Abstract
Stearoyl-coenzyme A desaturase-1 (SCD1) is a rate-limiting enzyme that catalyzes the biosynthesis of monounsaturated fatty acids from saturated fatty acids. Recently, SCD1 down-regulation has been implicated in the prevention of obesity, and the improvement of insulin and leptin sensitivity. In this study, we examined the effect of fucoxanthin, a marine carotenoid, on hepatic SCD1 in obese mouse models of hyperleptinemia KK-A y and leptin-deficiency ob/ob. In KK-A y mice, providing a diet containing 0.2 % fucoxanthin for 2 weeks markedly suppressed SCD1 mRNA and protein expressions in the liver. The fatty acid composition of liver lipids was also affected by an observed decrease in the ratio of oleic acid to stearic acid. Furthermore, serum leptin levels were significantly decreased in hyperleptinemia KK-A y mice after 2 weeks of fucoxanthin feeding. However, the suppressive effects of fucoxanthin on hepatic SCD1 and body weight gain were not observed in ob/ob mice. These results show that fucoxanthin down-regulates SCD1 expression and alters fatty acid composition of the liver via regulation of leptin signaling in hyperleptinemia KK-A y mice but not in leptin-deficient ob/ob mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fucoxanthin is a marine carotenoid present in edible brown seaweeds, such as Undaria pinnatifida, Laminaria japonica and Hijikia fusiformis. Compared to other carotenoids, fucoxanthin possesses unique structures such as an allenic bond, 5,6-monoepoxide, and acetyl residues. We have previously reported that fucoxanthin suppresses visceral fat accumulation and ameliorates hyperglycemia in diabetic/obese KK-A y mice [1]. Furthermore, we have also reported that dietary fucoxanthin increases the amount of docosahexaenoic acid in the liver of KK-A y mice [2]. In that study, we observed a change in the composition of liver lipid with an increase in stearic acid (18:0) and a decrease in oleic acid (18:1n-9). These results suggest that dietary fucoxanthin inhibits desaturation of 18:0 by stearoyl-coenzyme A desaturase (SCD) in the liver.
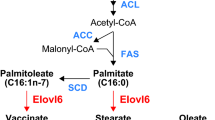
SCD is a rate-limiting enzyme that catalyzes the biosynthesis of monounsaturated fatty acids from saturated fatty acids. The main product of SCD reaction, 18:1n-9 is the preferable substrate for the formation of triglyceride, phospholipids and cholesterol esters [3, 4]. High expression of SCD in liver and adipose tissues is known to be associated with the development of obesity and the onset of metabolic syndrome [5–7]. Recently, it has been reported that mice with a disruption in the SCD1 gene, which expresses exclusively in the liver, present resistance to genetic and diet-induced obesity [8, 9]. In contrast, SCD1 deficiency in leptin-resistant obese A y/a and high-fat induced obese mice has been reported to improve leptin and insulin sensitivity [10]. The leptin produced by adipose tissue plays a critical role in the regulation of food intake, glucose metabolism, lipid metabolism and energy balance throughout the whole body both via central hypothalamic actions and directly action in peripheral tissues [11]. Human obesity is characterized by the inefficiency of leptin activity rather than the dysfunction of leptin secretion. Therefore, many studies have focused on the improvement in the leptin signaling pathway as a molecular target for the prevention of obesity [12]. In a noteworthy study on the leptin-mediated inhibition of obesity, Cohen et al. [13] have reported that the down-regulation of SCD1 is an important component of the metabolic response to leptin.
In this study, we examined the suppressive effect of fucoxanthin, which ameliorates both body weight gain and hyperglycemia in diabetic/obese KK-A y mice, on hepatic SCD1 expression. Two mouse lines presenting an obese phenotype, i.e., hyperleptinemia KK-A y mice and leptin-deficient ob/ob mice, were used to examine the regulation of hepatic SCD1 expression by fucoxanthin in leptin signaling. Dietary fucoxanthin attenuated hepatic SCD1 expression levels and hyperleptinemia in KK-A y mice, whereas there was no change in SCD1 expression or visceral fat accumulation in ob/ob mice without leptin secretion. Here our findings show the inhibitory effect of fucoxanthin on hepatic SCD1 through a metabolic response to leptin.
