary lycopene and tomato extract supplementations inhibit nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rats. Int J Cancer 126:1788–1796
McNally SJ, Harrison EM, Ross JA, Garden OJ, Wigmore SJ (2007) Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int J Mol Med 19:165–172
Lin HY, Shen SC, Lin CW, Yang LY, Chen YC (2007) Baicalein inhibition of hydrogen peroxide-induced apoptosis via ROS-dependent heme oxygenase 1 gene expression. Biochim Biophys Acta 1773:1073–1086
Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB (2008) General and abdominal adiposity and risk of death in Europe. N Engl J Med 359:2105–2120
See R, Abdullah SM, McGuire DK, Khera A, Patel MJ, Lindsey JB, Grundy SM, de Lemos JA (2007) The association of differing measures of overweight and obesity with prevalent atherosclerosis: the Dallas heart study. J Am Coll Cardiol 50:752–759
Katzmarzyk PT, Heymsfield SB, Bouchard C (2013) Clinical utility of visceral adipose tissue for the identification of cardiometabolic risk in white and African American adults. Am J Clin Nutr 97:480–486
Kimmons JE, Blanck HM, Tohill BC, Zhang J, Khan LK (2006) Associations between body mass index and the prevalence of low micronutrient levels among US adults. Med Gen Med 8:59
Renzi LM, Hammond BR Jr, Dengler M, Roberts R (2012) The relation between serum lipids and lutein and zeaxanthin in the serum and retina: results from cross-sectional, case-control and case study designs. Lipids Health Dis 29:11–33
Schoonjans K, Staels B, Auwerx J (1996) The peroxisome proliferator activated receptors (PPAR) and their effects on lipid metabolism and adipocyte differentiation. Biochim Biophys Acta 1302:93–109
Li AC, Binder CJ, Gutierrez A, Brown KK, Plotkin CR, Pattison JW, Valledor AF, Davis RA, Willson TM, Witztum JL, Palinski W, Glass CK (2004) Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARalpha, beta/delta, and gamma. J Clin Invest 114:1564–1576
Selvaraj RK, Shanmugasundaram R, Klasing KC (2010) Effects of dietary lutein and PUFA on PPAR and RXR isomer expression in chickens during an inflammatory response. Comp Biochem Physiol A Mol Integr Physiol 157:198–203
García-Rojas P, Antaramian A, González-Dávalos L, Villarroya F, Shimada A, Varela-Echavarría A, Mora O (2010) Induction of peroxisomal proliferator-activated receptor gamma and peroxisomal proliferator-activated receptor gamma coactivator 1 by unsaturated fatty acids, retinoic acid, and carotenoids in preadipocytes obtained from bovine white adipose tissue. J Anim Sc 88:1801–1808
Takayanagi K (2011) Prevention of adiposity by the oral administration of beta-Cryptoxanthin. Front Neurol 2:67
Trigatti B, Rayburn H, Viñals M, Braun A, Miettinen H, Penman M, Hertz M, Schrenzel M, Amigo L, Rigotti A, Krieger M (1999) Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci 96:9322–9327
Krieger M (1999) Charting the fate of the ‘good cholesterol’: identification and characterization of the high-density lipoprotein receptor SR-BI. Annu Rev Biochem 68:523–558
Braun A, Trigatti BL, Post MJ, Sato K, Simons M, Edelberg JM, Rosenberg RD, Schrenzel M, Krieger M (2002) Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ Res 90:270–276
Yang Y, Seo JM, Nguyen A, Pham TX, Park HJ, Park Y, Kim B, Bruno RS, Lee J (2010) Astaxanthin-rich extract from the green alga Haematococcus pluvialis lowers plasma lipid concentrations and enhances antioxidant defense in apolipoprotein E knockout mice. J Nutr 141:1611–1617
Acknowledgments
We are grateful to Wenzhong Wu and Yuan Huang of InnoBio Co., Ltd, Dalian, China for supplying the lutein samples. This work was supported by the National High Technology Research and Development Program of China (2010AA023003) and the National Natural Science Foundation of China (NSFC-81172657).
Conflict of interest
The authors have declared that no competing interests exist.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Han, H., Cui, W., Wang, L. et al. Lutein Prevents High Fat Diet-Induced Atherosclerosis in ApoE-Deficient Mice by Inhibiting NADPH Oxidase and Increasing PPAR Expression. Lipids 50, 261–273 (2015). https://doi.org/10.1007/s11745-015-3992-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-015-3992-1