Abstract
Mice were fed a control diet or a diet supplemented with hyodeoxycholic acid, the most abundant bile acid contained in pig bile, for 4 weeks, after which their serum and livers were collected. The contents of total fatty acids of serum and liver cholesteryl esters, and of liver triglycerides, were reduced following the administration of the hyodeoxycholic acid-supplemented diet, which was mainly due to the reductions in the contents of monounsaturated fatty acids. Free cholesterol contents in the serum and liver were not changed by hyodeoxycholic acid administration. Hyodeoxycholic acid administration reduced the gene expression levels of sterol regulatory element binding protein 1c, acetyl-CoA carboxylase, fatty acid synthase, and stearoyl-CoA desaturase-1. Hyodeoxycholic acid administration markedly changes the ratio of FXR-antagonist/FXR-agonist bile acids in the enterohepatic tissues of the mice (1.13 and 7.60 in hyodeoxycholic acid and control diet groups, respectively). Our findings demonstrate that hyodeoxycholic acid administration exerts the hypolipidemic effect in mice, in which downregulations of de novo lipogenesis and desaturation of saturated fatty acids are suggested to play important roles. In addition, regulation of FXR activation through the selective modification of the enterohepatic bile acid pool may be involved in the hypolipidemic effect of hyodeoxycholic acid administration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bile acids regulate the metabolism of lipids, carbohydrates, and bile acids themselves through the activation of their nuclear receptors [1, 2]. The important role of the farnesoid X receptor (FXR) in the regulation of lipid metabolism has most extensively been investigated. The activation of FXR by bile acids leads to the suppression of lipogenesis through the downregulation of sterol regulatory element binding protein 1c (SREBP1c) activation, which results in the reduction in triglyceride (TAG) contents in experimental animals [3, 4]. However, the activation of FXR is known to elevate cholesterol contents in the blood and liver as a result of the suppression of cholesterol mobilization for bile acid synthesis owing to the reduced expression level of cytochrome P450 7a1 (CYP7a1, cholesterol 7α-hydroxylase) (EC 1. 14. 13. 17) [5, 6]. However, the role of FXR activation in the elevation of cholesterol level is controversial because there have been reports showing the elevation in cholesterol level by FXR deficiency and the reduction by synthetic FXR agonist [7–9]. In addition to FXR, other nuclear receptors such as the pregnane X receptor, vitamin D receptor, and constitutive androstane receptor, as well as cell surface G-protein-coupled receptor (TGR5), are reported to be activated by bile acids [10, 11], although their roles in the regulation of lipid metabolism are as yet relatively less understood.
Bile preparations harvested from bears or domestic animals such as cattle and swine have long been used as traditional home remedies in Asian countries, including Japan. We have been investigating the effects of the administration of animal bile preparations on lipid levels using experimental animals because bile acids, major constituents of animal bile preparations, are potent regulators of lipid metabolism, as mentioned above. We have already demonstrated that the administration of cattle bile reduced serum TAG contents, but elevated serum and liver cholesterol contents in mice [12, 13]. However, the administration of pig bile was found to reduce liver TAG levels as well as serum cholesterol contents in our previous study using mice [14]. In addition, the proportions of palmitoleic acid (16:1n-7) and oleic acid (18:1n-9) in serum and liver lipids were elevated by cattle bile, but reduced by pig bile administration [13, 14]. The modifications of the contents and fatty acid composition in serum and liver lipid profiles in the mice treated with cattle bile were suggested to be mediated by cholic acid, the most abundant bile acid of cattle bile [13]. The bile acid composition of pig bile is quite different from that of cattle bile [13–15], which is considered to have opposite effects of cattle bile on the contents of cholesterol and monounsaturated fatty acids (MUFA) in the serum and liver [13, 14].
Hyodeoxycholic acid is the most abundant bile acid contained in pig bile [14, 15]. Hyodeoxycholic acid has been reported to reduce blood cholesterol levels in experimental animals through the inhibition of cholesterol absorption from the small intestine [16, 17]. This action is considered to underlie the reduction in serum total cholesterol contents in mice fed the pig bile-supplemented diet [14]. However, it has not yet been studied whether hyodeoxycholic acid administration reduces the contents of TAG and MUFA similarly to pig bile administration [14]. Therefore, we examined the effects of hyodeoxycholic acid administration on the contents of fatty acids in serum and liver lipids in mice. In addition, we examined the effects of hyodeoxycholic acid administration on the gene expression levels of proteins involved in de novo fatty acid synthesis and desaturation of saturated fatty acids and on the bile acid pool profile, which were contrasted with the changes in lipid and fatty acid profiles induced by hyodeoxycholic acid.
