Abstract
The current study examined the efficacy of graded doses of c9,t11 and t10,c12 CLA isomers on body composition, energy expenditure, hepatic and serum lipid liver biomarkers in hamsters. Animals (n = 105) were randomized to seven treatments (control, 1, 2, 3% of c9,t11; 1, 2, 3% of t10,c12) for 28 days. After 28 days treatment, 1–3% of t10,c12 lowered (p < 0.05) body fat mass compared to the control group. The 1–3% t10,c12 and 3% c9,t11 fed groups showed higher (p < 0.05) lean mass compared to other groups. We observed unfavorable changes in plasma total cholesterol and non-HDL cholesterol levels in animals fed with 3% t10,c12 CLA isomers. The 2%, 3% t10,c12 groups presented elevated (p < 0.05) ALT levels. The present data suggest that a diet enriched with more than 2% t10, c12 led to liver malfunction and poses unfavorable changes on plasma lipid profiles. The 1% t10,c12 CLA lowered (p < 0.05) body fat mass and increased (p < 0.05) lean body mass. The c9,t11 CLA has less potent actions than t10,c12 CLA. We conclude that the actions of CLA on energy and lipid metabolism are form and dose dependent in the hamster model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major isomers of CLA include cis-9,trans-11 (c9,t11) CLA and trans-10,cis-12 (t10,c12) CLA [1]. Over the last decade, health benefits of CLA have received a great deal of attention in various animal models and humans. Results have shown that c9,t11 and t10,c12 CLA mixtures possess anticancer effects both in vitro and in vivo [2–5]. Other effects including body composition modulation, atherosclerosis prevention, as well as immune system enhancement, have also been suggested in animal models [6–9]. CLA isomer enriched diets also effectively reduce body fat and increase lean body mass in several species [7, 10, 11]. However, previous studies have mainly focused on the effect of c9, t11 and t10,c12 CLA mixtures and conflicting results have been reported in many studies (Supplemental Table 1). Such sizable variation in results may due to the variability in animal model, duration of feeding as well as form, and the dosage of each isomer used. Indeed, the two CLA isomers may play different roles in body composition changes as well as lipid metabolism. The favorable modulation of body composition is mainly ascribed to the t10,c12 CLA isomer [12], while a less potent effect has been reported for the c9,t11 CLA isomer. Lack of effect on c9,t11 CLA consumption on body composition modulation may due to the use of mixture of isomers, where the effect of one isomer may overshadow another, or to the relative low dosage used in different trials.
In terms of lipid metabolism and safety, previous investigations have shown that CLA lowers total cholesterol and triglyceride levels in plasma [13, 14]; nevertheless, these appear to be highly species dependent. Less consistent results have been observed in hamster and human studies [13, 15]. Moreover, safety issues have been raised over CLA supplementation since increased spleen and liver weights have been observed in some animal studies [16, 17]. Indeed, a systematic comparison of the two major isomers at graded dosages has not been previously conducted. Very few studies have investigated the effect of highly enriched c9,t11 and t10,c12 CLA isomer effect solely (Supplement Table 1). Therefore, the current investigation was aimed to examine both isomer and dosage effect of c9, t11 and t10, c12 CLA on body composition changes and serum lipid content modulation in Golden Syrian hamsters. Hamsters are considered to be a suitable model for studying lipid changes due to their similar lipoprotein profile and metabolism compared to humans [18]. Additionally, safety evaluation was also assessed in the present study.
