Abstract
Camelina oil (CO) replaced 50 and 100 % of fish oil (FO) in diets for farmed rainbow trout (initial weight 44 ± 3 g fish−1). The oilseed is particularly unique due to its high lipid content (40 %) and high amount of 18:3n-3 (α-linolenic acid, ALA) (30 %). Replacing 100 % of fish oil with camelina oil did not negatively affect growth of rainbow trout after a 12-week feeding trial (FO = 168 ± 32 g fish−1; CO = 184 ± 35 g fish−1). Lipid and fatty acid profiles of muscle, viscera and skin were significantly affected by the addition of CO after 12 weeks of feeding. However, final 22:6n-3 [docosahexaenoic acid (DHA)] and 20:5n-3 [eicosapentaenoic acid (EPA)] amounts (563 mg) in a 75 g fillet (1 serving) were enough to satisfy daily DHA and EPA requirements (250 mg) set by the World Health Organization. Other health benefits include lower SFA and higher MUFA in filets fed CO versus FO. Compound-specific stable isotope analysis (CSIA) confirmed that the δ13C isotopic signature of DHA in CO fed trout shifted significantly compared to DHA in FO fed trout. The shift in DHA δ13C indicates mixing of a terrestrial isotopic signature compared to the isotopic signature of DHA in fish oil-fed tissue. These results suggest that ~27 % of DHA was synthesized from the terrestrial and isotopically lighter ALA in the CO diet rather than incorporation of DHA from fish meal in the CO diet. This was the first study to use CSIA in a feeding experiment to demonstrate synthesis of DHA in fish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Long chain n-3 fatty acids have been recognized for their health benefits for decades. This group of fatty acids is essential as they cannot be synthesized and therefore must be consumed in adequate amounts from the diet. Docosahexaenoic acid (DHA, 22:6n-3) and eicosapentaenoic acid (EPA, 20:5n-3) are mostly associated with benefits in heart, brain and eye health [46]. They are synthesized by marine microorganisms and travel through the ocean food chain. Therefore, the most efficient source of EPA and DHA is through consumption of lipid-rich seafood, particularly from cold-water [23]. Nearly half of the world’s seafood today is produced from aquaculture [16]. Salmonids like rainbow trout (Oncorhynchus mykiss) are an excellent source of DHA and EPA, and are also a popular farmed fish species. Like humans, rainbow trout also have a requirement for essential fatty acids, therefore DHA and EPA must be provided in the diet through other fish oils, such as herring [31]. Reducing the amount of fish oil in aquaculture feeds is critical for sustainability and economic reasons [50]; therefore research into alternative dietary oils is necessary.
Research in this area has focused on testing various terrestrial plant and animal lipids in aquaculture feeds and its effect on growth and lipid biochemistry. Most terrestrial plant oils, such as palm, sunflower, soybean, and linseed, have a higher proportion of n-6 and n-9 fatty acids and a lower proportion of n-3 fatty acids in comparison to fish oil. Although replacing fish oil with these oils generally does not affect overall health and growth of the fish, most studies have shown reduced levels of n-3 fatty acids in the fillet, particularly DHA and EPA [3, 4, 6, 26], which lowers health benefits normally provided to humans that consume fish. Therefore, research in terrestrial plant oils with significant amounts of n-3 is of particular interest.
The oilseed, Camelina sativa (commonly known as false flax or gold of pleasure) is a member of the Brassicaceae family which includes mustards, rapes, and canola [8]. The crop originated in southeastern Europe and southwestern Asia, however it has recently been reintroduced to Canadian agriculture on account of its agriculturally robust nature [53]. The oilseed presents many beneficial attributes useful to the agricultural sector, including ability to grow in low-fertility soils, tolerance to insects and weeds, and it can survive frost and freeze–thaw cycles [8]. The biochemical composition of camelina makes it an attractive nutritional source for the aquaculture feed industry. The oilseed is particularly unique due to its high lipid content (40 %) and high amount of 18:3n-3 (α-linolenic acid, ALA) (40 %), an essential medium chain n-3 PUFA which is a precursor to longer chain n-3 polyunsaturated fatty acids (PUFA) [32, 58]. Camelina oil is also naturally protected against lipid oxidation, despite increased PUFA content because it contains a high amount of γ-tocopherol, the most potent antioxidant tocopherol isomer [32]. Generally, camelina has more PUFA and monounsaturated fatty acids (MUFA) and lower saturated fatty acids (SFA) than other terrestrial plant oils, a fatty acid profile that benefits both fish and humans. These characteristics potentially give camelina a unique nutritional advantage over other plant oils that have been previously used in aquaculture. The use of camelina oil has been shown to be suitable in feeds for Atlantic cod [21, 27], and vegetable oil blends containing 20 % camelina oil have been tested in Atlantic salmon [5]; however it has not been tested in rainbow trout.
Although terrestrial plants do not naturally produce EPA and DHA, it is possible that the amount of ALA provided in the feeds may encourage synthesis of EPA and DHA in fish when these fatty acids are low or absent in the diet. Humans can synthesize DHA and EPA from ALA, however this process is inefficient with a conversion rate of <1 % [7]. Anadromous fish, those that migrate from sea water to fresh, are known to more efficiently convert ALA–DHA and EPA. For example, Atlantic salmon (Salmo salar) express the ∆5 and 6 desaturase genes that are responsible for desaturation of ALA [19, 57] and those fed a rapeseed oil diet showed even greater expression of these genes compared to the fish oil fed group [22]. In fact, this metabolic pathway is not just simply present and expressed, but is actually functional in the majority of the freshwater fish and salmonid species studied [49]. Consequently, studies have shown the n-3 LC PUFA input/n-3 LC-PUFA output ratio in fish fed vegetable oil diets is <1, indicating that salmonid aquaculture is a net producer of FO [14, 51]. Based on this premise, we hypothesized that rainbow trout will synthesize DHA and EPA to some degree from the high amount of ALA provided in the diet from camelina oil. As a tool to confirm this hypothesis, compound specific stable isotope analysis (CSIA) was used. Fatty acid isotopic signatures are frequently used in food web studies to determine the transfer of fatty acids from prey to predator based on their 13C/12C ratio [9]. In chemical and physical reactions, compounds containing the lighter 12C isotope preferentially react, resulting in a fractionation of 12C and 13C isotopes. The ratio of 13C/12C is expressed as δ13C. The δ13C of animal tissues is thought to reflect a mixture of δ13C from all diet items [52]. The ratio of carbon isotopes changes little as carbon moves through a food chain, therefore when dietary sources have distinctly different isotopic signatures, the ultimate sources of carbon and the relative contribution of different dietary items to a consumer can be determined [9, 38]. As a terrestrial plant, camelina fatty acids such as ALA and LNA have isotopic signatures distinctly different than the same fatty acids in marine sources like fish oil due to differences in the source of carbon (terrestrial carbon in the form of CO2 gas vs. marine carbon as carbonate), which results in differences in δ13C values due to how the stable isotopes are fractionated as CO2 dissolves [33]. Using CSIA, we can determine the dietary contribution of DHA and EPA in rainbow trout tissue fed a camelina oil diet and conclude whether these fatty acids were synthesized products from ALA in camelina oil or whether they have been incorporated in the tissue directly from the limited amount of DHA provided in the residual lipid in fish meal, depending on the δ13C values.
