Abstract
Palm oil that has been interesterified to produce a higher proportion of palmitic acid (16:0) in the sn-2 position reduces postprandial lipemia in young, normolipidemic men and women, but effects in older subjects with higher fasting triacylglycerol (TAG) concentrations are unknown. We tested the hypothesis that high-fat meals rich in interesterified palm olein (IPO) decrease lipemia and alter plasma lipoprotein fraction composition compared to native palm olein (NPO) in men aged 40–70 years with fasting TAG concentrations ≥1.2 mmol/L. Postprandial changes in plasma lipids following meals containing 75 g fat (NPO and IPO) were compared using a randomized, double-blind crossover design (n = 11). Although there were no significant differences in plasma TAG concentrations between meals over the total 6-h postprandial measurement period, IPO resulted in a decreased plasma TAG response during the first 4 h of the postprandial period (iAUC 1.65 mmol/L h, 95 % CI 1.01–2.29) compared to NPO (iAUC 2.33 mmol/L h, 95 % CI 1.58–3.07); meal effect P = 0.024. Chylomicron fraction TAG concentrations at 4–6 h were slightly reduced following IPO compared to NPO [NPO−IPO mean difference 0.29 mmol/L (95 % CI −0.01–0.59), P = 0.055]. There were no differences in IDL fraction TAG, cholesterol or apolipoprotein B48 concentrations following IPO compared with NPO. In conclusion, consuming a meal containing palm olein with a higher proportion of 16:0 in the sn-2 position decreases postprandial lipemia compared to native palm olein during the early phase of the postprandial period in men with higher than optimal fasting triacylglycerol concentrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Following consumption of a fat-containing meal, circulating triacylglycerol (TAG) concentrations are elevated for up to 8 h. As reviewed recently, three recent prospective studies have confirmed non-fasting TAG concentrations as a strong predictor of risk of coronary heart disease, and to be more discriminatory than fasting TAG concentrations [1–4]. Long chain saturated fatty acids such as palmitic acid (16:0) result in pronounced postprandial lipemia, but the magnitude of the increase in blood TAG concentrations may depend on the positional composition of the triacylglycerol [5].
Interesterification is an industrial process to shuffle the position of fatty acids within the TAG molecule without changing the fatty acid composition of the TAG and is commonly used by the food industry to produce fats with desirable physical characteristics. Interesterification of palm oil, which has 16:0 predominantly in the outer positions of the TAG molecule (sn-1 and sn-3), increases the proportion of 16:0 in the sn-2 position and results in a fat with a higher melting point than native palm olein (NPO), the latter being liquid at room temperature. The transition within the oils and fats industry away from animal fats and trans fatty acids towards interesterified palm olein (IPO), for use as a solid vegetable fat in bakery products and fat spreads, has recently prompted a number of scientific investigations into the acute and chronic health effects of interesterified fats, particularly in relation to their atherogenic potential [5–10]. Animal studies have demonstrated that consumption of TAG containing palmitic acid in the sn-2 position promotes atherogenesis to a greater extent than TAG containing palmitic acid the sn-1 or sn-3 positions [11]. However, chronic human studies have not found an effect of positional composition of palmitic acid on cholesterol concentrations [12, 13], and there have been no studies to date investigating longer term markers of atherogenesis.
With regard to postprandial lipemia, the evidence to date is fairly consistent in showing a decreased postprandial TAG response to IPO compared to NPO during the initial phase of the postprandial period. Specifically, Yli-Jokipii and colleagues reported decreased iAUC for plasma TAG concentrations after 55 g/m2 body surface area IPO compared to NPO in ten healthy premenopausal women with a mean age of 27 years (SD 3) [14], Berry et al. reported decreased plasma TAG concentrations 1–2 h after 50 g IPO compared to NPO in 20 healthy young men with a mean age of 29 years (SD 10) [7], and Sanders et al. reported decreased plasma TAG concentrations 1–4 h after 50 g IPO compared to NPO (and high oleic sunflower oil) in 50 healthy young men and women with a mean aged of 24 years (SD 3) [10]. These three study populations were all young, with mean fasting TAG from 0.7 to 1.1 mmol/L (SD 0.4–0.5), and mean body mass indices of 21–23 kg/m2 (SD 2–3). Sanders et al. concluded that fats that are solid at room temperature with a higher proportion of palmitic acid at the sn-2 position decrease postprandial lipemia compared with liquid oils, but that this requires replication in older subjects [10]. The relative postprandial lipemic response to IPO in a study population considered to be more “at-risk” of developing cardiovascular disease has not yet been characterized. Furthermore, differences in postprandial lipemia that may arise following IPO compared to NPO may result in compositional differences in circulating lipid fractions which may differ in their atherogenic potential; for example, a slower rate of increase of TAG-rich lipoproteins in the early phase of postprandial lipemia may be associated with a preponderance of TAG-rich lipoproteins and their remnants in the later postprandial phase.