Materials and Methods
Animals and Diets
Twenty male C57BL/6 mice (SLC, Shizuoka, Japan) at 6 weeks of age were used in this study. Ten mice were fed a powdered, high-caloric diet (QuickFat, Nippon Clea) as the control diet group. Another group of ten mice was assigned to a powdered, high-caloric diet supplemented with 0.3 wt % hyodeoxycholic acid (>98 % purity, Wako Pure Chem, Osaka, Japan). The composition of nutrients in the high-calorie diet was as follows (g in 100 g): moisture, 7.1; crude protein, 24.1; crude fat, 13.9; crude fiber, 2.9; crude ash, 5.2; nitrogen-free extracts, 46.8. The energy content of the high-caloric diet is reported as 415.1 kcal/100 g (http://www.clea-japan.com/en/diets/diet_a/a_07.html). Cholesterol content of the high-caloric diet was 0.068 (w/w). We have confirmed that the administration of the high-calorie diet for 4 weeks elevated the contents of serum and liver cholesterol and liver TAG contents in male C57BL/6 mice compared with that of a regular diet (344.9 kcal/100 g) [12–14]; these responses were suppressed by the addition of 1 wt % pig bile extract to the high-calorie diet [14]. Because the hyodeoxycholic acid content in the pig bile-supplemented diet was approximately 0.3 % [14], the effect of hyodeoxycholic acid at this concentration was examined in this study.
Dietary Protocols
The effects of hyodeoxycholic acid administration on body weight, intake of food, and water and lipid levels were evaluated in the two groups of mice (five mice/group) assigned to the control and hyodeoxycholic acid diets. These mice were individually housed in plastic cages (240 × 170 × 120 mm3) containing fresh paper bedding and were given the above-mentioned diets. Fresh diets were served every 2 or 3 days in round glass feeders (54 mm diameter × 47 mm height). The weight of powered diet and amount of water ingested by each mouse, as well as body weight gain were measured every week throughout the feeding period of 4 weeks. Another two groups of mice (five mice × two groups) were used for the assessment of the effects of hyodeoxycholic acid administration on bile acid contents of enterohepatic tissues. These groups of mice were group-fed (five mice/cage) for 4 weeks.
Sampling of Blood and Tissues
On the final day of the feeding period, the mice were fasted from 0900 h to 1300 h, and then anesthetized in a chamber filled with isoflurane gas (5 % in air). Blood was obtained from the anesthetized mice by cardiac puncture, allowed to clot, and then centrifuged to obtain serum. Two liver specimens (approximately 0.2 g each) were harvested from the mice and washed in ice-cold saline. Serum and one liver specimen for lipid analysis were quickly frozen in liquid nitrogen and stored at −70 °C. The other liver specimen for gene expression analysis was immersed in RNAlater (Life Technologies, Carlsbad, CA, USA), which was stored at 4 °C for 24 h and then at −70 °C until analysis. The enterohepatic tissues including the liver, gallbladder, and small intestine for the determination of bile acid content were harvested from the mice and stored at −70 °C until analysis. The protocol for the above animal treatments was in accordance with the Guidelines for Proper Conduct of Animal Experiments issued by the Science Council of Japan (June 1. 2006) (http://www.scj.go.jp/ja/info/kohyo/pdf/kohyo-20-k16-2e.pdf) and was approved by the Committee for Animal Care and Experiments of the University of Toyama (approval number, S-2012 INM-5).
Analysis of Fatty Acids in Serum and Liver
Total lipids were extracted from the serum and liver as described by Hara and Radin [18]. Cholesteryl esters (CE), TAG, and total phospholipids (PL) were purified by silica gel thin-layer chromatography (TLC) and their fatty acid contents were determined by gas–liquid chromatography, as described previously [13]. Heptadecanoic acid (17:0) was added to the lipid fractions as an internal standard for the quantification of their fatty acid contents. Free cholesterol concomitantly obtained in the above TLC was extracted from the silica gel adsorbents, and its content was determined by a clinical enzymatic assay (Wako Pure Chem, Osaka, Japan).
Gene Expression Analysis of Liver
Liver total RNA was extracted using RNAiso (Takara Bio, Tokyo, Japan). An aliquot of liver total RNA was reverse-transcribed to first-strand cDNA in the presence of oligo-deoxythymidine and random hexamer primers using a PrimeScript RT reagent kit (Takara Bio). An aliquot of the first-strand cDNA was subjected to real-time PCR in the presence of SYBR (SYBR Green Realtime PCR Master Mix, Toyobo Life Sciences, Osaka, Japan) and a set of gene-specific primers using a thermal cycler (MyiQ2 real-time PCR system, BioRad, Hercules, CA, USA). The sequences of the forward and reverse primers used for the amplification of specific cDNA sequences are shown as Electronic Supplementary Material. The amplified products were verified by checking their melting curves after the final cycle of each PCR. The relative content of cDNA derived from the mRNA of interest was expressed as a ratio with respect to that of 18S ribosomal RNA.