The purpose of this study was to evaluate camelina oil as a suitable lipid source for farmed rainbow trout. A nutritional feeding trial was conducted with diets containing camelina oil in order to determine lipid and tissue fatty acid composition in tissues in response to changes in diet composition. Using CSIA, this study determined the dietary contribution that camelina oil may have on the synthesis of DHA and EPA in rainbow trout.
Methods
Experimental Diets
Camelina (Calena cultivar) was grown and harvested by the Department of Plant and Animal Sciences, Dalhousie University at an off-campus location (Canning, NS, Canada). The seeds were single pressed using a KEK 0500 press at Atlantic Oilseed Processing, Ltd. (Summerside, Prince Edward Island, Canada) to extract the oil and ethoxyquin was added to the final product.
All diets were formulated as isonitrogenous, isolipidic, and isocaloric practical diets and were produced at the Faculty of Agriculture Campus, Dalhousie University (Truro, NS, Canada). The experimental treatments were as follows: a control diet with fish oil (FO); 50 % fish oil replacement with camelina oil (CO50); and 100 % fish oil replacement (CO100). Diets were formulated to meet nutritional requirements of rainbow trout [31]. All diets were steam pelleted using a laboratory pelleting mill (California Pellet Mill, San Francisco, USA). The initial size of the pellets was 1.5 mm and increased to 2.5 mm as the fish grew larger throughout each trial. Diets were stored at −20 °C until needed.
Experimental Fish
An experiment was conducted with juvenile rainbow trout (44.9 ± 10 g fish−1 mean initial weight ± SD; 15.7 ± 1.2 cm mean initial length) at the Faculty of Agriculture Campus, Dalhousie University (CCAC, 2011-016). Fish were received from Fraser Mill’s hatchery (Antigonish, NS, Canada). Fish were randomly distributed (837 total) into 9 experimental tanks (200 l capacity), each tank with 93 fish. Dietary treatments were administered to triplicate tanks for the 12-week trial. The fish were acclimated on the control diet for 1 week prior to initial sampling. A flow through system of freshwater was supplied to each tank at a rate of 10 l min−1 and a photoperiod of 12 h. The dissolved oxygen (10 mg l−1) and water temperature (14 °C) was monitored daily. Fish were fed to apparent satiation twice daily (0900 and 1600 h) and feed consumption was recorded weekly. Mortalities were weighed and recorded throughout the trial.
Sampling Methods
Sampling occurred at Dalhousie University Agriculture Campus (Truro, NS, Canada) at week 0 (the day before experimental diets were fed) and 12. Six fish per tank were randomly sampled on each sampling date and measured for length and weight. The skin was removed on the left side and muscle tissue was subsampled for dry matter and lipid analysis. The whole viscera and surrounding fat were weighed prior to sampling to measure the visceral somatic index. Lipid samples were stored on ice during sampling of each tank and were processed within an hour. Samples were collected in 50-ml test tubes that had been rinsed three times with methanol followed by three rinses with chloroform. The tubes were allowed to dry completely before they were weighed. The tubes were weighed again following the addition of the sample. After wet weights of samples were recorded, samples were covered with 8 ml of chloroform, the headspace in the tube was filled with nitrogen, the Teflon-lined caps sealed with Teflon tape and the samples were stored at −20 °C until shipment to the Ocean Sciences Centre, Memorial University (St. John’s, Newfoundland, Canada) for analysis (CCAC 12-09-MR, approved protocol for use of fish tissues from Dalhousie University).
Lipid Extracts
Lipid samples were extracted according to Parrish [36]. Samples were homogenized in a 2:1 mixture of ice-cold chloroform:methanol. Samples were homogenized with a Polytron PCU-2-110 homogenizer (Brinkmann Instruments, Rexdale, ON, Canada). Chloroform extracted water was added to bring the ratio of chloroform:methanol:water to 8:4:3. The sample was sonicated for 6 min in an ice bath and centrifuged at 4,000 rpm for 2 min. The bottom organic layer was removed using a double pipetting technique, placing a 2 ml lipid cleaned Pasteur pipette inside a 1 ml pipette, to remove the organic layer without disturbing the top aqueous layer. Chloroform was then added back to the extraction test tube and the entire procedure was repeated three times. All organic layers were pooled into a lipid-cleaned vial. The samples were concentrated using a flash-evaporator (Buchler Instruments, Fort Lee, NJ, USA).
Lipid Class Separation
Lipid class composition was determined using an Iatroscan Mark VI TLC–FID, silica coated Chromarods and a three-step development method [35]. The lipid extracts were applied to the Chromarods and focused to a narrow band using 100 % acetone. The first development system was hexane:diethyl ether:formic acid (99.95:1.0:0.05). The rods were developed for 25 min, removed from the system for 5 min and replaced for 20 min. The second development was for 40 min in hexane:diethyl ether:formic acid (79:20:1). The final development system had two steps, the first was 100 % acetone for two 15-min time periods, followed by two 10-min periods in chloroform:methanol:chloroform–extracted water (5:4:1). Before using each solvent system the rods were dried in a constant humidity chamber. After each development system, the rods were scanned in the Iatroscan and the data were collected using Peak Simple software (ver 3.67, SRI Inc.). The Chromarods were calibrated using standards from Sigma Chemicals (Sigma Chemicals, St. Louis, MO, USA).
Fatty Acid Methyl Ester (FAME) Derivatization
Lipid extracts were transesterified using the Hilditch reagent (1.5 H2SO4:98.5 anhydrous MeOH) for 1 h at 100 °C. Reagents were added in the proportion of 1.5 ml reagent per 4–16 mg of lipid [29]. Samples were vortexed half way through each derivatization reaction. To check the derivatization efficiency, samples were transesterified and then the lipid class composition of the methyl ester solution was determined by TLC–FID. The derivatization efficiency is calculated from the amount of underivatized acyl lipids compared to the amount of methyl esters in a sample.