The present study set out to test the hypothesis that interesterified palm olein, with an increased proportion of palmitic acid in the sn-2 position, would decrease postprandial lipemia in middle-aged/older men with higher than optimal fasting plasma TAG concentrations, but would increase the concentration of TAG in the lipoprotein fraction rich in TAG-rich lipoprotein remnants (the “IDL” fraction) in the later postprandial period. The primary outcome measure was incremental area under the curve (iAUC) for plasma TAG, and secondary outcome measures included TAG and cholesterol content of lipid subclasses. Other outcome variables included blood pressure and vascular tone assessed by digital volume pulse (DVP). This is the first study to date to investigate compositional changes (in TAG, cholesterol and apo-B48 concentrations) in lipid subclasses following a single meal of IPO compared to NPO.
Subjects and Methods
Subjects
Ethical approval for the study was obtained from a National Health Service research ethics committee (NRES Committee London, Dulwich reference 11/LO/0116), and written informed consent was given by participants. This trial was registered at clinicaltrials.gov as NCT01710280. Advertisements and a circular e-mail within King’s College London were used for recruitment, which was initiated in May 2011 by research investigators. A participant information sheet was provided to volunteers who expressed interest. Participants, recruited from King’s College London and the wider local community, attended the Metabolic Research Unit at the Diabetes & Nutritional Sciences Division, King’s College London in a fasting state for a screening appointment which included the measurement of height, weight, waist circumference, % body fat (by bioelectrical impedance using the Tanita™ body composition analyser), seated blood pressure, liver function tests, glucose, lipid profile and haematology. A small remuneration was given for participation in the postprandial study days. Since the target population was middle-aged/older men with higher than optimal fasting plasma TAG concentrations, inclusion criteria were: healthy males aged 40–70 years; non-smoking; and fasting plasma TAG ≥1.2 mmol/L. The initial aim was to recruit men with fasting plasma TAG ≥1.5 mmol/L but this was adjusted to ≥1.2 mmol/L due to difficulties recruiting sufficient numbers of volunteers. Exclusion criteria were as follows: reported medical history of cardiovascular disease, cancer, liver, kidney or bowel disease; fasting glucose ≥6.1 mmol/L or uncontrolled type 2 diabetes; presence of gastrointestinal disorder or use of drug which is likely to alter gastrointestinal motility or nutrient absorption; history of substance abuse or alcoholism; current self-reported weekly alcohol intake exceeding 28 units; allergy or intolerance to any component of the test meals; unwilling to restrict consumption of any source of fish oil for the duration of the study; weight change of >3 kg in preceding 2 months; body mass index <20 and >35 kg/m2; fasting blood cholesterol >7.8 mmol/L; and current use of lipid lowering medication. Sample size calculations were based on 80 % power at P = 0.05 to detect a 1.4 mmol/L h difference in the iAUC for plasma triacylglycerol concentrations, which gave a sample size of 11.
Study Design
A randomized, double-blind, crossover design was used to compare two test meals that did not differ in appearance, aroma or taste. Each test meal consisted of a muffin and a milkshake and provided 4.38 MJ (1047 kcal), 14 g protein, 93 g carbohydrate, and 75 g test fat. The two test fats were as follows: native palm olein (NPO, iodine value 56, Intercontinental Speciality Fats, Selangor, Malaysia) and interesterified palm olein (IPO, iodine value 56, Wilmar PGEO Edible Oils, Johor, Malaysia). Both palm olein (PO) fats had a similar fatty acid composition (shown in Table 1), but the IPO has 45.9 mol% of palmitic acid in the sn-2 position compared to 9.8 mol% in NPO [5]. The test fats were baked into muffins, labelled with a code A or B by an independent research technician, and stored frozen until consumed. The allocation of treatment was blinded from both the investigators and the study participants.