Bile Acid Determination
Frozen enterohepatic tissues were thawed and then homogenized with 10 ml of 50 % methanol. Three-hundred microliters of the resulting homogenate was used to extract total bile acids using an OASIS HLB SPE cartridge (30 mg) (Waters, Milford, MA, USA) after the treatment of the above homogenate with ice-cold alkaline acetonitrile, as described previously [19]. Bile acid-containing fractions were dried under nitrogen gas and the resultant residue was dissolved in a mixture of 100 μl of methanol and 1 ml of 1 N NaOH, which was heated for 4 h at 120 °C to hydrolyze conjugated bile acids. The reaction mixture was acidified to pH 1–2 with hydrochloric acid, and then bile acids were extracted three times with 2 ml of ethyl acetate. After the organic layer was evaporated to dryness under nitrogen gas, the resultant residue was dissolved in methanol. Bile acid contents in the samples were determined by liquid chromatography–mass spectrometry (LC–MS). The mobile phases used were water/methanol/acetonitrile (75:5:20, v/v/v) containing 10 mM ammonium acetate acidified with acetic acid (pH = 5.5) (A) and methanol:acetonitrile (5:95, v/v) (B). Gradient elution was initiated from A:B at 90:10 (v/v) and then changed to 10:90 (v/v) for 20 min. This proportion was kept for 10 min and then returned to the initial proportion, which was continued for 10 min before the next chromatographic run. Bile acids were detected as [M-H]− in a selected-ion monitoring mode at m/z = 407.2 and 391.2 to detect tri-hydroxylic (cholic, α-muricholic, β-muricholic, hyocholic acids) and di-hydroxylic bile acids (chenodeoxycholic, deoxycholic, and hyodeoxycholic, ursodeoxycholic acids), respectively, using an electrospray ionization-single quadrupole mass spectrometer (LCMS-2020, Shimadzu, Kyoto, Japan). Quantification was carried out using calibration curves derived from the known amounts of the above authentic bile acids purchased from Steraloids Inc. (Newport, RI, USA).
Statistical Analysis
The statistical significance of the differences in values between the control and hyodeoxycholic acid diet groups was determined by unpaired Student's t test. When p values were determined to be less than 0.05, the differences were considered statistically significant.
Results
Body Weight Gain and Food and Water Intake
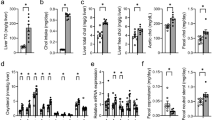
There was no significant difference in body weight gain, or food and water intake of mice between the control and hyodeoxycholic acid diet groups (Fig. 1a–c). The administration of hyodeoxycholic acid diet did not change the liver weight compared with that of the control diet (Fig. 1d).
Food intake (a), water intake (b), body weight change (c), and liver weight (d) of mice fed control and hyodeoxycholic acid (HDCA) diets. The total food and water intakes during the entire feeding period (4 weeks) are shown (a, b). Each column and each bar represent mean and SEM from five mice in each group, respectively. There was no significant difference in any of the above values between the control and HDCA diet groups
Serum Lipid Analysis
The content of total fatty acids of serum CE in the hyodeoxycholic acid diet group was significantly lower (1,512 ± 82.46 μg/ml) than that in the control diet group (1,898 ± 91.61 μg/ml) (p = 0.011) (Table 1). In addition, the contents of MUFA, such as 16:1n-7, 18:1n-9, and 18:1n-7, were significantly lower (p = 0.005, <0.001, and 0.008, respectively), but that of 18:0 tended to be higher (p = 0.059) than those in the control diet group. Hyodeoxycholic acid administration also reduced the contents of 18:2n-6 and 20:5n-3 in serum CE compared with the administration of the control diet (p = 0.042 and p = 0.005, respectively). The contents of free cholesterol in the serum and liver were not different between hyodeoxycholic acid-supplemented and control diet groups (Fig. 2a). The contents of total fatty acids in serum TAG were not significantly different between the control and hyodeoxycholic acid diet groups (p = 0.898), although the content of 20:4n-6 was slightly higher in the hyodeoxycholic acid diet group than in the control diet group (p = 0.035) (Table 1). The contents of several types of fatty acids (16:1n-9, 16:1n-7, 18:2n-6, 20:3n-6, and 20:5n-3) in serum PL were significantly lower in the hyodeoxycholic acid diet group than in the control diet group, although these differences were small and the total fatty acid contents of this lipid fraction were not significantly different between these dietary groups (p = 0.403).