All FAME were analyzed on a HP 6890 GC FID equipped with a 7683 autosampler. The GC column was a ZB wax + (Phenomenex, Torrance, CA, USA). The column length was 30 m with an internal diameter of 0.32 mm. The column temperature began at 65 °C at which it was held for 0.5 min. The temperature ramped to 195 °C at a rate of 40 °C min−1, held for 15 min then ramped to a final temperature of 220 °C at a rate of 2 °C min−1. This final temperature was held for 45 s. The carrier gas was hydrogen flowing at 2 ml min−1. The injector temperature started at 150 °C and was ramped to a final temperature of 250 °C at 120 °C min−1. The detector temperature stayed at 260 °C. Peaks were identified using retention times from standards purchased from Supelco: 37 component FAME mix (product number 47885-U), PUFA 3 (product number 47085-U) and PUFA 1 (product number 47033-U). Chromatograms were integrated using the Varian Galaxie Chromatography Data System, version 1.9.3.2 (Agilent Technologies, CO, USA). Percentage fatty acid data were calculated from the areas of chromatogram peaks. All fatty acids are expressed as mole percentages of total fatty acids.
Compound-Specific Stable Carbon Isotope Analysis
The δ13 values of 18:0, 18:1n-9, 18:2n-6, 18:3n-3, 20:5n-3, and 22:6n-3 were analyzed by GC-combustion-isotope mass spectrometry (GC-C-IRMS) at the Core Research Equipment and Instrument Training Network (CREAIT Network) at Memorial University of Newfoundland. FAME from dietary camelina oil, fish oil, and rainbow trout muscle tissue from FO and CO100 groups were analyzed. All δ13C values were reported relative to the Vienna Pee Dee Belemnite (VPDB) standard using standard delta notation:
An aliquot of the methanol used during methylation of fatty acids was analyzed for the δ13C composition. The average δ13C of methanol was used to correct for the additional methyl group added to fatty acids during transesterification, by subtracting the proportional contribution of methanol to the δ13C of FAME using the following equation:
where n is the number of C atoms in the fatty acid [10]. All FAME data were corrected for the contribution of this methyl group from this equation.
A two-end member mixing model [9] was used to determine the relative contribution of camelina oil and fish oil to DHA and EPA in muscle tissue:
where x k is the proportion of camelina oil C contribution to k, the fatty acid of interest, either DHA or EPA. δ13CCO is the isotope ratio of ALA in camelina oil, and δ13CFO is the isotope ratio of DHA in fish oil.
Statistical Methods
Statistical analysis followed methods outlined by Sokal and Rohlf [44]. To avoid committing a type II error (accepting a false null hypothesis), this study rigorously tested growth data in different ways to ensure conclusions made about camelina oil truly reflected the results. For analysis of growth data, lipid class data, and fatty acid data, where individual fish were weighed, measured and sampled, a three-way nested ANOVA was performed using the General Linear Model (Minitab 16 Statistical Software). The model was designed to test the effect of diet on the response variable and nested fish individuals within tanks to negate variability among tanks and individuals, while also testing for tank effects. For analysis of growth data that depend on comparison to an initial measurement and thus must be pooled per tank (i.e., mean weight gain and specific growth rate), a two-way ANOVA was performed to test the effect of diet and tank variability. In both cases, where significant differences occurred, treatment means were differentiated using the Tukey HSD multiple comparison test and all residuals were evaluated for homogeneity and normality. For analysis of growth data, t tests for each growth parameter were additionally performed between FO and CO100 treatment groups (highest camelina replacement) to verify results from the ANOVA. For analysis of stable isotope data, two-sample t tests were performed to detect the differences between δ13C values of specific fatty acids (fish oil vs. camelina oil; rainbow trout fed FO vs. rainbow trout fed CO100). For each model tested, the residuals were examined to evaluate the appropriateness of the model, therefore normality, homogeneity and independence of residuals were considered. If a p value was close to 0.05 and residuals were not normal, a p-randomization was conducted >1,000 times to test the data empirically.
Results
Experimental Diet Composition
The dry matter (89.1–90.3 %), ash (2.7–3.2 %), protein (44.4–46.9 %) and lipid (14.2–15.7 %) were similar across all diets and accurately reflected the formulation (Table 1). Camelina oil is composed mainly of PUFA (57 %) and MUFA (32 %) and is low in SFA (11 %) (Table 2). The unsaturated 18:3n-3 (30 %), 18:2n-6 (24 %), 18:1n-9 (15 %) and 20:1n-9 (11 %) were the most abundant fatty acids in the oil. The diets were mainly composed of neutral lipids (13–15 %), but camelina diets were significantly lower in polar lipids (0.3 %) than the fish oil diet (1 %) (Table 3). Triacylglycerol (TAG) and sterol (ST) significantly increased with the addition of camelina oil from 79 % (FO) to 83 % (CO50 and CO100) and 0.2 % (FO) to 4 % (CO50 and CO100). Phospholipid (PL) was significantly higher in FO (5 %) than CO diets (1 %). SFA significantly decreased when fish oil was replaced with camelina (FO > CO50 > CO100), but MUFA, PUFA and total n-3 increased significantly with the addition of camelina oil (FO < CO50 ≤ CO100). Fatty acids that were typically high in camelina oil increased significantly in the CO50 and CO100 diets compared to FO, particularly 18:1n-9, 18:2n-6 and 18:3n-3.