Subjects were randomly allocated to receive either meal A or B for their first visit by a single research investigator using a computer randomisation program. The participants consumed each of the two test meals in random order, and each test meal protocol was separated by at least 1 week. On the day preceding each test meal, participants were told not to participate in strenuous exercise and to avoid alcohol and foods high in fat. They were provided with a standardized low-fat meal (containing <10 g fat) as their evening meal, which they were required to consume before 2200 hours and then to avoid eating or drinking anything except for water. Participants attended the Metabolic Research Unit between 0800 and 1000 hours the next day. A cannula was inserted into the forearm antecubital vein, and blood was collected for baseline analysis. Following a 10 min supine rest, blood pressure and digital volume pulse measurements were taken in a supine position. The test meal was consumed within 10 min. Further venous blood samples were collected hourly until 6 h after the test meal and blood pressure/vascular measurements were made 2, 4 and 6 h after the test meal. Participants had access to water to sip as required over the 6 h period. After the blood sample at t + 3 h, they consumed a light, fat-free lunch (1.7 MJ consisting of 190 g fat-free yoghurt (<0.1 g fat) and a 120 g banana) as in previous studies [6, 7, 10, 15].
Methods
Blood samples for total plasma fatty acid composition, plasma TAG fraction fatty acid composition, plasma NEFA fraction fatty acid composition, plasma TAG, cholesterol, and NEFA concentrations were collected into EDTA tubes and centrifuged at 1,500×g for 15 min at 4 °C. Two millilitres plasma from blood samples taken at 4, 5 and 6 h time points was mixed with 0.77 g potassium bromide and 50 mg sucrose, then added to Ultra clear ultracentrifuge tubes (Beckman Coulter UK Ltd, catalogue number 344060), and over-layered with solutions of sodium chloride, potassium bromide and 100 mg/L EDTA sequentially at densities of 1.225 g/ml (2 ml) and 1.100 g/ml (4 ml), ending with distilled water containing 100 mg/L EDTA (4 ml), according to the method of Terpstra and colleagues [16, 17]. Ultracentrifugation using a SW40 Ti swinging bucket rotor (Beckman) in a Beckman-Coulter Optima L-90K ultracentrifuge at 160,070×g for 42 min at 20 °C with brake to 500 rpm was used to isolate the chylomicron-rich fraction (aspirating the top 1 ml layer), then the ultracentrifuge tubes were refilled with distilled water and ultra centrifuged at 230,501×g for 23 h at 22 °C to obtain the “IDL” layer which contains VLDL, IDL and chylomicron remnants. For ease of nomenclature these lipoprotein fractions will be referred to as the chylomicron fraction (density <1.006 kg/L) and IDL fraction respectively (density 1.006–1.019 kg/L), although it is recognised that both fractions will also contain VLDL. Further aliquots of plasma obtained from blood collected hourly 0–6 h were stored frozen at −80 °C until analysed. Enzymatic assays were used to determine concentrations of NEFA (WAKO NEFA-HR; WAKO Diagnostics), cholesterol, and TAG on an ILAB-650 analyser (Instrumentation Laboratory). The plasma TAG and NEFA fractions were separated by TLC and analyzed by GC to determine fatty acid composition [18, 19]. Apolipoprotein B48 concentrations in IDL fractions were measured using a human apo B48 ELISA kit (Shibayagi Co. Ltd., Shibukawa, Japan). Total apolipoprotein B concentrations were measured using a polyethylene glycol (PEG) enhanced immunoturbidimetric assay (Siemans Healthcare Diagnostics Ltd, Surrey, UK), however, results for this analysis are not available due to a technical error that occurred during analysis. The intra-assay coefficients of variation were: NEFA, 6.3 %; TAG, 3.4 %; cholesterol, 1.0 %; and apolipoprotein B48, 7.1 %.