Free cholesterol contents in serum and liver of mice fed control and hyodeoxycholic acid (HDCA) diets. Free cholesterol was isolated from the total lipids in serum and liver and its content was determined enzymatically. Each column and each bar represent mean and SEM from five mice in each group, respectively. There was no significant difference in free cholesterol levels in the serum and liver
Liver Lipid Analysis
The total fatty acid content of liver CE in the hyodeoxycholic acid diet group was 71.1 % of that in the control diet; this difference was statistically significant (p = 0.031) (Table 2). Hyodeoxycholic acid markedly reduced the contents of 16:1n-7 and 18:1n-9 in liver CE (p = 0.010 and <0.001, respectively). In addition, the content of 18:2n-6 was slightly, but significantly lower in the hyodeoxycholic acid diet group than in the control diet group (p = 0.029). The contents of free cholesterol in the liver were not significantly different between these two dietary groups (Fig. 2b). Hyodeoxycholic acid administration reduced the total fatty acid content of liver TAG to 63.8 % that of the control group (p = 0.018), which was mainly due to a large reduction in the content of 18:1n-9 (6,355 ± 715 vs. 3,813 ± 397 μg/g liver, respectively). The hyodeoxycholic acid diet also reduced the content of 16:1n-7 compared with the control diet (p = 0.015). The contents of 16:0 and 20:4n-6 were slightly, but significantly lower in the hyodeoxycholic acid diet group than in the control diet (p = 0.041 and 0.037, respectively). Similarly in serum CE and PL (Table 1), lower contents of 16:1n-7, 18:2n-6 and 20:5n-3 were observed in the liver PL of the hyodeoxycholic acid diet group than in the control diet group (p = 0.008, 0.003, and <0.001, respectively). However, these differences were relatively small and total fatty acid contents of liver PL were not significantly different between the two dietary groups (p = 0.073).
Gene Expression Levels in Liver
The expression level of SREBP1c mRNA was significantly lower in the liver of mice fed the hyodeoxycholic acid diet than that of the mice fed the control diet (p = 0.017) (Fig. 2). In addition, the hyodeoxycholic acid diet reduced the expression levels of the mRNAs of lipogenic enzymes upregulated by SREBP1c, such as acetyl-CoA carboxylase-1 (ACC) (EC 6. 4. 1. 2), fatty acid synthase (FAS) (EC 2. 3. 1. 85), and stearoyl-CoA desaturase-1 (SCD1) (EC 1. 14. 19. 1), compared with the control diet (p = 0.017, 0.003, 0.003, <0.001, respectively). The mRNA expression level of cytochrome P450 7A1 (CYP7a1) (EC 1. 14. 13. 17) was also markedly lower in the hyodeoxycholic acid diet group than in the control diet group. However, the expression levels of the mRNAs of 3-hydroxy-3-methylglutaryl coenzyme-A reductase (HMGCR) (EC 1. 1. 1. 34) and sterol-O-acyltransferase-2 (SOAT2) (EC 2. 3. 1. 26) in the hyodeoxycholic acid diet group were not significantly different from those in the control diet group.
Bile Acid Composition in Enterohepatic Tissues
β-Muricholic acid and cholic acid were the major bile acids found in the enterohepatic tissues of the mice fed the control diet (Fig. 4a). Hyodeoxycholic acid administration markedly reduced the contents of β-muricholic acid and cholic acid, and the extent of the reduction was greater in β-muricholic acid (−95 %) than in cholic acid (−81 %). Although the content of hyodeoxycholic acid was low in the tissue of the mice fed the control diet, it markedly increased upon hyodeoxycholic acid administration. Lower contents of α-muricholic acid, deoxycholic acid, and chenodeoxycholic acid were also found in the tissues of the control diet group, which did not significantly change following hyodeoxycholic acid administration (Fig. 4b). Ursodeoxycholic acid was also a minor bile acid in the enterohepatic tissues, but its content was significantly reduced following hyodeoxycholic acid administration. Hyocholic acid was not detected in the tissues of the control diet group, but was detectable in the hyodeoxycholic acid-supplemented diet group. The total bile acid content in the enterohepatic tissues of the hyodeoxycholic acid-supplemented diet group was not significantly different from that of the control diet group (p = 0.271).