Growth Performance
The initial weights of the fish were not significantly different among treatments at the start of the experiment (43–48 g fish−1; Table 4) and did not vary among tanks within treatments (F = 0.93; p = 0.49). The initial lengths (standard) of the fish did not vary significantly at the start of the experiment (15–16 cm) and did differ significantly among tanks (F = 0.65; p = 0.69). The final weight of the fish ranged from 168 g fish−1 (FO) to 184 g fish−1 (CO100), and did not vary among treatments or tanks within a treatment (F = 0.99; p = 0.45). Since the maximum difference in final weight was between FO and CO100 fish, the minimum detectable difference was calculated, which is the difference between means that could have been significantly different. The purpose of this calculation was twofold: to ensure that the difference in means between FO and CO100 fish was in fact different when compared directly without CO50 included, and to ensure that the statistical design was sufficient to detect differences between these treatments. Given the same variance and sample size as the original data, two-sample t tests were performed with two different means until a significant difference was observed. Each time a t test was performed the difference between the two means had increased. The difference between FO fish final mass (168 g) and CO100 fish final mass (184 g) was 16 g. If the final weight of FO fish was 164 ± 32 and CO100 fish 188 ± 35 (n = 18), the difference in weight would be 24 g and is significantly different (p = 0.039). A difference of 22 g between means was not significant (p = 0.057). The difference between FO and CO100 final weights would have to increase by 1.5 times before a difference could be detected. Alternatively, by increasing the sample size from 6 fish per tank (n = 18) to 12 fish per tank (n = 36), a significant difference between means could be observed (p = 0.047). Therefore, the experimental design was accurate in accepting the null hypothesis, since the design would need to alter dramatically and a significant biological difference would need to occur in order for a significant difference between FO and CO100 fish to be detected. At the end of the experiment, fish gained between 125 g fish−1 (FO) and 136 g fish−1 (CO100), again with no significant difference among treatments or tanks. The final length was the same across all treatments (23 cm) and no difference among tanks in a treatment (F = 0.98; p = 0.452). The condition factor did not vary among treatments or tanks (F = 0.66; p = 0.684). There was no difference in SGR among treatments (1.59–1.62 % day−1) and no difference among tanks in treatments. The visceral somatic index did not differ among treatments (12.2–12.5) or tanks (F = 2.18; p = 0.06). The FCR did not differ among treatments or tanks, however a two sample t test was performed between FO and CO100 fish which found that the FCR was significantly lower in FO than CO100 groups.
Lipid Class Tissue Composition
Initially, rainbow trout muscle contained 1.2 % total lipid (ww−1), composed of neutral lipid (0.8 %) and polar lipid (0.4 %) (Table 5). TAG was the dominant lipid class with 71 % of total lipid, followed by phospholipid (PL) (21 %), AMPL (6 %) and sterol (ST) (2 %). After the 12-week experiment, there was no significant difference in total lipid (2–4 % ww−1), neutral lipid (2–3 % ww−1) and polar lipid (0.4 % ww−1) among all treatments. TAG remained the same among treatments (75–86 %), however since the original p value was close to 0.05 and residuals were not homogenous or normal, a p value randomization was conducted 5,000 times. The randomized p value was 0.08, confirming the TAG was not significantly different among treatments. PL was significantly lower in CO50 (9 %) than FO (17 %).There were no significant differences among tanks within each treatment for muscle lipid classes.
Rainbow trout skin increased in total lipid from 2.2 % (ww−1) (FO) to 3.7 % (ww−1) (CO100) (Table 6). Neutral lipid was also significantly higher in CO100 (3.2 % ww−1) than FO (1.8 % ww−1). Polar lipid was significantly higher in CO50 (0.8 % ww−1) than FO (0.4 % ww−1) and CO100 (0.5 % ww−1). CO50 skin tissue was significantly lower in TAG (79 %) than FO skin (83 %) and CO100 skin (85 %). There were no significant differences among tanks within each treatment for skin lipid classes.
Fat surrounding the viscera in CO100 fish had significantly more total lipid (56 % ww−1) than CO50 fish (32 % ww−1) and FO fish (35 % ww−1) (Table 7). Neutral lipid was also significantly higher in fish fed CO100 (52 % ww−1) than fish fed CO50 (26 % ww−1) and FO (30 % ww−1). TAG was significantly higher in fish fed CO100 (83 %) than fish fed CO50 (72 %) and FO (74 %). AMPL was significantly higher in FO fish (12 %) and CO50 fish (15 %) than CO100 fish (3 %). There were no significant differences among tanks within each treatment for visceral fat lipid classes.
Fatty Acid Tissue Composition
Initial rainbow trout muscle tissue composition was composed mainly of PUFA (38 %) and MUFA (35 %), with some SFA (24 %) (Table 5). The n-3 fatty acids accounted for 23 % of the total fatty acid composition and the n-6 acids accounted for 13 %. Of the 18-carbon fatty acids, 18:1n-9 was highest (20 %) followed by 18:2n-6 (10 %).
Replacing fish oil with camelina oil significantly decreased the SFA content in muscle tissue from 27 % (FO) to 16 % (CO100) and increased the MUFA content from 31 % (FO) to 39 % (CO100). The PUFA content in the muscle was the same whether FO or CO diets were consumed. The total n-3 content of the muscle tissue decreased significantly and the n-6 content increased significantly comparing FO muscle and CO100 muscle. 20:5n-3 in the muscle tissue decreased significantly with the addition of camelina oil, from 10 % (FO) to 2 % (CO100). 22:6n-3 decreased by almost half with the addition of camelina oil, from 13 % (FO) to 7 % (CO100) in the muscle tissue. 20:3n-3 was ten times higher in CO100 (1 %) than FO (0.1 %) muscle tissue. In general, the muscle tissue of trout fed CO50 was significantly different from both FO and CO100, with values in between those of FO and CO100. There were no significant differences among tanks within each treatment for any of the muscle tissue fatty acids.
In the skin, including subcutaneous fat, SFA decreased significantly with the addition of camelina oil, while MUFA and PUFA and terrestrial fatty acids increased significantly with camelina oil inclusion (Table 6). Total n-3 fatty acids in the skin decreased significantly in CO100 (24 %) compared to FO (28 %). Total n-6 fatty acids in the skin increased significantly in CO100 (16 %) compared to FO (8 %). 22:6n-3 decreased significantly with the addition of camelina oil from 10 % (FO) to 5 % (CO100), as did 20:5n-3, from 10 % (FO) to 2 % (CO100). There were no significant differences among tanks within each treatment for any of the skin fatty acids.
As observed in both muscle tissue and skin, SFA in visceral fat decreased significantly in CO100 (16 %) compared to FO (26 %) (Table 7). MUFA, PUFA and terrestrial fatty acids in the visceral fat increased significantly with camelina oil inclusion. Total n-3 fatty acids in the viscera fat were not different among salmon in different dietary groups. 22:6n-3 was significantly higher in FO (8 %) compared to both CO50 (5 %) and CO100 (3 %). 20:5n-3, was significantly higher in FO (9 %) > CO50 (5 %) > CO100 (2 %). There were no significant differences among tanks within each treatment for any of the fatty acids in fat surrounding the viscera.
