Abstract
Conjugated linoleic acid (CLA) is a collection of octadecadienoic fatty acids that have been shown to possess numerous health benefits. The CLA used in our study was produced by the photoisomerization of soybean oil and consists of about 20% CLA; this CLA consists of 75% trans–trans (a mixture of t8,t10; t9,t11; t10,t12) isomers. This method could be readily used to increase the CLA content of all soybean oil used as a food ingredient. The objective of this study was to determine the effects of trans–trans CLA-rich soy oil, fed as a dietary supplement, on body composition, dyslipidemia, hepatic steatosis, and markers of glucose control and liver function of obese fa/fa Zucker rats. The trans–trans CLA-rich soy oil lowered the serum cholesterol and low density lipoprotein–cholesterol levels by 41 and 50%, respectively, when compared to obese controls. Trans–trans CLA-rich soy oil supplementation also lowered the liver lipid content significantly (P < 0.05) with a concomitant decrease in the liver weight in the obese rats. In addition, glycated hemoglobin values were improved in the group receiving CLA-enriched soybean oil in comparison to the obese control. PPAR-γ expression in white adipose tissue was unchanged. In conclusion, trans–trans CLA-rich soy oil was effective in lowering total liver lipids and serum cholesterol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity, cardiovascular disease, and type 2 diabetes are international health problems with their rates predicted to rise [1]. Research has shown that when human weight increases to the point of being classified as “obese,” the risk of health problems such as hypertension, type 2 diabetes, coronary heart disease, stroke, cancer, gallbladder disease, osteoarthritis, sleep apnea, and respiratory problems increase [2–7].
Conjugated linoleic acid (CLA) is a collection of octadecadienoic fatty acid isomers that have been shown to positively affect health in human and animal models [8]. Some of the health benefits of CLA include reduced adiposity, antidiabetogenic, antiatherogenic, and anticarcinogenic effects [9–12]. The benefits have been seen primarily with the cis-9,trans-11 (c9,t11) and trans-10,cis-12 (t10,c12) isomers. At present, it is known that the effects of CLA are dose-, time-, and species-dependent [13]. In addition, the activity of CLA is highly isomer specific; t10,c12 CLA isomer is antiadipogenic in differentiating human preadipocytes and the c9,t11 CLA isomer promotes adipogenesis [14]; therefore, it is important to identify isomer-specific responses.
Determination of the isomer-specific effects of CLA is necessary before CLA can be used in fortified foods or supplements. The major sources of CLA in the human diet are meat and dairy products derived from ruminants, and in these products the predominant CLA isomer (>90%) is c9,t11 [15]. However, to obtain the estimated optimum dietary CLA levels of about 3 g/d, from natural sources it would be necessary to increase the animal or dairy products which would increase saturated fat intake [16, 17]. Therefore, a concentrated source of dietary CLA that is low in saturated fat is desirable [18]. Jain and Proctor [18] showed that CLA isomers can be rapidly produced in large quantities in a pilot scale by efficient photoisomerization of soy oil. This reaction produces mainly trans–trans CLA isomers, the nutritional effects of which have not been studied. This CLA-rich soy oil produced by the pilot-scale system contained 180 times the total CLA isomers obtained from beef or dairy products [18]. Hence, the objectives of this study were to determine the effects of dietary supplementation of trans–trans CLA-rich soy oil on body composition, dyslipidemia, hepatic steatosis, and markers of glucose control and liver function of obese fa/fa Zucker rats.
Experimental Procedures
Study Design
This study was conducted using 3-month old female obese fa/fa Zucker rats (Harlan Laboratories, Indianapolis, IN), a commonly used model for studying obesity. Thirty-six rats were divided into the following treatment groups (n = 12): lean control (L-Ctrl), obese control (O-Ctrl), and obese CLA (O-CLA). The L-Ctrl and O-Ctrl groups were fed AIN-93M purified rodent diet. The O-CLA group was fed AIN-93M modified to contain ~0.5% trans–trans CLA isomers by diet mass. All animals were pair-fed to the mean intake of the L-Ctrl group and the food intake was measured three times a week. Although pair-feeding may cause stress in hyperphagic obese Zucker rats, in this study we did not observe any visual signs of stress. Pair-feeding was necessary to match the macronutrient intake of all the groups. The body weight of the animals was recorded once per week. The rats had free access to deionized water. After 100 days of treatment, the rats were fasted for 12 h before being sacrificed by exsanguination via cardiac puncture. University of Arkansas Institutional Animal Care and Use Committee guidelines for the treatment and care of the animals were followed throughout the study duration.