Discussion
Cyp7a1 converts cholesterol to 7α-hydroxycholesterol as the first step of the bile acid synthetic pathway [20], and its expression level is downregulated in a feedback manner by bile acids [21]. As we observed, the expression level of Cyp7a1 mRNA in the liver was markedly reduced following HDCA administration, suggesting that hyodeoxycholic acid might suppress cholesterol mobilization for bile acid synthesis. Nevertheless, hyodeoxycholic acid administration did not lead to the elevation in free cholesterol level in the serum and liver (Fig. 2). Moreover, CE levels in the serum and liver were rather reduced following hyodeoxycholic acid administration (Tables 1, 2). It has already been demonstrated that dietary administration of HDCA could suppress the absorption and augment the secretion of cholesterol in the small intestine [17]. On the other hand, we observed that the reductions in serum and liver CE contents following hyodeoxycholic acid administration were associated with significant reductions in the contents of 16:1n-7 and 18:1n-9 and a trend for the elevation in the content of 18:0 (Tables 1, 2). These responses might reflect the limited availability of MUFA for the synthesis of CE due to a lower expression level of SCD1 in the livers of mice fed the hyodeoxycholic acid diet (Fig. 3). MUFA synthesis mediated by SCD1 is reported to be required for the efficient synthesis of CE in the liver [22, 23]. However, serum CE is also generated by the esterification of free cholesterol to fatty acids derived from serum phosphatidylcholine through the action of lecithin-cholesterol acyltransferase [24, 25]. The CE species synthesized through this pathway contains polyunsaturated fatty acids such as 18:2n-6 and 20:4n-6 [24, 25]. As shown, the content of 18:2n-6 was elevated, but that of 20:5n-3 was reduced in serum CE in the mice fed the hyodeoxycholic acid-supplemented diet compared with those in the mice fed the control diet, as similarly observed for serum PL (Table 1). Thus, a significant portion of serum CE is suspected to be synthesized through the action of LCAT. Determination of the activity of LCAT in serum would be necessary to assess its role in the reduction in serum CE contents by hyodeoxycholic acid. Thus, multiple steps in the regulation of CE synthesis are suspected to be involved in the reduction in serum and liver CE contents.
Expression levels of mRNA in the livers of mice fed the control and hyodeoxycholic acid (HDCA) diets. Total RNA extracted from the livers of the mice fed the test diets and the specific sequences of cDNA derived from the above mRNA were amplified by PCR using the respective primers shown in Table 1. The relative values compared with the value of 18S ribosomal RNA are shown. Each column and each bar represent mean and SEM from five mice in each group, respectively. The p values obtained from Student’s t test are shown above each panel. NS not significant (p > 0.05)
Hyodeoxycholic acid administration decreased the content of liver TAG, which was associated with marked reductions in the contents of 18:1n-9 and 16:1n-7 (Table 2). These results also suggest that the suppression of fatty acid desaturation due to a reduced expression of SCD1 mRNA in liver (Fig. 3) play a significant role in the reduction in liver TAG contents following hyodeoxycholic acid administration. However, the suppression of de novo fatty acid synthesis, as suggested by the reduced expression levels of ACC and FAS mRNA (Fig. 3), could contribute to the reduction in the contents of 16:0 as well as liver TAG following hyodeoxycholic acid administration. Thus, more extensive studies are necessary to assess the role of other enzymatic steps involved in the synthesis and degradation of TAG in the reduction in liver TAG contents by hyodeoxycholic acid administration.
Analysis of gene expression levels in the liver (Fig. 2) suggests that hyodeoxycholic acid administration suppressed SREBP1c-mediated de novo lipogenesis and fatty acid desaturation leading to reduced MUFA synthesis in the liver. It has already been reported that the administration of FXR-agonist bile acids such as chenodeoxycholic acid [23] and cholic acid [3] can reduce TAG level through the suppression of SREBP1c-mediated lipogenesis. We showed that hyodeoxycholic acid administration exclusively elevated hyodeoxycholic acid content, although hyodeoxycholic acid has not been reported to be inert as an FXR activator [27]. Concomitantly, the content of cholic acid was markedly reduced, but those of chenodeoxycholic acid and deoxycholic acid did not change following hyodeoxycholic acid administration (Fig. 4). Chenodeoxycholic acid is recognized to be the most potent endogenous FXR activator, whereas the potency of deoxycholic acid is moderate, and cholic acid is recognized to be inactive [11, 28]. Interestingly, recent reports have demonstrated that α- and β-muricholic acids act as endogenous antagonists of FXR activation in the mouse enterohepatic tissues [29, 30]. Furthermore, it has also been shown that ursodeoxycholic acid, although its level was relatively low and further reduced following HDCA administration (Fig. 4), was shown to possess a property similar to that of muricholic acids in terms of being an antagonist of FXR [30]. The ratios of FXR-antagonist to FXR-agonist bile acids in the enterohepatic tissue (total contents of α-muricholic acid, β-muricholic acid, and ursodeoxycholic acid to total contents of chenodeoxycholic acid and deoxycholic acid) were 1.13 and 7.60 in the hyodeoxycholic acid diet and control diet groups, respectively. These results imply that hyodeoxycholic acid administration could increase FXR activity through preferential reduction in the contents of FXR-antagonist bile acids over those of FXR-agonist bile acids. However, we should be careful for the above assumption because it has not been defined yet whether hyodeoxycholic acid is an FXR antagonist or not, which might largely influence its effect on the activity level of FXR in the enterohepatic tissues. Thus, the effect of bile acid administration in vivo should also be investigated from the viewpoint of the balance of bile acids with different potencies to FXR in the enterohepatic tissues.