Materials and Methods
Animals and Study Design
One hundred and five male Golden Syrian hamsters (Charles River Laboratories, Montreal, Quebec) weighing between 90 and 100 g were housed individually in plastic cages and subjected to a 12 h light/dark cycle at constant room temperature 25 °C. Upon arrival, the hamsters were provided with free access to rodent chow diet (Nestle, Purina, USA) and water for 3 weeks, and then switched to a semi-purified hypercholesterolemic diet containing 5% fat and 0.25% cholesterol for 3 more weeks. The study used a completely randomized design. Hamsters were randomized into 7 groups of 15 animals. Seven experimental diets were tested, including a control diet with no CLA and treatment diets enriched with 1, 2, 3% of c9, t11 CLA as well as 1, 2, 3% of t10,c12 CLA, each provided for 4 weeks. CLA isomers were provided in free fatty acid form and prepared by low temperature crystallization. Both CLA isomers were supplied by Lipid Nutrition (Wormerveer, Netherlands). Table 1 presents the macronutrient and fatty acid composition of experimental diets. Dietary ingredients were purchased from Harland Laboratories Inc. (Indiana, USA) except cornstarch and sucrose which were purchased locally. Butylated hydroxytoluene (Sigma-Aldrich, Inc. ON, Canada) was added as an antioxidant. Food intake was measured every 2 days and body weight was measured weekly. On day 28, hamsters were anesthetized by isoflurane inhalation. Blood samples were taken by cardiac puncture then transferred into pre-coated heparin tubes. Hamsters were sacrificed followed by evisceration. Organ samples were wrapped and snap frozen by liquid nitrogen then stored at −80 °C until further analysis. The study was approved by the University of Manitoba Animal Care Protocol Review Committee, and carried out in accordance with the guidelines of the Canadian Council on Animal Care [19].
Body Composition Measurement
Body composition was measured by DEXA from Lunar Prodigy Advance. Tissue analyses were conducted by software Encore version 9.30.044. Percentage body fat, body fat mass and lean body mass were assessed using this software.
Lipid Profile and Hepatic Biomarker Measurement
Blood samples were thawed at room temperature then centrifuged at 3,500 rpm for 20 min. Red blood cells (RBC), plasma and serum samples were collected separately. Serum samples were used for lipid profile and liver enzyme assessment. Analyses were conducted by the Vitro Chemistry System 350 (Ortho-Clinical Diagnostics, Inc. Rochester, NY, USA). Triglyceride, total cholesterol and HDL-cholesterol levels were measured directly, whereas non-HDL cholesterol levels were calculated by subtracting HDL-cholesterol from total cholesterol content. Serum concentrations of liver enzymes AST, ALT and GGT were also measured.
Cholesterol Synthesis Measurement
Hamsters received 0.5 ml of deuterium oxide (D2O, 99.9%, Cambridge Isotope Laboratories, Inc MA, USA) by intraperitoneal injection 2 h prior to sacrifice to assess cholesterol synthesis rate. Deuterium-enriched cholesterol was used as an indication of cholesterol synthesis [20]. Isotope ratios of DH/H2 of plasma cholesterol were expressed in δ per mil, relative to Vienna Standard Mean Ocean Water. Enrichment of deuterium in both plasma water and RBC cholesterol were measured. Cholesterol samples extracted from RBCs were analyzed by online GC/pyrolysis/isotope ratio mass spectrometry (IRMS) equipped with an Agilent 6890N GC and Finnigan Delta V Plus IRMS (Bremen, Germany) through a Finnigan combustion interface (Combustion Interface III, Bremen, Germany). Cholesterol fractional synthesis rates were measured and calculated as previously described [20].
Energy Expenditure Assessment
On day 25, energy expenditure was assessed by the MM100-metabolic monitor system (CWE, Inc Ardmore, USA). Animals were kept in individual air chambers. Oxygen consumption was measured indirectly by monitoring oxygen and carbon dioxide concentrations in the chamber for 2 h per animal.
Hepatic Lipid Content Analysis
Liver lipids were extracted according to the Folch method [21] by chloroform and methanol in a ratio of 2:1 (v/v). Analysis of hepatic triglyceride and cholesterol concentrations was conducted by commercial available enzymatic kits (Roche Diagnostics, QC, Canada).
Statistics Analysis
All statistical analyses were performed by Statistical Analysis System (version 8.1; SAS Institute Inc Cary, NC). Data from different diet groups were analyzed by one-way ANOVA for overall significance then followed by Tukey’s post-hoc tests to identify the significant difference between treatment groups. Results were expressed as means ± SEM (standard error mean). Regression analysis was also performed. Treatment effects and differences between means were considered significant when p < 0.05.