Blood pressure was measured according to British Hypertension Society guidelines using an automated upper arm blood pressure monitor, the Omron 705IT (Omron Healthcare Europe B.V.). The digital volume pulse was obtained by photoplethysmography (PulseTrace, Micro Medical Ltd., Kent, UK) and used to calculate stiffness index (DVP-SI, m/s) and reflection index (DVP-RI, %).
Statistical Analyses
An incremental AUC (iAUC) was calculated for plasma TAG, NEFA and cholesterol concentrations and paired T test carried out to compare meal differences. Repeated measures ANOVA was performed (meal and time as within-subject factors) on change from baseline data for total plasma analytes, or raw data for lipoprotein fraction data which was only collected at 4–6 h. A P value < 0.05 was considered statistically significant.
Results
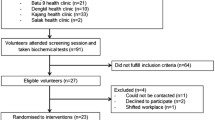
Twenty-five volunteers who met the initial eligibility criteria following a telephone questionnaire attended screening sessions at the Metabolic Research Unit, Diabetes & Nutritional Sciences Division, King’s College London, of whom 14 did not meet inclusion criteria (Fig. 1). The screening characteristics of the 11 participants who completed the study (June–December 2011) are shown in Table 2.
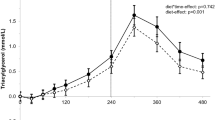
The postprandial changes in plasma TAG and the iAUC after the test meals are shown in Fig. 2. There were no significant differences between meals although both the treatment effect for plasma TAG changes from baseline (1–6 h) (P = 0.067) and the iAUC 0–6 h (P = 0.09) showed a trend towards a smaller increase in postprandial plasma TAG concentrations following IPO compared to NPO. This was more marked in the early phase of postprandial lipemia and post hoc analysis of the iAUC from 1 to 4 h was conducted to determine the early phase postprandial TAG response. There was a significant difference between meals, with a decrease in Δ plasma TAG 1–4 h (P = 0.020) and in the iAUC 0–4 h (P = 0.024) observed following IPO compared to NPO. There were no differences between meals for changes or iAUC in plasma cholesterol or NEFA concentrations.
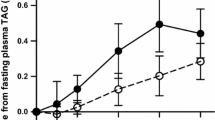
a Plasma and chylomicron fraction triacylglycerol (TAG) concentrations following 75 g native palm olein (closed symbols) and interesterified palm olein (open symbols). Mean with 95 % CI, n = 11. Plasma TAG data were analysed by ANOVA on changes from fasting values: 1–6 h meal effect P = 0.067, meal × time interaction P = 0.469, and time effect P < 0.0005; 1–4 h meal effect P = 0.020, meal × time interaction P = 0.316, and time effect P < 0.0005. Chylomicron fraction data were analysed by ANOVA on raw values: 4–6 h meal effect P = 0.055, meal × time interaction P = 0.147, and time effect P = 0.037. b Paired T test of the iAUC 1–6 h for plasma TAG showed a non-significant difference between meals (P = 0.090) but paired T test of the iAUC 1–4 h for plasma TAG showed a significant reduction following IPO compared to NPO (P = 0.024); error bars are 95 % CI. c Plasma non-esterified fatty acids (NEFA) concentrations following 75 g native palm olein (closed symbols) and interesterified palm olein (open symbols). Mean with 95 % CI, n = 10. Data were analyzed by ANOVA on changes from fasting values: meal effect P = 0.928, meal × time interaction P = 0.572 and time effect P < 0.0005. d Plasma cholesterol concentrations following 75 g native palm olein (closed symbols) and interesterified palm olein (open symbols). Mean with 95 % CI, n = 11. Data were analyzed by ANOVA on changes from fasting values: meal effect P = 0.613, meal × time interaction P = 0.102 and time effect P = 0.001
The TAG concentration of the chylomicron fraction (4–6 h) was decreased following IPO compared to NPO but this did not quite reach significance (P = 0.055) (Table 3). However, a paired T test at the point of peak lipemia (4 h) showed that chylomicron TAG was significantly lower after IPO compared to NPO (mean difference NPO-IPO 0.463 mmol/L, 95 % CI 0.032–0.894 mmol/L, P = 0.038). There were no differences in the TAG concentration of the IDL fraction, nor the cholesterol concentrations of the chylomicron and IDL fractions. Furthermore, no differences in IDL fraction apolipoprotein B48 concentrations were observed, although there was a large amount of inter-individual variability (Table 3). The fatty acid composition of the plasma TAG fraction was close to the test meal fat composition at 4–5 h, the point of peak lipemia (Table 4). The proportion of plasma NEFA and TAG fractions that was 16:0 increased (P < 0.0005 and P < 0.005, respectively) and the TAG fraction 18:1n-9 proportion increased (P < 0.005) following the meals, but the proportion of the NEFA fraction that was 18:1n-9 initially decreased at 1–2 h (P < 0.005). Comparison between the test meals revealed that 16:0,18:0, 18:1n-9 and 18:2n-6 profiles following NPO and IPO were similar.