Diets
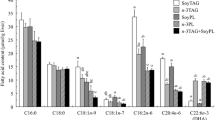
Animals in the control group were fed AIN-93M purified rodent diet formulated in accordance to the American Institute of Nutrition committee report [19]. Animals in the O-CLA group received AIN-93M containing 40 g/kg of trans–trans CLA-rich soybean oil substituted for regular soybean oil. The rat diet composition is given in Table 1. Refined, bleached deodorized soy oil was photo-irradiated in a pilot-plant regime using the optimal conditions discussed by Jain and Proctor [18]. The total CLA and isomer content of the oil was measured as fatty acid methyl esters (FAMES) by base-catalyzed conversion [20]. The CLA isomer composition of the CLA-rich soybean oil is presented in Table 2.
Body Composition
Dual-energy X-ray Absorptiometry (DXA; GE Lunar DXA, Waukesha, WI) was used to analyze the body composition of the rats at baseline and immediately prior to sacrifice. The animals were anesthetized and placed stomach down on the scan bed of the DXA. The absorbance of two X-ray beams was measured and percent lean tissue and percent fat tissue were calculated by software (enCORE, GE Lunar, Waukesha, WI) appropriate for the body composition assessment of small animals.
Necropsy and Tissue Collection
Organs and tissues of interest were removed from the animal immediately after sacrifice. The liver and white adipose tissue were placed in cryogenic storage containers and flash-frozen in liquid nitrogen before being stored at −80 °C. Approximately 7 mL of blood collected from the animal via cardiac puncture during sacrifice was stored on ice before being centrifuged to separate serum from whole blood. Aliquots of serum were transferred to microcentrifuge tubes and stored at −80 °C until analysis. A small amount of blood collected from the heart during sacrifice was placed in a microtube containing K2 EDTA (an anticoagulant) and stored at −80 °C.
Biochemical Analysis
Serum triglycerides (TG), total cholesterol (TC), high density lipoprotein–cholesterol (HDL–C), low density lipoprotein–cholesterol (LDL–C), aspartate transaminase (AST), blood urea nitrogen (BUN), glycated hemoglobin (HbA1c), and glucose concentrations were determined using commercially available kits from Alfa Wassermann Diagnostic Technologies (West Caldwell, NJ). An ACE Alera clinical chemistry system (Alfa Wassermann Diagnostic Technologies, West Caldwell, NJ) was used according to the manufacturer’s instructions to perform these tests.
Insulin
Serum insulin was quantified using a commercially available ELISA kit (Alpco Immunoassays, Salem, NH). An aliquot of serum was thawed at 2–8 °C before use. A 96-well plate in the kit was prepared per manufacturer instructions to include duplicate standards, appropriate controls, and sample duplicates. Absorbance was measured using a BioTek ELx808 microplate reader (Winooski, VT) attached to a PC running BioTek Gen5 data analysis software (Winooski, VT).
Liver Lipids
One gram of liver was homogenized in a 20-fold volume of 2:1 chloroform–methanol (v/v) mixture. Following homogenization, 0.58% NaCl solution was added to achieve separation of the phases and centrifuged for 20 min at 500×g. Supernatant was discarded and the organic phase was filtered and washed with chloroform through fat free filter paper (3.2 cm Whatman, Whatman International Ltd, Maidstone, England). The filtered organic phase containing the tissue lipids was then transferred to a pre-weighed scintillation vial. Liver lipids were determined using the Folch gravimetric method [21]. Liver total cholesterol was determined using the method described by Searcy and Bergquist [22].
Gene Expression in White Adipose Tissue
RNA Extraction and cDNA Synthesis
RNA was extracted from approximately 100 mg of white adipose tissue by TRIzol Reagent method using RNeasy Lipid Tissue Mini Kit (Qiagen, USA) by following the supplier’s instructions. The total amount of RNA present in each sample was quantitated using Nanodrop (Thermo Scientific, Wilmington, DE) and 1 μg/sample of total RNA was used for cDNA synthesis using a QuantiTect Rev. Transcription Kit (Qiagen, USA).