Results
Effects of CLA Consumption on Food Intake, Body Composition and Energy Expenditure
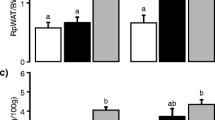
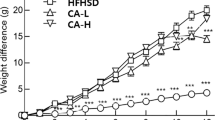
Hamsters fed with t10,c12 CLA enriched diets showed a lower (p < 0.05) food intake in comparison to control and other groups (Table 2). No differences were noted in average daily food intake between the control animals and c9,t11 treatment groups. Energy expenditure data suggested a strong tendency towards animals on three dosages of t10,c12 CLA supplementation having greater oxygen consumption rate compared to control group and others (p = 0.0657). At the end of the 28-day experimental period, hamsters in all seven treatment groups exhibited similar body weights. Body composition results are presented in Table 2. DEXA analyses data indicate that hamsters fed with 1, 2, and 3% t10, c12 CLA enriched diets showed 27, 30 and 23% less (p < 0.0001) body fat mass compared to control animals, respectively. Additional, higher lean body mass was found in groups fed with all three dosages of t10,c12 CLA supplemented diets. Such effects were also observed in animals fed with 3% c9,t11 CLA compared to control (p = 0.0002). Both 1 and 3% t10,c12 CLA fed animals had a lower body weight gain over 4 weeks, whereas opposite effects were observed in animals on 3% c9,t11 CLA enriched diet (Fig. 1).
Effect of CLA Consumption on Lipid Profiles
Serum lipid content was affected by t10,c12 CLA isomer in hamsters (Table 3). Neither the serum HDL-cholesterol concentration nor the triglyceride concentration differed among treatment groups. However, animals consuming 3% t10,c12 CLA enriched diets displayed the highest total cholesterol and non-HDL cholesterol levels, in contrast to the rest of the treatment groups after 28 days of dietary intervention (p < 0.05).
Effect of CLA Consumption on Liver Weight and Hepatic Lipid Content
Data on effects of CLA consumption on liver weight and hepatic lipid content are presented in Table 4. Hamsters in the 2 and 3% t10,c12 CLA diet groups exhibited higher liver weight (p = 0.001).With regard to hepatic lipid content, neither liver cholesterol nor the triglyceride concentration differed between groups after 28 days of consumption of CLA enriched diets. No differences were observed between dosages. Moreover, there was no difference in serum total protein level across the treatment groups (data not shown).
Effect of CLA Consumption on Hamster Liver Enzyme Level
After the 4-week experimental period, elevated ALT levels were observed in hamsters fed 2 and 3% t10,c12 CLA diets (p < 0.0001), however, such results were not noted in any of the other treatment groups. Additionally, ALT levels in animals on 3% t10,c12 CLA enriched diet were higher (p = 0.0155) than those in animals fed with 2% t10,c12 CLA enriched diet. No differences were noticed in hepatic AST or GGT levels across the seven experimental diets (Table 5).
Effect of CLA Consumption on Cholesterol Synthesis
Hamsters displayed similar cholesterol synthesis rates regardless of CLA intervention after 28 days (Fig. 2). Regression analysis suggested that the elevated serum cholesterol level of 2 and 3% t10, c12 CLA treatment groups occurred independently of the individual rates of cholesterol synthesis across animals.
Discussion
The primary finding of the present study was that 1% t10,c12 CLA effectively altered body composition without exhibiting potential adverse effects on the lipid profile and liver health. Similar body composition changes were also observed at 2 and 3% of t10,c12 CLA. However, data from both serum lipid profiles and hepatic biomarkers suggest that 3% t10,c12 CLA led to unfavorable increases in total as well as non-HDL cholesterol levels. Moreover, both 2 and 3% t10,c12 CLA treatment induced elevated ALT levels. Such changes may suggest health concerns associated with consuming high dosages of t10,c12 CLA.