Materials and Methods
Preparation of Fucoxanthin
Dried brown seaweed (U. Pinnatifida) was purchased from a local market in Hakodate, Japan. Crude lipid from seaweed containing fucoxanthin was extracted with acetone from dried seaweed, and then fucoxanthin was separated using silicagel column chromatography with n-hexane/acetone (7:3, v/v) as our previous report [2]. Purified fucoxanthin (all-trans- and cis- fucoxanthin, >95 % purity) was used for animal experiments.
Animal Care and Experimental Design
Diabetic/obese KK-A y mice (4-week old, female) and B6.V-Lep ob/J (ob/ob) mice (5-week old, male) were purchased from CLEA Japan, Inc. (Tokyo, Japan) and Charles River Laboratories Japan, Inc. (Kanagawa, Japan), respectively. Mice were housed individually and had freely access to food and tap water. Room temperature and humidity were controlled at 23 ± 1 °C and 50 % with a 12 h light/12 h dark cycle. Mice were fed normal rodent diet MF (Oriental Yeast Co.,Ltd. (Tokyo, Japan)), for a week during acclimation and switched to experimental diets. The KK-A y mice were fed the diet containing 0.1 or 0.2 % fucoxanthin for 1 or 2 weeks. The ob/ob mice were fed the diet containing 0.2 % fucoxanthin for 4 weeks. Previously, we reported that fucoxanthin suppresses the weight gain of white adipose tissue in KK-A y mice at 0.2 % in the diet [14]. Therefore, in this study, we fed 0.1 and 0.2 % fucoxanthin-containing diets to KK-A y mice. After 12-h fasting, blood samples were collected from the caudal vena cava under anesthesia with diethyl ether. Mice were euthanized, and liver and white adipose tissue (WAT) were removed and weighed. Livers were then frozen for lipid analysis and Western blotting or soaked in RNA later™ (Sigma Chemical Co., St. Louis, MO) for quantitative real time polymerase chain reaction (PCR) analysis. All procedures for the use and care of animals for this research were approved by the Ethical Committee of Experimental Animal Care at Hokkaido University.
Western Blotting
Total protein was prepared by homogenization of liver in lysis buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 % Triton X-100, 0.5 % sodium deoxycholate, 0.1 % SDS and 1 mM phenylmethylsulfonyl fluoride) supplemented protease inhibitor cocktail (Sigma Chemical Co., St. Louis, MO) on ice. Liver lysate was centrifuged at 10,000×g for 30 min at 4 °C, and the supernatant was harvested and stored at −30 °C for assay. Protein concentration was measured using a DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). The lysates mixed with the loading buffer (Sigma Chemical Co., St. Louis, MO) were boiled at 95 °C for 5 min. Protein samples were then loaded at 20 μg protein per lane, size fractionated by SDS-polyacrylamide (10 %) gel electrophoresis, and transferred onto polyvinylidene difluoride membrane. The membranes were incubated with primary goat anti-SCD1 antibody (sc14719; 1:1,000) in 1 % skimmed milk in TBS buffer for 1 h at room temperature. Thereafter, the membrane was incubated with horseradish peroxidase-conjugated anti-goat IgG (sc2020; 1:5,000). These antibodies were purchased from Santa Cruz Biotechnology, Inc., Santa Cruz, California, USA. After washing, immunoreactive bands were visualized with a ImmunoStar® LD (Wako pure chemicals, Osaka, Japan). β-Actin (mouse anti-β-actin antibody (Ab-1; 1:15,000), Calbiochem, Darmstadt, Germany) was detected as the loading control.
Quantitative Real-Time PCR
Total RNA was extracted from the liver with the RNeasy Mini Kit (Qiagen, Tokyo, Japan), according to the manufacturer’s protocol and was then reversed transcribed into cDNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems Japan Ltd., Tokyo, Japan). cDNA was added to the FastStart Universal Probe Master (Rox) (Roche Diagnostics Japan Ltd., Tokyo, Japan), and quantitative real-time PCR analysis was performed using the ABI Prism 7500 (Applied Biosystems Japan Ltd., Tokyo, Japan). The mRNA expression levels were measured using the Taqman® Gene Expression Assays (Applied Biosystems Japan Ltd, Tokyo, Japan). The gene-specific primers Mm00772290_m1 (SCD1 mRNA) and Mm00607939_s1 (β-actin mRNA) were used, respectively. The gene expression level was normalized for the β-actin transcript level.