Quantitative Real-Time PCR
Following cDNA synthesis, qRT-PCR was performed using the Mastercycler ep realplex with SYBR Green system (Eppendorf, Hamburg, Germany). Concentrations of reagents were used per the manufacturer’s instructions. Real-time PCR primers were designed using Primer3 primer design software and all primer sets were synthesized by Invitrogen (Invitrogen, Carlsbad, CA). Primer sequences used for this study are summarized in Table 3. The following experimental conditions were used for all target gene expression including generation of standard curves. The initial denaturation cycle was performed at 95 °C for 5 min. All subsequent denaturation and annealing cycles were repeated 45 times at 95 °C for 15 s and 60 °C (55 °C annealing for reference gene β-actin) for 45 s, respectively. The relative gene expression ratio by qRT-PCR was calculated using “REST” software.
Statistical Methods
The data analysis involved estimation of means and standard error (SE) using JMP 8 (2009 SAS Institute Inc. Cary, NC). The effects of treatment were analyzed by one-way ANOVA model followed by post hoc analysis using the Fisher’s least squares means separation test when F values were significant. For all analyses, a P-value less than 0.05 was considered significant.
Results
Food Intake, Body Weight, Body Composition, and Organ Weights
There were no significant differences (P > 0.05) in the mean food intake between the three experimental groups, as the rats were pair-fed to the mean intake of the L-Ctrl group. The effects of treatment on body weights, body composition, and organ weights are shown in Table 4. There were no significant differences in the final body weights or body composition of O-Ctrl and O-CLA groups. A significant difference was found between the body weights and body composition of the L-Ctrl group when compared to the O-Ctrl and O-CLA group. The liver weights of the O-CLA group were significantly lower than those of the O-Ctrl, but were significantly higher than the liver weights for the L-Ctrl group (P < 0.05). These results provide evidence that trans–trans CLA enriched soybean oil reduces hepatomegaly in fa/fa obese Zucker rats.
Serum Lipid Profiles
The serum lipid profiles are presented in Table 5. The serum TC and LDL-C concentrations of the rats in the O-CLA group were significantly lower compared to the obese control, 41 and 50%, respectively. There was no difference in the TG and HDL-C levels in rats in the O-CLA and O-Ctrl group. This indicates that CLA was able to reduce the total cholesterol concentration without lowering the HDL-C. All serum lipid parameters measured (TC, LDL-C, HDL-C, and TG) were significantly lower in the L-Ctrl group when compared to the O-CLA and O-Ctrl groups (P < 0.05).
Liver Lipids
The liver lipid data is presented in Table 5. Percent total liver lipids were significantly different among the experimental groups. Percent liver lipids in the O-CLA group were significantly lower than the percent liver lipids in the O-Ctrl group. The reduced liver lipid content of the O-CLA group could explain the lower liver weights in the O-CLA group compared to the O-Ctrl group, and also support a claim that trans–trans CLA-rich soy oil supplementation lowers the accumulation of fat in the liver. The percentage of liver cholesterol was reported as the ratio of cholesterol to total liver lipids. No significant differences were found between the percent liver cholesterol in rats in the O-CLA and O-Ctrl group, and rats in the L-Ctrl group showed significantly (P < 0.05) lower liver cholesterol values than the O-Ctrl and O-CLA groups.
Serum Glucose, HbA1c, AST and Insulin
The serum and whole blood metabolite results are presented in Table 6. Glucose and AST levels in the L-Ctrl group were significantly lower than in the O-Ctrl and O-CLA groups. There was no significant difference in the AST level between the O-CLA and O-Ctrl group. Rats in the L-Ctrl group also had serum insulin values significantly lower than the O-Ctrl group. CLA supplementation had an intermediary effect in lowering the serum insulin levels as the values were not different from either L-Ctrl or O-Ctrl groups. The HbA1c levels of the rats in the O-CLA treatment were found to be significantly lower than the O-Ctrl group, indicating that CLA supplementation effectively regulates the blood sugar levels.