There were no significant meal effects or meal × time interactions for blood pressure or digital volume pulse (Supplementary Fig. 1).
Discussion
Evidence points to an independent role for non-fasting (postprandial) triglyceride concentrations as a risk factor for cardiovascular disease [20], and calculated non-fasting remnant cholesterol concentrations have been associated with risk of coronary heart disease [21]. This study aimed to investigate whether the interesterification of palm olein resulted in adverse effects on postprandial lipemia in healthy men aged 40–70 years with higher than optimal fasting TAG concentrations. As previously reported in younger men and women [7, 10, 14], a blunted increase in plasma TAG during the early, pre-peak phase of postprandial lipemia was observed following IPO compared to NPO. We decided to investigate an older, male population who had high-normal levels of fasting TAG and were likely to have a more pronounced postprandial lipemia [22], to confirm that the same response occurs in those who do not have overt cardiovascular disease or type 2 diabetes, but may have raised metabolic risk factors.
Importantly, these results confirm that interesterification influences postprandial lipemic responses even in individuals with higher fasting TAG levels and a more pronounced lipemic response. The reduction in iAUC for plasma TAG during the early phase was in the order of 30 %, which is considered clinically relevant. However, due to the lack of clinical prevention trials using standardized postprandial TAG measurements the implications of this size of reduction on risk of cardiovascular events is unknown. Furthermore the relative importance of the magnitude versus the duration of postprandial lipemia on cardiovascular risk is unknown. Quintiles of random measurements of non-fasting TAG concentrations that differed by a mean of ~0.3–0.5 mmol/L were associated with significantly increasing hazard ratios for coronary heart disease mortality in men from the Norwegian Counties Study, suggesting that postprandial differences of a similar size to that observed here could be clinically significant if these fats form a major component of a habitual high-fat diet [4]. The postprandial TAG response was approximately 1.5 mmol/L higher at the 4 h peak concentration compared with our previous study [10], but it is also important to note that the total amount of fat was greater in the current study: 75 g compared to 50 g used previously [10]. A meal containing 75 g fat was administered because an objective of the study was to analyse the lipid composition of the chylomicron and IDL fractions, and by inducing a maximal increase in postprandial TAG-rich lipoprotein (TRL), the detection of any meal-induced differences would be optimized.
Prolonged lipemia is expected to result in compositional changes in lipid fractions due to the reciprocal exchange of TAG and cholesterol via CETP between chylomicron and HDL and LDL particles. It was hypothesised that although IPO might elicit a beneficial reduction in the early postprandial phase response (0–4 h) a subsequently prolonged postprandial lipemic response (>4 h) would result in greater lipoprotein remodelling by CETP and therefore a higher remnant TAG concentration and greater TRL cholesterol content. The cholesterol content of TAG-rich lipoproteins is thought to be an important causal factor in the development of atherosclerosis [23]. The concentration of TAG in the chylomicron fraction was moderately reduced 4–6 h following IPO compared to NPO, reflecting the reduction in total TAG concentration in plasma. This did not result in differences in the TAG:cholesterol ratio in the chylomicron and IDL fractions from 4 to 6 h. Furthermore, the IDL fraction apolipoprotein B48 concentrations at 4–6 h did not appear to be affected by interesterification of palm olein, suggesting that clearance of chylomicron remnants is not improved nor impaired as a consequence of the reduced lipemia in the earlier postprandial phase. This agrees with our earlier study showing that total plasma apolipoprotein B48 concentrations did not differ over an 8 h postprandial period following 50 g IPO and NPO in younger men and women [10].