As a secondary effect of CLA, data from the current study suggest that t10,c12 CLA decreases energy intake in hamsters. These data are in line with results reported by others [12, 22, 23]. Data from the present study show that animals on the t10,c12 CLA treatment had a lower fat body mass which was in accordance with previous observations [10, 22, 24, 25]. In earlier studies, t10,c12 CLA isomers were identified as being responsible for biological effects on body composition [13]. Results from the current study also provide evidence supporting a physiological effect of CLA on fat mass in a manner that is isomer dependent. Several possible mechanisms may explain body fat reduction effects in response to CLA supplementation. Much evidence suggests that t10,c12 CLA may induce fat mass reduction by decreasing energy intake, inhibiting adipogenesis or lipogenesis by suppressing gene expression of sterol regulatory element binding protein (SREBP), liver X-receptor (LXR) α, PPAR-α and PPAR-γ target genes [26–29]. Additional evidence supports a t10,c12 CLA-induced down regulation of the hypothalamic appetite regulating gene expression which suppresses appetite, leading to reduced energy intakes [30, 31]. Data from current study indicate that reductions in fat mass following the three doses of t10,c12 CLA were associated with decreased energy intake as well as a tendency towards increased energy expenditure.
Notably, animals fed with 3% c9,t11 CLA displayed increased lean body mass. The same observation was also noticed in animals fed all three dosages of t10,c12 CLA. To our knowledge, the current study is the first evidence supporting an effect on hamster body composition by consumption of the c9,t11 CLA isomer solely. Other work suggests that a CLA mixture including 25% c9,t11 CLA induced increased carcass lean tissue in growing pigs [32]. Previous works reported that CLA consumption increases lean body mass in several species (reviewed in [33]). However, the lack of evidence of such an effect on c9,t11 CLA isomer may be explained by the relative low dose of c9,t11 CLA isomer or c9,t11 containing mixture used in previous studies. Recently, Nall et al. [34] demonstrated that CLA and arginine increased lean body mass, due possibly to a depression in muscle protein turnover, however, the mechanism of CLA supplementation increasing lean body mass remains to be fully understood and requires further investigation.
The effects of c9,t11 and t10,c12 CLA on serum lipid profiles vary between animals and humans [14, 35]. In the present study, animals fed 3% t10,c12 enriched diet exhibited the highest total as well as non-HDL cholesterol levels, in comparison with the rest of the groups. These results are in accordance with some studies published by other authors when animals were fed diets with either a CLA mixture or the t10,c12 CLA isomer. Bissonauth et al. [36] reported an increase in LDL-cholesterol levels induced by 2% highly enriched t10,c12 CLA. Kritchevsky et al. [9] demonstrated elevated total cholesterol levels induced by CLA mixtures (43:44%, c9,t11:t10,c12) in rabbits. In contrast, evidence supports either a positive action or no effects on total cholesterol and non-HDL cholesterol levels by either pure or mixed CLA consumption [37–39]. Current data fail to provide any further positive indication of CLA improving serum lipid profile. Most human studies report no effect of supplementation with CLA mixtures on lipid profiles [35]. Tricon et al. [40] reported that serum concentration of total cholesterol, LDL cholesterol, TG and the ratio of total to HDL cholesterol were elevated after consumption of CLA capsules containing 80% t10, c12 in healthy subjects. Moloney et al. [41] also demonstrated an increased LDL to HDL ratio in subjects with type II diabetes. Moreover, a recent review has suggested that all fatty acids with a double bond in the trans-configuration raise the LDL to HDL ratio [15]. Data from the present work partially reflects this adverse effect of high dose t10,c12 CLA isomer on serum lipid content. The isotope work indicates that increased total serum cholesterol is not the result of augmented endogenous cholesterol synthesis. Navarro et al. [42] found that t10,c12 CLA significantly reduced the LDL-receptor number when expressed as an arbitrary value of per mg of protein in hamster liver. One possible theory offered was that t10,c12 CLA induced an increase in free cholesterol pool size, perhaps related to down regulation of the LDL receptor. In comparison with the aforementioned study, the high dosage of t10,c12 CLA provided in the current study significantly increased serum total and non-HDL cholesterol levels, with a lack of change in hepatic lipid content. Taken together, growing evidence suggests that t10,c12 CLA does not favorably change serum lipid levels.
Recently, the safety of CLA consumption has become a concern. In the present study, the high dosages of t10,c12 CLA (2 and 3%) increased hamster liver weights compared with control and c9,t11 CLA treatments. This finding is consistent with those of a number of other studies [13, 21, 43]. Along with this observation, our study has shown that CLA supplementation did not affect lipogenesis in liver since the triglyceride concentrations failed to differ across treatment groups. The increased liver weight in animals fed with t10,c12 isomer are not necessarily due to liver lipid accumulation but rather from a greater number of hepatocytes [13, 44]. Future histological analysis may provide a better understanding of the underlying cause of elevated liver weight.