Expression of PPAR-γ in White Adipose Tissue in Zucker Rats
We measured the expression of PPAR-γ in white adipose tissue (WAT). We found no significant difference in the expression of PPAR-γ in the O-CLA group when compared to the O-Ctrl group (Figure 1). The expression of PPAR-γ mRNA in both O-Ctrl and O-CLA groups were significantly lower than the L-Ctrl group.
Expression of PPAR-γ in Zucker rat adipose tissue treated with trans–trans CLA compared to obese control by qRT-PCR. The experiment was normalized to β-actin as an internal control and results were expressed as a percentage ratio of the control value. Values are means, standard errors represented by vertical bars (n = 4). * Represents significant difference (P < 0.05) compared to the obese control. PPAR-γ Peroxisome proliferator-activated receptor-γ, L-Ctrl lean + control diet, O-Ctrl obese + control diet, O-CLA obese + conjugated linoleic acid diet (0.5%)
Discussion
Natural CLA sources, such as dairy fats, reportedly contain over twenty-five CLA isomers; chemical synthesis and modification of existing isomers are adding to this list [23]. Most studies examining the biological activities of CLA have used c9,t11 CLA and t10,c12 CLA which are primarily derived from an alkaline catalyzed reaction of linoleate [10]. To the authors’ knowledge, no studies have been done to examine the effects of trans–trans CLA-enriched soybean oil on the body composition, cardiovascular risk factors, and diabetes risk factors in animal models or humans. Lack of knowledge on the health effects of dietary supplementation of trans–trans CLA isomers is due to fiscal impracticalities in obtaining enough trans–trans CLA isomers to conduct a feeding trial. The development of a new method of CLA production by the photoisomerization of soy oil that creates primarily trans–trans CLA isomers by Jain and Proctor [18, 24] allowed us to examine the possible in vivo health benefits of trans–trans CLA isomers. Unfortunately, at the time of study it was not possible to produce CLA-rich soybean oil containing only trans–trans CLA isomers. However, trans–trans CLA isomers make up 75% of the CLA isomers in the oil, and we think it unlikely that the small portion of the remaining cis–trans and trans–cis could have confounded our results. This study was designed to determine whether dietary supplementation of soybean oil rich with trans–trans CLA isomers is effective in improving body composition, dyslipidemia, hepatic steatosis, and markers of glucose control and liver function in obese fa/fa Zucker rats that are associated with an increased risk for type 2 diabetes and cardiovascular disease.
The present study demonstrated that trans–trans CLA rich soybean oil had no effect on the body composition, which is in agreement with the findings of Sanders and colleagues [25], who also reported that the body fat percentage of female obese Zucker rats treated with CLA (50:50 t9,c11:c10,t12 or t9,c11 or c10,t12) via intragastric gavage at 1.5 g CLA/kg bwt did not differ from those receiving the control corn oil vehicle. In contrast, Sisk and others [26] reported that supplementing with 0.5% CLA consisting of primarily c9,t11 and c10,t12 isomers caused an increase in adiposity in obese Zucker rats. Others have shown that dietary supplementation of CLA is effective in reducing the percentage of body fat and increasing the percentage of body protein [27, 28]. These contradictory results can be explained due to the sex of the rats and the type of CLA isomers used in the study. Poulos and others [29] demonstrated that dietary CLA can have sex-dependent effects on the body composition of rats. With dietary CLA supplementation of c9,t11 and c10,t12 isomers at 0.5% concentration, the CLA was found to have positive effects on male rats by increasing muscle mass and bone growth, but similar changes were not observed in the females [29]. Nonetheless, we observed that the trans–trans CLA isomers are effective in reducing hepatic steatosis which was in contrast to the results reported by Sanders and colleagues [25] who indicated that supplementation of c9,t11 CLA, t10,c12 CLA or a mixture of the two isomers did not lower hepatic steatosis. Our study shows that dietary supplementation of CLA rich soybean oil significantly (P < 0.05) reduced the total liver lipid content. This is in agreement with similar findings by Noto et al. [30] who reported a similar decrease (~60% Noto, ~40% current study) in the liver lipid content of Zucker rats supplemented with a 1.5% (w/w) CLA. Although we saw no improvement in liver function like was reported by Noto et al., the lack of significant difference in the AST, BUN, or total protein, supports a claim that liver function was not adversely affected by CLA supplementation. The discrepancy in improved liver function and reduction in liver lipid percentage could be attributed to the higher CLA level used by Noto et al. and the fact that CLA intake in the present study was controlled by pair-feeding.