Bile acid contents in enterohepatic tissues of mice fed control and hyodeoxycholic acid (HDCA) diets. Total bile acid was extracted from the enterohepatic tissues including those in the liver, gallbladder, and small intestines and treated with alkaline to hydrolyze conjugated forms of bile acids. The contents of unconjugated bile acids above were determined using liquid chromatography–mass spectrometry. Bile acids with higher and lower contents are separately shown in a and b, respectively. Each column and each bar represent mean and SEM from five mice in each group, respectively. The p values obtained from Student’s t test are shown above each panel. CA cholic acid, CDCA chenodeoxycholic acid, DCA deoxycholic acid, HCA hyocholic acid, MCA muricholic acid, UDCA ursodeoxycholic acid, NS not significant (p > 0.05)
The contents of cholic acid and β-muricholic acid as well as chenodeoxycholic acid were markedly reduced upon hyodeoxycholic acid administration (Fig. 4), whereas those of chenodeoxycholic acid, deoxycholic acid and α-muricholic acid were not significantly reduced. Interestingly, the content of hyocholic acid in the control mice was negligibly low, but increased upon HDCA administration. It has been reported that hyocholic acid could be synthesized from hyodeoxycholic acid and chenodeoxycholic acid through 7α-hydroxylation [31] and 6α-hydroxylation, respectively [32]. Thus, hyodeoxycholic acid seems to modify differentially the levels of individual bile acids in the enterohepatic tissues, which is considered to be mediated through the differential effects on the activities of various bile acid-metabolizing enzymes. In addition, the levels of bile acid transporters expressed in the liver as well as the intestines are involved in the regulation of the levels of bile acids in the enterohepatic tissues [33]. Both the changes in the expression levels of enzymes and transporters regulating the enterohepatic levels of bile acids are also regulated by FXR activity [11]. As mentioned above, hyodeoxycholic acid itself is recognized as an inert FXR agonist [27], although this bile acid could augment FXR activity levels though the modification of FXR-antagonist/FXR-agonist balance in enterohepatic tissues (Fig. 4). A similar mechanism might be involved in the regulation of bile acid profiles by HDCA administration. On the other hand, we should also focus on the role of hyocholic acid, which appeared in the enterohepatic tissues following hyodeoxycholic acid administration (Fig. 4). Similar to hyodeoxycholic acid, hyocholic acid has been reported to be an inert bile acid for FXR activation [34], although its interaction with FXR-agonist bile acids is also unknown. Thus, we need more detailed data on how hyodeoxycholic acid alters bile acid compositions in differential parts of enterohepatic tissues from viewpoints of the activities of bile acid-metabolizing enzymes and their transporters as well as of the rates of biliary bile acid output.
Note that small, but significant reductions in the contents of 18:2n-6 and 20:5n-3 in the extensive lipid fractions from serum and liver were induced in the hyodeoxycholic acid-supplemented diet group compared with the control diet group (Tables 1, 2). Similar changes in the contents of 18:2n-6 and 20:5n-3 were shown in the mice fed the pig bile-supplemented diet [14], suggesting that these changes are mediated by hyodeoxycholic acid contained in pig bile. The contents of these fatty acids largely change depending on the differences in the contents of n-6 and n-3 PUFA in the diet [35], whereas these contents in the control and hyodeoxycholic acid diets were quite similar. On the other hand, 20:5n-3 is also generated from dietary 18:3n-3 through chain elongation and desaturation mainly in the liver [36]. Therefore, further studies are necessary to elucidate the reason for the difference in the contents of 18:2n-6 and 20:5n-3 between the control and hyodeoxycholic acid diet groups in terms of the effects on the incorporation of diet-derived n-6 and n-3 precursor fatty acids as well as their elongation and desaturation. We should also mention that the reduction in the contents of 20:5n-3 in tissues would augment the synthesis of 20:4n-6-derived eicosanoids with higher proinflammatory potencies [37] and reduce the synthesis of anti-inflammatory 20:5n-3-derived eicosanoids [38]. Therefore, the effect of the administration of hyodeoxycholic acid on inflammatory responses should be further investigated.