Fatty Acid Analysis
Total lipid was extracted from the liver by Folch method [15]. Fatty acid methyl esters of total liver lipids were prepared by the method of Christopher and Glass as described by Prevot and Mordret [16]. The fatty acid composition was analyzed with a gas chromatograph (GC-14B, Shimadzu Corporation., Kyoto, Japan) equipped with FID and Omegawax 320 fused silica capillary column (30 m × 0.32 mm i.d.). The relative amount of each fatty acid was expressed as mol% of total fatty acids. Individual fatty acids were identified by comparison with the retention times of standards (Nu-Check-Prep, Inc, Elysian MN) and were quantified by an online Shimadzu Chromatopack C-R8A integrator.
Measurement of Serum Leptin and Blood Glucose
The serum leptin concentration was measured with a commercial assay kit, Lbis Leptin-Mouse ELISA Kit (Shibayagi Co., ltd, Gunma, Japan), according to the manufacturer’s instruction. Blood glucose was measured using a blood glucose monitor, the Glutest Neo Sensor (Sanwa Kagaku Kenkyusyo Co. Ltd., Aichi, Japan), in mice without fasting before a day of dissection.
Statistical Analysis
Data are presented as the means ± SEM. In animal experiments, the statistical significance between control and fucoxanthin groups was analyzed using one-way ANOVA, followed by Dunnett’s multiple comparison test for Fig. 1a and Student’s t tests for other experiments.
Hepatic SCD1 expression in KK-A y mice fed fucoxanthin. KK-A y mice were fed a diet supplemented with or without fucoxanthin for 1 or 2 weeks. a Mice were fed the diet containing 0.1 and 0.2 % fucoxanthin for 1 week. b Mice were fed 0.2 % fucoxanthin-containing diet for 2 weeks. SCD1 expressions were analyzed by Western blotting using the respective primary antibody. Primary antibody binding was detected using chemiluminescence with the appropriate secondary antibody. SCD1 mRNA expression levels were measured by quantitative real-time PCR. Each value represents the mean ± standard error. ** p < 0.01, * p < 0.05 versus control group. SCD1 stearoyl-coenzyme A desaturase-1
Results
Dietary fucoxanthin suppressed an increase in body weight (final body weight, control group: 35.5 ± 0.9 g, fucoxanthin group: 32.9 ± 0.7 g) and visceral WAT mass (final weight, control group: 5.5 ± 0.3 g, fucoxanthin group: 3.7 ± 0.3 g) in KK-A y mice after a 2-week feeding, as described in previous studies [1]. To examine the effect of fucoxanthin on SCD1 expression in the liver, we conducted quantitative real-time PCR and Western blot analysis. As shown in Fig. 1a, a 1-week feeding of fucoxanthin suppressed hepatic SCD1 expression in a dose-dependent manner. In KK-A y mice that were fed 0.2 % fucoxanthin for 2 weeks, mRNA and protein expression levels of SCD1 were decreased to 40 and 30 % of those of the control group, respectively (Fig. 1b).
A substrate for SCD1, 18:0, was significantly increased within the fatty acid composition of the liver lipid of fucoxanthin-fed mice. In contrast, dietary fucoxanthin significantly decreased the main product of SCD1 reaction, 18:1n-9, compared to the control group, despite a lack of change in 18:1n-7 levels (Fig. 2a). The ratio of 18:1n-9 to 18:0 was also markedly decreased in the fucoxanthin group compared to the level in the control group (Fig. 2b). These results show that fucoxanthin suppresses desaturation of 18:0 into 18:1n-9 through down-regulation of hepatic SCD1 mRNA expression in KK-A y mice.
Fatty acid composition in the liver of KK-A y mice fed fucoxanthin. KK-A y mice were fed a diet supplemented with or without fucoxanthin for 2 weeks. a Fatty acid composition in liver lipid of KK-A y mice. Liver lipid was prepared as described in “Materials and Methods” and analyzed using gas-chromatography. b The desaturation index was expressed as 18:1n-9/18:0. Each value represents the mean ± standard error. ** p < 0.01 versus control group
The KK-A y mouse is well-characterized to impair leptin sensitivity with increasing fat accumulation, and present with hyperleptinemia [17]. The serum leptin levels of fucoxanthin-fed mice were lowered significantly compared to those in control mice after 1 week of feeding (Fig. 3a). Furthermore, fucoxanthin suppressed an increase in leptin concentration, which is associated with the development of obesity. In the fucoxanthin group, the serum leptin levels were 6.0 ± 0.4 ng/mL after 2 weeks of feeding, whereas the control group had serum leptin levels of 12.2 ± 1.7 ng/mL (Fig. 3b). In KK-A y mice fed 0.2 % fucoxanthin for 2 weeks, the blood glucose levels were also lower than those of the control mice (control group: 331 ± 32 mg/dL, fucoxanthin group: 202 ± 21 mg/dL). We previously showed that fucoxanthin attenuated serum insulin levels of KK-A y mice with hyperinsulinemia [18]. These results suggest that fucoxanthin impairs leptin resistance with a decrease in the development of obesity in KK-A y mice. In a previous study, we reported that 0.2 % fucoxanthin suppresses body weight gain and fat accumulation in female KK-A y mice [1, 14]. Therefore, the same female KK-A y mice were used in this study. We also confirmed that 0.2 % fucoxanthin suppresses body weight gain and hepatic SCD1 mRNA expression, and decreases serum leptin levels in male KK-A y mice after 2 weeks of feeding (data not shown).