This observed difference in the postprandial lipemic response might be due to differences in the rate of intestinal absorption of the test fats. Palmitic acid profiles in the plasma TAG fraction and the proportions of palmitic acid in isolated chylomicron TAG fractions were similar following NPO and IPO as observed in our previous studies [7, 10], suggesting that both fats were absorbed at similar rates. However, this is inconclusive unless tracer-labelled palmitic acid is ingested, as total plasma TAG fraction palmitic acid also reflects VLDL palmitic acid and/or chylomicron palmitic acid released from stored TAG within the enterocytes derived from a previous meal. Evidence suggests that palmitic acid in the sn-2 position is unlikely to delay or reduce absorption in adults compared with TAG containing palmitic acid in the sn-1,3 positions [9]. It is therefore likely that the reduced lipemia observed following interesterification of palmitic acid-rich fats is due to changes in the physical characteristics of the fats, whereby the process of interesterification generates TAG species such as tri-palmitin and di-saturated TAG, which have melting points higher than body temperature. Indeed, in the current study the IPO contained a greater proportion of solid fat at 37 °C compared to NPO which was liquid at 37 °C and it is likely that the different melting points of the two test fats influenced the rate of digestion and absorption in the gut [5].
A slower rate of absorption may have initially lowered the lipemic response but ultimately resulted in increased plasma TAG concentrations in the very late postprandial period, beyond the 6 h period monitored in the current study; this was observed by Sanders et al. where plasma TAG concentrations remained at about 28 % above fasting concentrations 8 h following IPO, compared to only about 10 % above fasting concentrations 8 h following NPO [10]. The duration of postprandial measurements is a limitation of the present study design as the effects on lipemia/TAG-rich lipoprotein composition beyond 6 h, and following subsequent meals, would further elucidate any differences as a result of interesterification of palm olein. Future studies should investigate compositional changes in the lipoprotein fractions at 8 and 10 h postprandially to achieve a better understanding of the influence of prolonged lipemia on the atherogenic potential of lipoproteins.
In summary, these compositional data from the lipoprotein fractions suggest that remnant triacylglycerol-rich lipoproteins are not differentially affected by palm oil interesterification up to 6 h, and imply that there is unlikely to be a greater risk of atherosclerosis when habitually consuming interesterified palm olein compared to native palm olein. However, further detailed investigations into lipoprotein fractions including the later postprandial phase (up to 8 or 10 h) and during chronic dietary interventions are required to confirm that compositional changes do not occur due to slower absorption and prolonged lipemia.
Abbreviations
- iAUC:
-
Incremental area under the curve
- DVP:
-
Digital volume pulse
- IPO:
-
Interesterified palm olein
- NEFA:
-
Non-esterified fatty acids
- NPO:
-
Native palm olein
- TAG:
-
Triacylglycerol
References
Jackson KG, Poppitt SD, Minihane AM (2012) Postprandial lipemia and cardiovascular disease risk: interrelationships between dietary, physiological and genetic determinants. Atherosclerosis 220:22–33
Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM (2007) Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 298:309–316
Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A (2007) Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 298:299–308
Lindman AS, Veierod MB, Tverdal A, Pedersen JI, Selmer R (2010) Nonfasting triglycerides and risk of cardiovascular death in men and women from the Norwegian Counties Study. Eur J Epidemiol 25:789–798
Berry SE (2009) Triacylglycerol structure and interesterification of palmitic and stearic acid-rich fats: an overview and implications for cardiovascular disease. Nutr Res Rev 22:3–17
Berry SE, Miller GJ, Sanders TA (2007) The solid fat content of stearic acid-rich fats determines their postprandial effects. Am J Clin Nutr 85:1486–1494
Berry SE, Woodward R, Yeoh C, Miller GJ, Sanders TA (2007) Effect of interesterification of palmitic acid-rich triacylglycerol on postprandial lipid and factor VII response. Lipids 42:315–323