In this study, we demonstrated that hyodeoxycholic acid exerts the hypolipidemic effect in mice, in which differential regulations of lipogenesis and fatty acid desaturation in different lipid fractions of the serum and liver are suggested to be involved. We also mention that significant portions of the effects of hyodeoxycholic acid administration overlap with those of the administration of pig bile containing hyodeoxycholic acid [14], suggesting that hyodeoxycholic acid plays an important role in the hypolipidemic effect of pig bile. However, the effects of hyodeoxycholic acid administration on the expression levels of proteins of lipogenic enzymes as well as on the rates of lipogenesis in the liver should be essentially investigated. These assessments might be difficult in in vivo experiments, but could be carried out more precisely in in vitro systems using cultured liver cells. In addition, the estimation of the intrinsic activity of hyodeoxycholic acid for FXR as well as its interaction with other endogenous bile acids in the enterohepatic tissues including liver are necessary in future studies. As above, further studies are necessary to define the mechanism underlying the hyodeoxycholic acid-induced hypolipidemic effect. However, the therapeutic efficacy of hyodeoxycholic acid may be expected for diseases mediated by the excess accumulation of hepatic lipids such as steatosis and steatohepatitis [39].
Abbreviations
- ACC:
-
Acetyl-CoA carboxylase
- CE:
-
Cholesteryl ester
- CYP7a1:
-
Cytochrome P450 7a1
- FXR:
-
Farnesoid X receptor
- FAS:
-
Fatty acid synthase
- HMGCAR:
-
3-Hydroxy-3-methylglutaryl coenzyme-A reductase
- LCMS:
-
Liquid chromatography–mass spectrometry
- MUFA:
-
Monounsaturated fatty acid
- PL:
-
Phospholipid
- SCD1:
-
Stearoyl-CoA desaturase-1
- SOAT2:
-
Sterol-O-acyltransferase-2
- SREBP1c:
-
Sterol regulatory element binding protein 1c
- TAG:
-
Triglyceride
References
Keitel V, Kubitz R, Häussinger D (2008) Endocrine and paracrine role of bile acids. World J Gastroenterol 14:5620–5629
Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B (2009) Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 89:147–191
Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J (2004) Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest 113:1408–1418
Bilz S, Samuel V, Morino K, Savage D, Choi CS, Shulman GI (2006) Activation of the farnesoid X receptor improves lipid metabolism in combined hyperlipidemic hamsters. Am J Physiol Endocrinol Metab 290:E716–E722
Einarsson C, Hillebrant CG, Axelson M (2001) Effects of treatment with deoxycholic acid and chenodeoxycholic acid on the hepatic synthesis of cholesterol and bile acids in healthy subjects. Hepatology 33:1189–1193
Song KH, Li T, Owsley E, Strom S, Chiang JY (2009) Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology 49:297–305
Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ (2000) Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102:731–744
Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, Edwards PA (2006) Identification of novel pathways that control farnesoid X receptor-mediated hypocholesterolemia. Proc Natl Acad Sci USA 103:1006–1011
Zhang Y, Yin L, Anderson J, Ma H, Gonzalez FJ, Willson TM, Edwards PA (2010) Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. J Biol Chem 285:3035–3043
Fiorucci S, Cipriani F, Baldelli A, Mencarelli A (2010) Bile acid-activated receptors in the treatment of dyslipidemia and related disorders. Prog Lipid Res 49:171–185
Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM (1999) Bile acids: natural ligands for an orphan nuclear receptor. Science 284(5418):1365–1368
Watanabe S, Tsuneyama K (2010) A triglyceride-lowering effect of cattle bile is associated with elevation of cholesterol levels and liver injury in mice. J Trad Med 27:179–185
Watanabe S, Tsuneyama K (2012) Cattle bile but not bear bile and pig bile induce lipid profile changes and fatty liver injury in mice. J Toxicol Sci 37:105–121
Watanabe S, Fujita K (2013) Pig bile reduces hepatic triglyceride content in mice. J Trad Med 30:190–197
Qiao X, Ye M, Pan DL, Miao WJ, Xiang C, Han J, Guo DA (2011) Differentiation of various traditional Chinese medicines derived from animal bile and gallstone: simultaneous determination of bile acids by liquid chromatography coupled with triple quadrupole mass spectrometry. J Chromatogr A 1218:107–117