Compound-Specific Stable Carbon Isotope Analysis
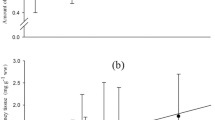
The δ13C values for 18:0, 18:1n-9, 18:2n-6 and 18:3n-3 were significantly different between dietary camelina oil and herring oil used in CO100 and FO diets (Table 8). The δ13C values in muscle tissue were significantly different for 18:0, 18:1n-9, 18:2n-6, 18:3n-3, 22:6n-3 between camelina-fed and fish-oil fed rainbow trout. The δ13C values for muscle tissue fed either FO or CO100 differed significantly for all selected fatty acids, except for 20:5n-3. The tissue δ13C for 18:1n-9 was most different between FO (−24.2 ‰) and CO100 (−29.5 ‰) than any other fatty acid. The δ13C value for 22:6n-3 in muscle tissue was significantly higher in CO100-fed fish than FO-fed fish; which is a 1.3 ‰ difference from 22:6n-3 in muscle fed FO and a 2.4 ‰ difference from dietary FO.
Discussion
Replacing 100 % of fish oil with camelina oil did not negatively affect growth of rainbow trout after a 12-week feeding trial. Several different vegetable oils have been fed to farmed rainbow trout partially or totally substituting fish oil without compromising growth [11, 18, 30], including camelina oil in Atlantic cod [21, 27]. This is the first study to use camelina oil to replace fish oil in diets for rainbow trout.
The higher FCR values observed in trout fed diets CO50 and CO100 could be due to slightly higher feed consumption by these fish, since growth rate and final weight were not significantly different among treatments. However, FCR values for rainbow trout that are <1 indicate successful feed conversion [2, 13, 47]; therefore all treatments are comparable to this standard for this species, fed at 15 °C. Weight gain, condition factor, growth rate and visceral somatic index were not different among treatments, and typical for farmed rainbow trout at this size and grown at this temperature. In terms of growth performance, camelina oil can fully replace fish oil in farmed rainbow trout without negative effects.
Viscera was the preferential lipid deposition site, in agreement with other studies [12, 17], but the total lipid content with CO100 feeding was much higher (56 % ww−1) than in FO (35 % ww−1). Similarly in the skin, total lipid was higher in camelina-fed groups than the control. Salmon fed a vegetable oil blend containing 20 % camelina oil had significantly higher lipid deposition and increased TAG content [24], and since viscera were the primary lipid deposition sites, it is not surprising that increased lipid was stored around these organs. The muscle lipid composition was unaffected by dietary oil type, a result also observed by other studies that fed different vegetable oils to rainbow trout and salmon [17, 26]. The lipid composition of the viscera fat most reflected that of the diet, because it was accumulated throughout the experiment from dietary fat and also accounted for most of the stored lipid. However, it should also be mentioned that several studies noted that fat content in salmonid tissues responded differently to different lipid sources. For example, parallel studies with salmon of the same size revealed that differences in flesh adiposity depended on whether fish were fed palm, rapeseed or linseed oils [26]. Therefore, it is difficult to compare changes in lipid composition in this study to other studies that tested different types of vegetable oils, since the response may be different, even when comparing fish of the same species and size.
In general, fatty acid profiles of all three tissues were significantly affected by the addition of camelina oil, and the differences between FO- and CO-fed fish showed similar proportions and trends across all three tissues. In the muscle, SFA were significantly lower, MUFA significantly higher and PUFA the same across all diets. From a human health perspective, consuming rainbow trout fillets fed 100 % camelina oil diets is more beneficial than consuming rainbow trout fed fish oil, in terms of lower SFA and higher MUFA and equivalent PUFA, on a proportion and as well as a quantitative basis. Although, the proportion of n-3 fatty acids in the muscle was significantly lower in CO-fed fish compared to FO-fed fish, these differences were very small. There was a much greater difference in total n-6 fatty acids, which were about twice as high in CO100 muscle as FO muscle, which inevitably reduced the n-3/n-6 ratio by about one-third, from 4.4 to 1.7. Despite the lower n-3/n-6 ratio, there is still almost twice as much n-3 as n-6 which is enough to help increase the low n-3/n-6 ratio in the typical Western diet, which is as low as 0.06 and the cause of many diseases, particularly cardiovascular [42]. Terrestrial fatty acids in the tissues were about four times higher in CO100 fish compared to FO fish. Increases in these fatty acids is a common result when feeding vegetable oils to farmed salmonids [4, 15, 39, 48]. High levels of ALA in fish fillets are still beneficial for human health, as it is an essential fatty acid in human nutrition [7, 42]. However, proportions of DHA were about half the amount in CO100 fed trout compared to FO fed trout; and EPA was about one-fifth the level in CO100 fed trout compared to FO fed trout. Most studies have shown that the proportions of EPA and DHA are decreased in fish fed diets containing vegetable oils; however the quantitative amount of DHA and EPA in fatty fish filets like rainbow trout probably still meets human nutritional requirements for these EFA. According to the World Health Organization (WHO), the daily requirement for DHA + EPA is 250 mg [56]. Based on Canada’s Food Guide, one serving of cooked fish is 75 g [20]. The sum of DHA and EPA levels in CO100 fillets is 10 %, or 7.5 mg g−1 (wet weight tissue). Therefore, if one serving (75 g) of rainbow trout fed 100 % camelina oil was consumed, the amount of DHA + EPA (563 mg) would be more than sufficient to meet the daily requirement recommended by the WHO (250 mg).
Including high levels of ALA in the diet did not prevent reductions in DHA and EPA in any tissue when fish oil was removed. Previous studies on rainbow trout and salmon have reported increases in DHA when fed a vegetable oil diet and have implied some conversion of ALA–DHA [11, 26]; but in the present study, loss of DHA was half compared to the fish oil diet, for all tissues. Studies have suggested a possible metabolic competition between 18:2n-6 (linoleic acid, LNA) and ALA, since both fatty acids are substrates for the same Δ6 desaturase enzymes [11, 43].Therefore high content of dietary LNA in camelina oil may inhibit metabolism of ALA into EPA and DHA or high content of ALA may inhibit metabolism of LNA into ARA. Either situation may have been the case in the present study, since neither n-3 or n-6 fatty acids showed extensive desaturation or elongation products in fish fed the CO100 diet. Limited substrate availability also inhibits the efficiency of Δ6 desaturase [47]; however 40 % of fatty acids (LNA + ALA) in the present study were available substrates, so this may not be the issue. But, excessive C18 PUFA substrates could limit the availability of Δ6 desaturase to act on C24 fatty acids, and thus potentially limit final DHA production [48]. However, a few fatty acids were observed that suggest elongation and desaturation had occurred, despite decreased levels of EPA and DHA. In muscle, viscera fat and skin, 18:4n-3, 18:5n-3, 20:3n-3 and 20:3n-6 increased significantly with the addition of camelina oil, although some of these fatty acids increased in very small proportions, therefore their biological significance is questionable. Also, some of these fatty acids are not typically known as intermediates in the n-3 pathway. The conventional n-3 pathway begins with Δ6 desaturation of ALA to 18:4n-3, elongation to 20:4n-3 and then Δ5 desaturation to EPA and two elongations followed by a second Δ6 desaturation and β-oxidation to DHA [45]. Wei-Chun et al. [54] postulated a bypass in the n-3 pathway whereby ALA is chain elongated to 20:3n-3. The study found that the barramundi elongase has the capacity for conversion of ALA and LNA to their C20 products, suggesting an alternative pathway for PUFA synthesis bypassing the first Δ6 desaturation step, which is rate limiting and may slow the pathway [34]. Desaturation of 20:3n-3 continues in the usual pathway with Δ5 desaturase. Therefore PUFA synthesis may be more efficient with the option of an alternative pathway when there is ALA and LNA competition at this rate limiting step of Δ6 desaturation, which may explain the increase in 20:3n-3 when camelina oil is supplied in the diet. However, this alternative pathway is yet to be confirmed with rainbow trout.