Sundram K, Karupaiah T, Hayes KC (2007) Stearic acid-rich interesterified fat and trans-rich fat raise the LDL/HDL ratio and plasma glucose relative to palm olein in humans. Nutr Metab 4:3
Hayes KC, Pronczuk A (2010) Replacing trans fat: the argument for palm oil with a cautionary note on interesterification. J Am Coll Nutr 29:253S–284S
Sanders TA, Filippou A, Berry SE, Baumgartner S, Mensink RP (2011) Palmitic acid in the sn-2 position of triacylglycerols acutely influences postprandial lipid metabolism. Am J Clin Nutr 94:1433–1441
Kritchevsky D, Tepper SA, Chen SC, Meijer GW, Krauss RM (2000) Cholesterol vehicle in experimental atherosclerosis. 23. Effects of specific synthetic triglycerides. Lipids 35:621–625
Zock PL, de Vries JH, de Fouw NJ, Katan MB (1995) Positional distribution of fatty acids in dietary triglycerides: effects on fasting blood lipoprotein concentrations in humans. Am J Clin Nutr 61:48–55
Nestel PJ, Noakes M, Belling GB, McArthur R, Clifton PM (1995) Effect on plasma lipids of interesterifying a mix of edible oils. Am J Clin Nutr 62:950–955
Yli-Jokipii K, Kallio H, Schwab U, Mykkanen H, Kurvinen JP, Savolainen MJ, Tahvonen R (2001) Effects of palm oil and transesterified palm oil on chylomicron and VLDL triacylglycerol structures and postprandial lipid response. J Lipid Res 42:1618–1625
Sanders TA, Oakley FR, Cooper JA, Miller GJ (2001) Influence of a stearic acid-rich structured triacylglycerol on postprandial lipemia, factor VII concentrations, and fibrinolytic activity in healthy subjects. Am J Clin Nutr 73:715–721
Terpstra AH, Woodward CJ, Sanchez-Muniz FJ (1981) Improved techniques for the separation of serum lipoproteins by density gradient ultracentrifugation: visualization by prestaining and rapid separation of serum lipoproteins from small volumes of serum. Anal Biochem 111:149–157
Terpstra AH (1985) Isolation of serum chylomicrons prior to density gradient ultracentrifugation of other serum lipoprotein classes. Anal Biochem 150:221–227
Lepage G, Roy CC (1986) Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 27:114–120
Sanders TA, Berry SE, Miller GJ (2003) Influence of triacylglycerol structure on the postprandial response of factor VII to stearic acid-rich fats. Am J Clin Nutr 77:777–782
Boren J, Matikainen N, Adiels M, Taskinen MR (2014) Postprandial hypertriglyceridemia as a coronary risk factor. Clinica Chimica Acta; Int J Clin Chem 431C:131–142
Varbo A, Benn M, Tybjaerg-Hansen A, Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG (2013) Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol 61:427–436
Jackson KG, Knapper-Francis JME, Morgan LM, Webb DH, Zampelas A, Williams CM (2003) Exaggerated postprandial lipaemia and lower post-heparin lipoprotein lipase activity in middle-aged men. Clin Sci (Lond, Engl: 1979) 105:457–466
Varbo A, Benn M, Tybjaerg-Hansen A, Nordestgaard BG (2013) Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation 128:1298–1309
Acknowledgments
This study was funded by King’s College London. We thank Tracy Dew (King’s College Hospital), and Robert Gray, Anne Catherine-Perz, Sarah Cottin, Fawaz Alzaid and Jai David (King’s College London) for their assistance with sample processing and analysis during the study. Fats were provided by Intercontinental Specialty Fats, Selangor, Malaysia and Wilmar PGEO Edible Oils, Johor, Malaysia. The authors’ responsibilities were as follows—WLH, SEB, and TABS: conceived and devised the study and contributed to the running of the study and analysis and writing of the manuscript; MFB, JH, LVW, AF: organized and conducted the study (including recruitment, enrolment, assignment to intervention, conducting study days) at King’s College London and contributed to sample analysis and writing the manuscript. Thomas Sanders is a member of the Programme Advisory Committee of the Malaysian Palm Oil Board, a member of the Scientific Advisory Committee of the Global Dairy Platform.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Hall, W.L., Fiuza Brito, M., Huang, J. et al. An Interesterified Palm Olein Test Meal Decreases Early-Phase Postprandial Lipemia Compared to Palm Olein: a Randomized Controlled Trial. Lipids 49, 895–904 (2014). https://doi.org/10.1007/s11745-014-3936-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-014-3936-1