Serum leptin levels in KK-A y mice. KK-A y mice were fed 0.2 % fucoxanthin-containing diet or control diet for 1 week (a) or 2 weeks (b). Serum leptin levels were measured using a commercial assay kit as described in “Materials and Methods”. Each value represents the mean ± standard error. ** p < 0.01 versus control group
In leptin-deficient ob/ob mice, a diet containing 0.2 % fucoxanthin did not affect body weight after 4 weeks of feeding (Fig. 4a). In addition, fucoxanthin did not attenuate weight gain of visceral WAT, although this weight gain slightly increased. The blood glucose levels of control and fucoxanthin groups were the same (Fig. 4b, c). Dietary fucoxanthin thus did not attenuate obesity or blood glucose levels in leptin-deficient ob/ob mice.
Effect of fucoxanthin on body weight, visceral WAT weight and blood glucose in leptin-deficient ob/ob mice. Leptin-deficient ob/ob mice were fed with a diet supplemented with or without 0.2 % fucoxanthin for 4 weeks. Blood glucose was measured as described in “Materials and Methods”. Each value represents the mean ± standard error. * p < 0.05 versus control group. WAT white adipose tissue
The expression levels of SCD1 mRNA and protein were not changed in ob/ob mice fed 0.2 % fucoxanthin for 4 weeks (Fig. 5a, b). Consistent with hepatic SCD1 expression, there was no observed increase in 18:0 and decrease in 18:1n-9 in the liver of ob/ob mice that were fed fucoxanthin (Fig. 6). These results suggest that the suppressive effect of fucoxanthin on hepatic SCD1 expression was not present in the leptin-deficient mice.
Hepatic SCD1 expression in leptin-deficient ob/ob mice fed fucoxanthin. Leptin-deficient ob/ob mice were fed a diet supplemented with or without 0.2 % fucoxanthin for 4 weeks. a mRNA and b protein expression levels of SCD1 in the liver. SCD1 mRNA expression levels were analyzed using quantitative real-time PCR. SCD1 expressions were analyzed by Western blotting using respective primary antibody. Primary antibody binding was detected by chemiluminescence with the appropriate secondary antibody. Each value represents the mean ± standard error. SCD1 Stearoyl-coenzyme A desaturase-1
Fatty acid composition in the liver of leptin-deficient ob/ob mice fed fucoxanthin. Leptin-deficient ob/ob mice were fed a diet supplemented with or without 0.2 % fucoxanthin for 4 weeks. Liver lipids were prepared as described in “Materials and Methods” and analyzed using gas-chromatography. Each value represents the mean ± standard error
Discussion
SCD catalyzes delta 9-desaturation in saturated fatty acids. Four isoforms of SCD have been identified, and SCD1 represents the predominant isoform in the liver in mice [5]. SCD1 is highly expressed in obesity-model mice that are fed a high-fat diet and in leptin-deficient [3, 19]. Interestingly, SCD1-deficient mice present a lean phenotype and are resistant to obesity. Furthermore, SCD1 deficiency has been reported to improve leptin and insulin sensitivity in both genetically and diet-induced obese mice [10]. Recently, the down-regulation of SCD1 has been considered to mediate leptin signaling [13, 20, 21] and as an important target for the prevention and management of obesity by regulating energy metabolism.