Although the level of DHA in the muscle was half that compared to the initial DHA level, it was still twice the level provided in the diet. It is possible that DHA was selectively incorporated in the muscle throughout the duration of the trial because levels in the diet were low, which has been suggested in other studies [24, 55]. Catabolism of DHA may have also occurred in order to meet the metabolic needs of the fish, which explains lowered levels of DHA in the tissue compared to the initial samples. However, higher proportions of DHA were observed in the tissue than was provided in the diet, therefore it is also possible that some DHA was synthesized de novo. In order to confirm this, CSIA was used. Camelina oil and fish oil were isotopically distinct with differences in 18:0, 18:1n-9, LNA and ALA. In terrestrial ecosystems, δ13C is based on plants with different photosynthetic pathways (e.g., c3, c4 and CAM) and in pelagic marine ecosystems δ13C is based on phytoplankton [38]. These fatty acids (18:0, 18:1n-9, LNA and ALA) were slightly more isotopically enriched in fish oil than camelina oil, as camelina is a c3 plant and has δ13C values between −25 and −35 ‰ [33] and marine origins are isotopically heavier or enriched with 13C [37].
Different δ13C values for DHA in tissues fed CO100 indicate that substantial fractionation occurred that resulted in isotopically lighter DHA in trout fed CO100 (29.7 ‰) compared to FO (28.4 ‰), a difference of 1.3 ‰. The δ13C of a consumer is assumed to be equivalent to the weight proportion of the δ13C of all dietary components, therefore the significant shift in DHA δ13C indicates mixing of a terrestrial and marine isotopic signature and significant fractionation. The mixing model estimates camelina oil contributed 27 % to DHA in muscle tissue of trout fed CO100, and 14 % to EPA. One assumption however, is that the fractionation which occurred during elongation and desaturation was minimal. Kinetic fractionation of carbon is associated with the formation or breaking of a carbon bond. Elongation and desaturation of precursors results in fractionation, because enzymes preferentially utilize the lighter precursor resulting in depleted 13C products [10, 28]; however this fractionation likely contributed very little to the overall shift toward the terrestrial isotopic signature, as it has been suggested that desaturation of fatty acids does not have any significant carbon isotopic fractionation effects [41]. The kinetic isotope effect expected for chain elongation from ALA to DHA should result in lighter DHA [1], which was observed in this experiment. The degree to which the shift in δ13C in DHA was due to fractionation caused by biosynthesis and/or conservation of the δ13C of ALA is not distinguishable, but fatty acids synthesized de novo are expected to reflect the δ13C values of the carbon utilized from the diet [40], in this case from ALA. Lipid-transport processes supplying TAG to tissues involve hydrolysis and re-esterification could explain the fractionation that occurred. There are many potential sources of fractionation of fatty acids, but most work to date in real systems has found little evidence to support it.
Therefore, the isotopically lighter DHA in CO100 fed trout compared to the significantly heavier DHA in FO fed trout suggests that the origins of DHA may have been terrestrial, i.e., DHA was likely synthesized from the terrestrial and isotopically lighter ALA in the CO100 diet. Although some residual DHA from fish meal in the CO100 diet may have been selectively incorporated in tissues, the δ13C is assumed to be equivalent to that of fish oil. Other dietary components provide 1 % lipid to the diet, therefore their δ13C values contributes very minimally to the δ13C values in the muscle tissue. Therefore the isotopic shift observed in CO100 tissues indicates that some DHA was synthesized de novo (~27 %); otherwise the δ13C of DHA in CO100 muscle tissue would have matched the δ13C in FO muscle tissue. Also, it is highly unlikely that DHA in the fish fed CO100 was simply DHA remaining from the originally fed diets because of its δ13C. If DHA was simply being catabolized and removed from the tissues, the isotopically lighter DHA would be removed preferentially, consequently leaving the more enriched DHA in the tissue; however, DHA remaining in CO100 muscle tissue was in fact isotopically lighter. Biosynthesis in fish has been demonstrated using CSIA in a previous study. A controlled feeding experiment with mummichogs (Fundulus heteroclitus) found that shifts in non-essential amino acid δ13C values in muscle tissue indicate a high degree of de novo biosynthesis, particularly in a diet that was low in protein content, and it was suggested that such a diet required biosynthesis to meet muscle composition demand [25]. Similarly, in the present study it is reasonable to suggest the biosynthesis of DHA by rainbow trout, as this fatty acid was depleted in the CO100 diet. This is the first study to use CSIA in a controlled feeding experiment to demonstrate synthesis of DHA in fish.
Camelina oil used to replace 100 % of fish oil in diets for rainbow trout shows promise for the aquaculture industry. Growth performance of fish fed camelina oil diets was not affected compared to a typical commercial diet. Although fatty acid proportions were significantly altered after 12 weeks of feeding, final DHA and EPA amounts in a 75 g filet were enough to satisfy daily DHA and EPA requirements set by the WHO. Other human health benefits include lower SFA and higher MUFA in filets fed camelina oil versus fish oil. Biosynthesis of LC PUFA was demonstrated by evidence of elongated products and by the unique isotopic signature of DHA in CO100 tissue, confirmed by CSIA. Feeding camelina oil diets for a full production cycle, including first feeding, would be beneficial to monitor fatty acid profiles to determine if DHA and EPA levels remain the same as the levels observed after 12 weeks.