It is well known that serum liver enzyme activity exists as an indicator of liver function. In our study, elevated ALT concentrations in liver were observed in animals fed 2 and 3% t10,c12 CLA. Such results suggest that high doses of t10,c12 may lead to liver malfunction since ALT serves as a biomarker for hepatocellular necrosis [45]. Elevated ALT level may also indicate increased inflammation and oxidation subsequent to t10,c12 CLA consumption. In contrast, animal studies conducted by Macarulla et al. [44] reported no changes in hepatic ALT concentration after 6-week consumption of t10,c12 CLA. The discrepancy could be attributed to the dosage variances between studies (0.5 vs. 2% and higher).
In a recent investigation on the safety of dietary CLA consumption, Iwata et al. [46] reported slight increases in ALT activities in the high dose CLA (6.8/d) group after 12-week intervention in healthy overweight Japanese subjects. Taken together, c9,t11 CLA and 1% t10,c12 CLA did not exert any adverse effect on liver health in the hamster model. Present data show clearly that the degree of impact of CLA supplementation on liver health is isomer dependent. Recently, the European Food Safety Authority has issued positive safety opinions about two CLA products as a safe ingredient for food and beverage [47]. In the present study, 1, 2 and 3% CLA-enriched diet correspond to human diet equivalents (2,500 kcal intake daily) of 2, 4 and 6 g/d. These levels of intakes are very similar to what EFSA has recommended. The 3% CLA treatment represented the higher dosages than what has been recommended by EFSA (6 g at 3% vs. 3.5–4.5 g) which provided a better idea for safety evaluation for CLA consumption. Iwata et al. [46 reported mild to moderate adverse effects in overweight male Japanese subjects supplemented with either 3.4 or 6.8 g/d CLA (50:50, c9,t11:t10,c12), where slight increases in AST and ALT activity levels were observed at 12 weeks. The author indicated that such elevations were small and within the normal range. Moreover, one of the most recent reviews conducted by Katan and colleagues [15] has suggested that CLA exerts a negative effect on the circulation lipid profile.
In summary, the present study provided a systematic comparison of two CLA isomers at three different dosages. The most important finding is that low dose intakes of t10,c12 CLA effectively lower body fat mass and increase lean body mass without posing unfavorable changes in serum lipid profile. Higher dosages (2 and 3%) of t10,c12 CLA supplementation produce adverse effects in liver function and 3% t10,c12 CLA has negative effects on serum lipid content. 3% c9,t11 CLA also increased lean body mass; however, no body fat lowering effects of the c9,t11 CLA isomers were seen at any of the three dosages. Additionally, c9,t11 CLA did not have any adverse effect in liver function in the hamster model. Data from the present study suggests that 1% t10,c12 CLA, which was close to the EFSA recommended level, effectively modulates body composition whereas 2 and 3% cause unfavorable physiological changes.