Fucoxanthin is one of the major marine carotenoids contained in edible brown seaweeds. We and others have reported that dietary fucoxanthin suppresses WAT weight gain and ameliorates hyperglycemia in mouse models of genetic and diet-induced obesity [1, 22]. In diabetic/obese KK-A y mice that were fed 0.2 % fucoxanthin, SCD1 expression levels in the liver were lower than those in the control mice even after 1 week of feeding. In the fatty acid composition of the liver, the decrease of 18:1n-9, a product of SCD1, was accompanied by an increase in 18:0, a substrate for SCD1 reaction, after 2 weeks of fucoxanthin feeding. In addition, fucoxanthin decreased serum leptin levels and suppressed WAT weight gain after 2 weeks of fucoxanthin feeding in KK-A y mice, an obese mouse model presenting hyperleptinemia and hyperglycemia with the development of obesity. Miyazaki et al. [10] showed that increased leptin signaling due to SCD1 deficiency plays a part in increased insulin sensitivity in agouti-induced and high-fat diet-induced obese mice. In addition, leptin has been reported to down-regulate SCD1 mRNA expression in the liver of obese mice [23]. Taken together the present results and previous reports, it is suggested that decreased SCD1 expression in KK-A y mice by fucoxanthin contributes to the suppression of WAT weight gain and the improvement of blood glucose levels by regulating leptin sensitivity.
Conversely, SCD1 expression levels were not changed in leptin-deficient ob/ob mice fed fucoxanthin. The composition of 18:1n-9 in liver lipids did not significantly decrease, although 18:1n-9 and 18:0 tend to decrease and increase, respectively. These results suggest that the down-regulation of hepatic SCD1 is associated with leptin response. It is noteworthy that the anti-obesity effect of fucoxanthin was nonexistent in leptin deficient ob/ob mice. These data show that the anti-obesity effect and the improved blood glucose levels of fucoxanthin in KK-A y mice are partly associated with the down-regulation of hepatic SCD1 expression in leptin-dependent action.
In the present study, we have shown for the first time that dietary fucoxanthin has an inhibitory effect on hepatic SCD1 expression through the leptin signaling pathway. However, the mechanism of SCD1 down-regulation by fucoxanthin was not determined in this study. Previously, it had been demonstrated that leptin inhibits SCD1 transcription in HepG2 cells [23]. In addition, obese leptin-deficient ob/ob mice with extremely high levels of SCD1 were normalized by leptin-treatment [13, 24]. These reports indicate that leptin is responsible for the down-regulation of SCD1 expression. Therefore, improved leptin sensitivity by fucoxanthin is thought to largely contribute to the suppression of hepatic SCD1 in KK-A y mice. Furthermore, the inefficiency of fucoxanthin in ob/ob mice indicates that leptin plays a critical role in anti-obesity and anti-diabetic effects through the down-regulation of hepatic SCD1.
In conclusion, fucoxanthin down-regulated hepatic SCD1 expression through the regulation of leptin signaling in KK-A y mice with hyperleptinemia, but it did not do so in leptin-deficient ob/ob mice. A large proportion of human obesity is characterized by leptin resistance and not leptin deficiency; therefore, fucoxanthin is expected to show preventive effects on obesity by maintaining a satisfactory homeostatic mechanism.
Abbreviations
- SCD:
-
Stearoyl-coenzyme A desaturase
- mRNA:
-
Messenger RNA
- 18:0:
-
Stearic acid
- 18:1n-9:
-
Oleic acid
- WAT:
-
White adipose tissue
- PCR:
-
Polymerase chain reaction
References
Hosokawa M, Miyashita T, Nishikawa S, Emi S, Tsukui T, Beppu F, Okada T, Miyashita K (2010) Fucoxanthin regulates adipocytokine mRNA expression in white adipose tissue of diabetic/obese KK-Ay mice. Arch Biochem Biophys 504:17–25
Tsukui T, Konno K, Hosokawa M, Maeda H, Sashima T, Miyashita K (2007) Fucoxanthin and fucoxanthinol enhance the amount of docosahexaenoic acid in the liver of KKAy obese/diabetic mice. J Agric Food Chem 55:5025–5029
Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD (2002) Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci USA 99:11482–11486
Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM (2000) The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem 275:30132–30138