References
Abrajano T, Murphy D, Fang J, Comet P, Brooks J (1994) 13C/12C ratios in individual fatty acids of marine mytilids with and without bacterial symbionts. Org Geochem 21:611–617
Bailey J, Alanara A (2006) Effect of feed portion size on growth of rainbow trout, Oncorhynchus mykiss (Walbaum), reared at different temperatures. Aquaculture 253:728–730
Bell G, Henderson R, Tocher D, McGhee F, Dick J, Porter A, Smullen R, Sargent J (2002) Substituting fish oil with crude palm oil in the diet of Atlantic salmon (Salmo salar) affects muscle fatty acid composition and hepatic fatty acid metabolism. J Nutr 132:222–230
Bell G, Tocher D, Henderson R, Dick J, Crampton V (2003) Altered fatty acid compositions in Atlantic salmon (Salmo salar) fed diets containing linseed and rapeseed oils can be partially restored by a subsequent fish oil finishing diet. J Nutr 122:2793–2801
Bell G, Pratoomyot J, Stachan F, Henderson R, Fontinallas R, Hebard A, Guy D, Hunter D, Tocher D (2010) Growth, flesh adiposity and flesh fatty acid composition of Atlantic salmon (Salmo salar) families with contrasting flesh adiposity: effects of replacement of dietary fish oil with vegetable oils. Aquaculture 306:225–232
Brandsen M, Carter C, Nichols P (2003) Replacement of fish oil with sunflower oil in feeds for Atlantic salmon (Salmo salar L.): effect on growth performance, tissue fatty acid composition and disease resistance. Comp Biochem Physiol B 135:611–625
Brenna J, Salem N, Sinclair A, Cunnane S (2009) α-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids 80:85–91
Budin J, Breene W, Putnam D (1995) Some compositional properties of camelina (Camelina sativa L. Crantz) seeds and oils. J Am Oil Chem Soc 72:309–315
Budge S, Wooller M, Springer A, Iverson S, McRoy C, Divoky G (2008) Tracing carbon flow in an arctic marine food web using fatty acid stable isotope analysis. Oecologia 157:117–129
Budge S, Wang S, Hollmen T, Wooler M (2011) Carbon isotopic fractionation in eider adipose tissue varies with fatty acid structure: implications for trophic studies. J Exp Biol 214:3790–3800
Caballero M, Obach A, Rosenlund G, Montero D, Gisvold M, Izquierdo M (2002) Impact of different dietary lipid sources on growth, lipid digestibility, tissue fatty acid composition and histology of rainbow trout, Oncorhynchus mykiss. Aquaculture 214:253–271
Corraze G, Kaushik S (1999) Les lipides des poisson marins et d’eau douce. Oleagineux Corps Gras Lipide 6:111–115
Cowx I (2005) Cultured aquatic species information programme. Oncorhynchus mykiss. In: FAO Fisheries and Aquaculture Department, Rome, Italy (online). http://www.fao.org/fishery/culturedspecies/Oncorhynchus_mykiss/en. Cited 17 Nov 2012
Crampton V, Nanton D, Ruohonen D, Skjervold P, El-Mowafi A (2010) Demonstration of salmon farming as a net producer of fish protein and oil. Aquac Nutr 16:437–446
Drew M, Ogunkoya A, Janz D, Van Kessel A (2007) Dietary influence of replacing fish meal and oil with canola protein concentrate and vegetable oils on growth performance, fatty acid composition and organochlorine residues in rainbow trout (Oncorhynchus mykiss). Aquaculture 267:260–268
FAO (Food and Agriculture Organization) (2012) World review of fisheries and aquaculture. In: State of the world fisheries and aquaculture 2012. (FAO Fish Tech Paper), Rome, p 209
Figueiredo-Silva A, Rocha E, Dias J, Silva P, Rema P, Gomes E, Valente L (2005) Partial replacement of fish oil by soybean oil on lipid distribution and liver histology in European sea bass (Dicentrarchus labrax) and rainbow trout (Oncorhynchus mykiss) juveniles. Aquac Nutr 11:147–155
Greene S, Selivonchick D (1990) Effects of dietary vegetable, animal and marine lipids on muscle lipid and hematology of rainbow trout (Oncorhynchus mykiss). Aquaculture 89:165–182
Hastings N, Agaba M, Tocher D, Zheng X, Dickson C, Dick J, Teale A (2004) Molecular cloning and functional characterization of fatty acyl desaturase and elongase cDNAs involved in the production of eicosapentaenoic and docosahexaenoic acids from α-linolenic acid in Atlantic salmon (Salmo salar). Mar Biotechnol 6:463–474
Health Canada (2011) Eating well with Canada’s food guide. In: Food and nutrition, Ottawa, Canada (online). http://www.hc-sc.gc.ca/fn-an/food-guide-aliment/basics-base/serving-portion-eng.php. Cited 17 Nov 2012
Hixson S, Parrish C, Anderson D (2013) Effect of replacement of fish oil with camelina (Camelina sativa) oil on growth, lipid class and fatty acid composition of farmed juvenile Atlantic cod (Gadus morhua). Fish Physiol Biochem. 39:1441–1456
Jordal A, Torstensen B, Tsoi S, Tocher D, Lall S, Douglas S (2005) Dietary rapeseed oil affects the expression of genes involved in hepatic lipid metabolism in Atlantic salmon (Salmo salar L.). J Nutr 135:2355–2361
Kris-Etherton P, Harris W, Appel L (2002) Fish consumption, fish oil, omega-3 fatty acids and cardiovascular disease. Circulation 106:2747–2757
Leaver M, Taggart J, Villeneuve L, Bron J, Guy D, Bishop S, Houston R, Matika O, Tocher D (2011) Heritability and mechanisms of n-3 long chain polyunsaturated fatty acid deposition in the flesh of Atlantic salmon. Comp Biochem Physiol D 6:62–69
McMahon K, Fogel M, Elsdon T, Thorrold S (2010) Carbon isotope fractionation of amino acids in fish muscle reflects biosynthesis and isotopic routing from dietary protein. J Anim Ecol 79:1132–1141
Menoyo D, Lopez-Bote C, Obach A, Bautista J (2005) Effect of dietary fish oil substitution with linseed oil on the performance, tissue fatty acid profile, metabolism, and oxidative stability of Atlantic salmon. J Anim Sci 12:2853–2862
Morais S, Edvardsen R, Tocher D, Bell G (2012) Transcriptomic analyses of intestinal gene expression of juvenile Atlantic cod (Gadus morhua) fed diets with camelina oil as replacement for fish oil. Comp Biochem Physiol B 161:283–293