Abbreviations
- DEXA:
-
Dual energy X-ray absorptiometry
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- GGT:
-
γ-Glutamyltranspeptidase
References
MacDonald HB (2000) Conjugated linoleic acid and disease prevention: a review of current knowledge. J Am Coll Nutr 19(2 Suppl):111S–118S
Thomas Yeung CH et al (2000) Dietary conjugated linoleic acid mixture affects the activity of intestinal acyl coenzyme A: cholesterol acyltransferase in hamsters. Br J Nutr 84(6):935–941
Durgam VR, Fernandes G (1997) The growth inhibitory effect of conjugated linoleic acid on MCF-7 cells is related to estrogen response system. Cancer Lett 116(2):121–130
Cesano A et al (1998) Opposite effects of linoleic acid and conjugated linoleic acid on human prostatic cancer in SCID mice. Anticancer Res 18(3A):1429–1434
Liew C et al (1995) Protection of conjugated linoleic acids against 2-amino-3-methylimidazo [4,5-f]quinoline-induced colon carcinogenesis in the F344 rat: a study of inhibitory mechanisms. Carcinogenesis 16(12):3037–3043
Drury B et al (2009) Dietary trans,cis-12 conjugated linoleic acid reduces early glomerular enlargement and elevated renal cyclooxygenase-2 levels in young obese fa/fa zucker rats. J Nutr 139(2):285–290
Tarling EJ et al (2009) Effect of dietary conjugated linoleic acid isomers on lipid metabolism in hamsters fed high-carbohydrate and high-fat diets. Br J Nutr 101(11):1630–1638
Parra P, Serra F, Palou A (2010) Moderate doses of conjugated linoleic acid isomers mix contribute to lowering body fat content maintaining insulin sensitivity and a noninflammatory pattern in adipose tissue in mice. J Nutr Biochem 21:107–115
Kritchevsky D et al (2000) Influence of conjugated linoleic acid (CLA) on establishment and progression of atherosclerosis in rabbits. J Am Coll Nutr 19(4):472S–477S
West DB et al (2000) Conjugated linoleic acid persistently increases total energy expenditure in AKR/J mice without increasing uncoupling protein gene expression. J Nutr 130(10):2471–2477
Park Y et al (1997) Effect of conjugated linoleic acid on body composition in mice. Lipids 32(8):853–858
Park Y et al (1999) Evidence that the trans-10,cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids 34(3):235–241
De Deckere EAM et al (1999) Effects of conjugated linoleic acid (CLA) isomers on lipid levels and peroxisome proliferation in the hamster. Br J Nutr 82(4):309–317
Mitchell PL, McLeod RS (2008) Conjugated linoleic acid and atherosclerosis: studies in animal models. Biochem Cell Biol 86(4):293–301
Brouwer IA, Wanders AJ, Katan MB (2010) Effect of animal and industrial trans-fatty acids on HDL and LDL cholesterol levels in humans–a quantitative review. PLoS One 5(3):e9434. doi:10.1371/journal.pone.0009434
Clement L et al (2002) Dietary trans-10,cis-12 conjugated linoleic acid induces hyperinsulinemia and fatty liver in the mouse. J Lipid Res 43(9):1400–1409
Perez-Matute P et al (2007) Conjugated linoleic acid inhibits glucose metabolism, leptin and adiponectin secretion in primary cultured rat adipocytes. Mol Cell Endocrinol 268(1–2):50–58
Spady DK, Dietschy JM (1983) Sterol synthesis in vivo in 18 tissues of the squirrel monkey, guinea pig, rabbit, hamster, and rat. J Lipid Res 24(3):303–315
Canadian Council on Animal Care (2006) Guide to the care and use of experimental animals, vol 2, chap XV. Hamsters
Jones PJ et al (2000) Modulation of plasma lipid levels and cholesterol kinetics by phytosterol versus phytostanol esters. J Lipid Res 41(5):697–705
Folch JM, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226(1):497–509
West DB et al (1998) Effects of conjugated linoleic acid on body fat and energy metabolism in the mouse. Am J Physiol 275(3 Pt 2):R667–R672
Takahashi Y et al (2002) Dietary conjugated linoleic acid reduces body fat mass and affects gene expression of proteins regulating energy metabolism in mice. Comp Biochem Physiol B Biochem Mol Biol 133:395–404
DeLany JP, West DB (2000) Changes in body composition with conjugated linoleic acid. J Am Coll Nutr 19(4):487S–493S
Azain MJ et al (2000) Dietary conjugated linoleic acid reduces rat adipose tissue cell size rather than cell number. J Nutr 130(6):1548–1554