Cohen-Solal C, Parquet M, Férézou J, Sérougne C, Lutton C (1995) Effects of hyodeoxycholic acid and alpha-hyocholic acid, two 6alpha-hydroxylated bile acids, on cholesterol and bile acid metabolism in the hamster. Biochim Biophys Acta 1257:189–197
Wang DQ, Tazuma S, Cohen DE, Carey MC (2003) Feeding natural hydrophilic bile acids inhibits intestinal cholesterol absorption: studies in the gallstone-susceptible mouse. Am J Physiol Gastrointest Liver Physiol 285:G494–G502
Hara A, Radin N (1978) Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem 90:420–426
Alnouti Y, Csanaky IL, Klaassen CD (2008) Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J Chromatogr B 873:209–217
Russell DW, Setcehll KD (1992) Bile acid biosynthesis. Biochemistry 31:4737–4749
Li-Hawkins J, Gåfvels M, Olin M, Lund EG, Andersson U, Schuster G, Björkhem I, Russell DW, Eggertsen G (2002) Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J Clin Invest 110:1191–1200
Miyazaki M, Bruggink SM, Ntambi JM (2006) Identification of mouse palmitoyl-coenzyme A Delta9-desaturase. J Lipid Res 47:700–704
Miyazaki M, Kim YC, Ntambi JM (2001) A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J Lipid Res 42:1018–1024
Jonas A (1998) Regulation of lecithin cholesterol acyltransferase activity. Prog Lipid Res 37:209–234
Jonas A (2000) Lecithin cholesterol acyltransferase. Biochim Biophys Acta 1529:245–256
Bilz S, Samuel V, Morino K, Savage D, Choi CS, Shulman GI (2005) Activation of the farnesoid X receptor improves lipid metabolism in combined hyperlipidemic hamsters. Am J Physiol Endocrinol Metab 290:E716–E722
Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ (2002) Vitamin D receptor as an intestinal bile acid sensor. Science 296:1313–1316
Howard WR, Pospisil JA, Njolito E, Noonan DJ (2000) Catabolites of cholesterol synthesis pathways and forskolin as activators of farnesoid X-activated unclear receptor. Toxicol Appl Pharmacol 163:195–202
Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F (2013) Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 17:225–235
Hu X, Bonde Y, Eggertsen G, Rudling M (2014) Muricholic bile acids are potent regulators of bile acid synthesis via a positive feedback mechanism. J Intern Med 275:27–38
Cohen BI, Singhal AK, Mongelli J, Rothschild MA, McSherry CK, Mosbach EH (1983) Hydroxylation of secondary bile acids in the perfused prairie dog liver. Lipids 18:909–912
Bodin K, Lindbom U, Diczfalusy U (2005) Novel pathways of bile acid metabolism involving CYP3A4. Biochim Biophys Acta 1687:84–93
Alrefai WA, Gill RK (2007) Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res 24:1803–1823
Wang H, Chen J, Hollister K, Sowers LC, Forman BM (1999) Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 3:543–553
Okuyama H, Kobayashi T, Watanabe S (1996) Dietary fatty acids -the N-6/N-3 balance and chronic elderly diseases. Excess linoleic acid and relative N-3 deficiency syndrome seen in Japan. Prog Lipid Res 35:409–457
Jakobsson A, Westerberg R, Jacobsson A (2006) Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res 45:237–249
Smith WL (2005) Cyclooxygenases, peroxide tone and the allure of fish oil. Curr Opin Cell Biol 17:174–182
Serhan CN, Chiang N, Van Dyke TE (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8:349–361
Ferré P, Foufelle F (2010) Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab Suppl 2:83–92
Acknowledgments
This work was supported in part by a Grant-in-Aid in Regional Innovation R&D Program from the Ministry of Economy, Trade and Industry, Japan and by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Research Project Number: 23590873). The authors wish to thank Mr. Shunsuke Komado and Mr. Atsushi Yoneyama (University of Toyama) for their help in analyzing gene expression and fatty acid composition.
Conflict of interest
S.W. received financial support from Kokando Co., Ltd., Japan. K.F. has no conflict of interest directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Watanabe, S., Fujita, K. Dietary Hyodeoxycholic Acid Exerts Hypolipidemic Effects by Reducing Farnesoid X Receptor Antagonist Bile Acids in Mouse Enterohepatic Tissues. Lipids 49, 963–973 (2014). https://doi.org/10.1007/s11745-014-3947-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-014-3947-y