Monson K, Hayes J (1982) Biosynthetic control of the natural abundance of carbon 13 at specific positions within fatty acids in Saccharomyces cerevisiae: isotopic fractionations in lipid synthesis is evidence for peroxisomal regulations. J Biol Chem 257:5568–5575
Morrison W, Smith L (1964) Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J Lipid Res 5:600–608
Nadege R, Kaushik S, Larroquet S, Panserat S, Corraze G (2006) Replacing dietary fish oil by vegetable oils has little effect on lipogenesis, lipid transport and tissue lipid uptake in rainbow trout (Oncorhynchus mykiss). Br J Nutr 96:299–309
National Research Council (NRC) (2011) Nutritional requirements of fish and shrimp. National Academies Press, Washington, DC
Ni Eidhin D, Burke J, O’Beirne D (2003) Oxidative stability of n-3-rich camelina oil and camelina oil-based spread compared with plant and fish oils and sunflower spread. J Food Sci 68:345–353
O’Leary M (1988) Carbon isotopes in photosynthesis. Bioscience 38:328–336
Park W, Kothapalli K, Lawrence P, Tyburczy C, Brenna J (2009) An alternate pathway to long chain polysaturates: the FADS2 gene product D8-desaturates 20:2n-6 and 20:3n3. J Lipid Res 50:1195–1202
Parrish C (1987) Separation of aquatic lipid classes by Chromarod thin-layer chromatography with measurement by Iatroscan flame ionization detection. Can J Fish Aquat Sci 44:722–731
Parrish C (1999) Determination of total lipid, lipid classes and fatty acids in aquatic samples. In: Arts M, Wainman B (eds) Lipids in freshwater ecosystems. Springer, New York, pp 4–10
Phillips D, Newsome S, Gregg J (2005) Combining sources in stable isotope mixing models: alternative methods. Oecologia 144:520–527
Post D (2002) Using stable isotopes to estimate trophic position: models, methods and assumptions. Ecology 83:703–718
Rosenlund G, Obach A, Sandberg M, Standal H, Tveit K (2001) Effect of alternative lipid sources on long-term growth performance and quality of Atlantic salmon (Salmo salar L.). Aquac Res 2001:323–328
Ruess L, Tiunov A, Haubert D, Richnow H, Haggblom M, Scheu S (2005) Carbon stable isotope fractionation and trophic transfer of fatty acids in fungal based soil food chains. Soil Biol Biochem 37:945–953
Schouten S, Breteler W, Blokker P, Schogt N, Rijpstra W, Grice K, Baas M, Damsté J (1998) Biosynthetic effects on the stable carbon isotopic compositions of algal lipids: implications for deciphering the carbon isotopic biomarker record. Geochim Cosmochim Acta 62:1397–1406
Simopoulus A (2002) The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 56:365–379
Skonberg D, Rasco B, Dong F (1994) Fatty acid composition of salmonid muscle changes in response to a high oleic diet. Nutr Metab 124:1628–1638
Sokal R, Rohlf F (1994) Biometry. W. H. Freeman Company, New York
Sprecher H (2000) Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim Biophys Acta 1486:219–231
Swanson D, Block R, Mousa S (2012) Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr 3:1–7
Thanuthong T, Francis D, Senadheera S, Johns P, Turchini G (2011) LC-PUFA biosynthesis in rainbow trout is substrate limited: use of the whole body fatty acid balance method and different 18:3n-3/18:2n-6 ratios. Lipids 46:1111–1127
Thanuthong T, Francis D, Manicham E, Senadheera S, Cameron-Smith D, Turchini G (2011) Fish oil replacement in rainbow trout diets and total dietary PUFA content: II) Effects on fatty acid metabolism and in vivo fatty acid bioconversion. Aquaculture 322:99–108
Tocher D (2003) Metabolism and functions of lipids and fatty acids in teleost fish. Res Fish Sci 11:107–184
Turchini G, Torstensen B, Wing-Keong N (2009) Fish oil replacement in finfish nutrition. Rev Aquac 1:10–57
Turchini T, Francis D, Keast R, Sinclair A (2011) Transforming salmonid aquaculture from a consumer to a producer of long chain omega-3 fatty acids. Food Chem 124:609–614
Voight C, Rex K, Michener R, Speakman J (2008) Nutrient routing in omnivorous animals tracked by stable carbon isotopes in tissues and exhaled breath. Physiol Ecol 157:31–40
Warwick S (2011) Brassicaceae in agriculture. In: Schmidt R, Bancroft I (eds) Genetics and genomics of the Brassicaceae. Springer, New York, pp 33–65
Wei-Chun T, Muhlhausler B, James M, Stone D, Gibson R (2012) An alternative n-3 fatty acid elongation pathway utilising 18:3n-3 in barramundi (Lates calcarifer). Biochem Biophys Res Commun 423:176–182
Wijekoon M (2012) Effect of water temperature and diet on cell membrane fluidity and fatty acid composition of muscle, liver, gill and intestine mucosa of adult and juvenile steelhead trout, Oncorhynchus mykiss. PhD thesis, Memorial University of Newfoundland, St. John’s, Newfoundland, Canada
World Health Organization (WHO) (2008) Fats and fatty acids in human nutrition: report of an expert consultation. FAO Food and Nutrition Paper, Geneva, Switzerland, p 3
Zheng X, Tocher D, Dickson C, Bell G, Teale A (2005) Highly unsaturated fatty acid synthesis in vertebrates: new insights with the cloning and characterization of a Δ6 desaturase of Atlantic salmon. Lipids 40:13–24
Zubr J (1997) Oil-seed crop: camelina sativa. Ind Crop Prod 6:113–119
Acknowledgments
This study was supported by Genome Atlantic, the Atlantic Canada Opportunities Agency (ACOA)—Atlantic Innovation Fund (AIF), the Research and Development Corporation of Newfoundland (RDC) and the NSERC. The authors would like to acknowledge Dr. Matthew Rise for conceptual contribution to the project; Christina Bullerwell for feeding and maintenance of fish; Jeanette Wells, John J. Heath and Geert Van Biesen for technical support; Dr. Marije Booman, Jamie Fraser, Zhiyu Chen for assistance with fish sampling.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hixson, S.M., Parrish, C.C. & Anderson, D.M. Changes in Tissue Lipid and Fatty Acid Composition of Farmed Rainbow Trout in Response to Dietary Camelina Oil as a Replacement of Fish Oil. Lipids 49, 97–111 (2014). https://doi.org/10.1007/s11745-013-3862-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-013-3862-7