Kang K et al (2003) trans-10,cis-12 CLA inhibits differentiation of 3T3–L1 adipocytes and decreases PPAR gamma expression. Biochem Biophys Res Commun 303(3):795–799
Brown JM et al (2003) Isomer-specific regulation of metabolism and PPARgamma signaling by CLA in human preadipocytes. J Lipid Res 44(7):1287–1300
Granlund L, Pedersen JI, Nebb HI (2005) Impaired lipid accumulation by trans10,cis CLA during adipocyte differentiation is dependent on timing and length of treatment. Biochim Biophys Acta 1687(1–3):11–22
LaRosa PC et al (2007) trans-10,cis-12 Conjugated linoleic acid activates the integrated stress response pathway in adipocytes. Physiol Genomics 14(31):544–553
So M, Tse I, Li E (2009) Dietary fat concentration influences the effects of trans-10,cis-12 conjugated linoleic acid on temporal patterns of energy intake and hypothalamic expression of appetite-controlling genes in mice. J Nutr 139:145–151
Cao Z et al (2007) Intracerebroventricular administration of conjugated linoleic acid (CLA) inhibits food intake by decreasing gene expression of NPY and AgRP. Neurosci Lett 418:217–221
Ostrowska E et al (1999) Dietary conjugated linoleic acids increase lean tissue and decrease fat deposition in growing pigs. J Nutr 129(11):2037–2042
Wang YW, Jones PJH (2004) Conjugated linoleic acid and obesity control: efficacy and mechanisms. Int J Obes Relat Metab Disord 28(8):941–955
Nall JL et al (2009) Dietary supplementation of l-arginine and conjugated linoleic acid reduces retroperitoneal fat mass and increases lean body mass in rats. J Nutr 137(7):1279–1285
Salas-Salvado J, Marquez-Sandoval F, Bullo M (2006) Conjugated linoleic acid intake in humans: a systematic review focusing on its effect on body composition. Glucose, and lipid metabolism. Crit Rev Food Sci Nutr 46(6):479–488
Bissonauth V et al (2006) The effect of t10,c12 CLA isomer compared with c9,t11 CLA isomer on lipid metabolism and body composition in hamsters. J Nutr Biochem 17:597–603
Lee KN, Kritchevsky D, Pariza MW (1994) Conjugated linoleic acid and atherosclerosis in rabbits. Atherosclerosis 108(1):19–25
Nicolosi RJ et al (1997) Dietary conjugated linoleic acid reduces plasma lipoproteins and early aortic atherosclerosis in hypercholesterolemic hamsters. Artery 22(5):266–277
LeDoux M et al (2007) Rumenic acid significantly reduces plasma levels of LDL and small dense LDL cholesterol in hamsters fed a cholesterol- and lipid-enriched semi-purified diet. Lipids 42(2):135–141
Tricon S et al (2004) Opposing effects of cis-9,trans-11 and trans-10, cis-12 conjugated linoleic acid on blood lipids in healthy humans. Am J Clin Nutr 80(3):614–620
Moloney F et al (2004) Conjugated linoleic acid supplementation, insulin sensitivity, and lipoprotein metabolism in patients with type 2 diabetes mellitus. Am J Clin Nutr 80(4):887–895
Navarro V et al (2007) Effects of trans-10,cis-12 conjugated linoleic acid on cholesterol metabolism in hypercholesterolaemic hamsters. Eur J Nutr 46(4):213–219
Navarro V et al (2003) Effects of conjugated linoleic acid on body fat accumulation and serum lipids in hamsters fed an atherogenic diet. J Physiol Biochem 59(3):193–199
Macarulla MT et al (2005) Effects of conjugated linoleic acid on liver composition and fatty acid oxidation are isomer-dependent in hamster. Nutrition 21(4):512–519
45. Meeks RG, Harrison SD, Bull RJ (1991) Liver function tests in the differential diagnosis of hepatotoxicity. In: Meeks RG (ed) Hepatotoxicology. CRC Press
Iwata T et al (2007) Safety of dietary conjugated linoleic acid (CLA) in a 12-weeks trial in healthy overweight Japanese male volunteers. J Oleo Sci 56(10):517–525
EFSA, Scientific Opinion on the safety of “conjugated linoleic acid (CLA)-rich oil” (Tonalin® TG 80) as a Novel Food ingredient EFSA Journal, 2010. 8:43.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Liu, X., Joseph, S.V., Wakefield, A.P. et al. High Dose trans-10,cis-12 CLA Increases Lean Body Mass in Hamsters, but Elevates Levels of Plasma Lipids and Liver Enzyme Biomarkers. Lipids 47, 39–46 (2012). https://doi.org/10.1007/s11745-011-3616-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-011-3616-3