Kaestner KH, Ntambi JM, Kelly TJ Jr, Lane MD (1989) Differentiation-induced gene expression in 3T3-L1 preadipocytes. A second differentially expressed gene encoding stearoyl-CoA desaturase. J Biol Chem 264:14755–14761
Bjermo H, Risérus U (2010) Role of hepatic desaturases in obesity-related metabolic disorders. Curr Opin Clin Nutr Metab Care 13:703–708
Miyazaki M, Bruggink SM, Ntambi JM (2006) Identification of mouse palmitoyl-coenzyme A Delta9-desaturase. J Lipid Res 47:700–704
Ntambi JM, Miyazaki M (2003) Recent insights into stearoyl-CoA desaturase-1. Curr Opin Lipidol 14:255–261
Jiang G, Li Z, Liu F, Ellsworth K, Dallas-Yang Q, Wu M, Ronan J, Esau C, Murphy C, Szalkowski D, Bergeron R, Doebber T, Zhang BB (2005) Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1. J Clin Invest 115:1030–1038
Miyazaki M, Sampath H, Liu X, Flowers MT, Chu K, Dobrzyn A, Ntambi JM (2009) Stearoyl-CoA desaturase-1 deficiency attenuates obesity and insulin resistance in leptin-resistant obese mice. Biochem Biophys Res Commun 380:818–822
Gautron L, Elmquist JK (2011) Sixteen years and counting: an update on leptin in energy balance. J Clin Invest 121:2087–2093
Myers MG, Cowley MA, Münzberg H (2008) Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 70:537–556
Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM (2002) Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 297:240–243
Maeda H, Hosokawa M, Sashima T, Funayama K, Miyashita K (2005) Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem Biophys Res Commun 332:392–397
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Prevot AF, Mordret FX (1976) Utilisation des colonnes capillaries de verre pour l’analyse des corps gras par chromotographie en phase gazeuse. Rev Fse Corps Gras 23:409–423 (in French)
Masuzaki H, Ogawa Y, Aizawa-Abe M, Hosoda K, Suga J, Ebihara K, Satoh N, Iwai H, Inoue G, Nishimura H, Yoshimasa Y, Nakao K (1999) Glucose metabolism and insulin sensitivity in transgenic mice overexpressing leptin with lethal yellow agouti mutation: usefulness of leptin for the treatment of obesity-associated diabetes. Diabetes 48:1615–1622
Nishikawa S, Hosokawa M, Miyashita K (2012) Fucoxanthin promotes translocation and induction of glucose transporter 4 in skeletal muscles of diabetic/obese KK-A y mice. Phytomedicine 19:389–394
Flowers JB, Rabaglia ME, Schueler KL, Flowers MT, Lan H, Keller MP, Ntambi JM, Attie AD (2007) Loss of stearoyl-CoA desaturase-1 improves insulin sensitivity in lean mice but worsens diabetes in leptin-deficient obese mice. Diabetes 56:1228–1239
Rahman SM, Dobrzyn A, Dobrzyn P, Lee SH, Miyazaki M, Ntambi JM (2003) Stearoyl-CoA desaturase 1 deficiency elevates insulin-signaling components and down-regulates protein-tyrosine phosphatase 1B in muscle. Proc Natl Acad Sci USA 100:11110–11115
Rahman SM, Dobrzyn A, Lee SH, Dobrzyn P, Miyazaki M, Ntambi JM (2005) Stearoyl-CoA desaturase 1 deficiency increases insulin signaling and glycogen accumulation in brown adipose tissue. Am J Physiol Endocrinol Metab 288:E381–E387
Woo MN, Jeon SM, Kim HJ, Lee MK, Shin SK, Shin YC, Park YB, Choi MS (2010) Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6 N mice. Chem Biol Interact 186:316–322
Mauvoisin D, Prévost M, Ducheix S, Arnaud MP, Mounier C (2010) Key role of the ERK1/2 MAPK pathway in the transcriptional regulation of the stearoyl-CoA desaturase (SCD1) gene expression in response to leptin. Mol Cell Endocrinol 319:116–128
Biddinger SB, Miyazaki M, Boucher J, Ntambi JM, Kahn CR (2006) Leptin suppresses stearoyl-CoA desaturase 1 by mechanisms independent of insulin and sterol regulatory element-binding protein-1c. Diabetes 55:2032–2041
Acknowledgments
This work was supported by Grants-in Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (M.H., 23380120) and by the Japan Society for the Promotion of Science (JSPS) Grant-in Aid for JSPS Fellows (F.B., 2867).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Beppu, F., Hosokawa, M., Yim, MJ. et al. Down-Regulation of Hepatic Stearoyl-CoA Desaturase-1 Expression by Fucoxanthin via Leptin Signaling in Diabetic/Obese KK-A y Mice. Lipids 48, 449–455 (2013). https://doi.org/10.1007/s11745-013-3784-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-013-3784-4