Excess accumulation of lipid in the liver is associated with insulin resistance [30]. Interestingly, our study showed that dietary supplementation of trans–trans CLA lowered the HbA1c, a long-term measure of glucose control and reduced the circulating insulin levels, moderately but not significantly, in the obese rats. However, we did not see any improvements in fasting blood glucose. We feel that the elevated glucose values were caused by both the anesthetic mixture, which is known to elevate fasting glucose values and the stress response of the animal model [31–33]. Similar insulin-sensitizing effects of CLA were reported by Houseknecht and coworkers in the ZDF rat [34]. The insulin-sensitizing effect of CLA is believed to be by activation of peroxisome proliferator-activated receptor-γ (PPAR-γ) [35]. Polyunsaturated fatty acids and their metabolites have been identified as PPAR-γ ligands [36]; hence, based on the data discussed above, insulin sensitizing effects of trans–trans CLA rich soy oil may be due to prevention of hepatic steatosis and moderate upregulation of PPAR-γ. However, upregulation of PPAR-γ in white adipose tissue was not found to be significant (Fig. 1).
CLA rich soybean oil supplementation in obese rats also reduced the serum cholesterol and LDL-C levels. Our results are in agreement with other studies that have shown that dietary CLA can lower the serum cholesterol levels [30, 37]. This decreased serum cholesterol and LDL levels by CLA rich soybean oil can be due to a possible transcriptional activation of the LDL receptor gene which in turn enhances the uptake of VLDL and LDL cholesterol via hepatic LDL receptors [38]. Other possible cholesterol lowering effects of CLA could be due to inhibition of secretion of apolipoprotein B or by inhibiting cholesterol absorption by down-regulating the intestinal sterol O-acyltransferase activity [39, 40].
CLA has been reported to be a potent ligand activator of PPAR-α [41, 42], however, further studies are necessary to evaluate the role of trans–trans CLA in inducing hepatic fatty acid oxidation by ligand activation of PPAR-α. Increased fatty acid oxidation is associated with reduced LDL secretion rate [43], which is in agreement with our findings that show lower serum LDL levels in the obese rats supplemented with trans–trans CLA. In addition, it is plausible that hepatic fatty acid synthesis is strongly downregulated by trans–trans CLA, which is in agreement with others who reported that polyunsaturated fats strongly downregulated hepatic lipogenesis [44–46]. CLA also enhances the mRNA levels of lipogenic enzymes and their activity with concomitant reduction in the body fat content [47]. However, the trans–trans isomer in CLA enriched soy bean oil in our study did not lower the body fat percentage which further supports our hypothesis that trans–trans CLA prevents hepatomegaly, lowers the hepatic lipid content and improves the serum lipid profiles by increasing the hepatic fatty acid oxidation rather than compensatory increase in the hepatic lipogenesis.
Conclusion
In summary, dietary supplementation with soybean oil rich in trans–trans CLA isomers is effective in lowering serum cholesterol in obese Zucker rats. Trans–trans CLA-rich soy oil supplementation also lowered the liver lipid content. Glycated hemoglobin values for rats receiving trans–trans CLA were also significantly lower, indicating that trans–trans CLA may have a role in regulating blood sugar.
Abbreviations
- AIN:
-
American Institute of Nutrition
- AST:
-
Aspartate transaminase
- BUN:
-
Blood urea nitrogen
- CLA:
-
Conjugated linoleic acid
- DXA:
-
Dual-energy X-ray absorptiometry
- HbA1C:
-
Glycated hemoglobin
- HDL-C:
-
High density lipoprotein cholesterol
- LDL-C:
-
Low density lipoprotein cholesterol
- NFκB:
-
Nuclear factor κ B
- PPAR:
-
Peroxisome proliferator activator receptor
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- VLDL:
-
Very low density lipoprotein
References
Kopelman PG (2000) Obesity as a medical problem. Nature 404(6778):635–643
Kopelman P (2007) Health risks associated with overweight and obesity. Obes Rev 8(Suppl 1):13–17
Van Itallie TB (1985) Health implications of overweight and obesity in the United States. Ann Intern Med 103(6 (Pt 2)):983–988
Giovannucci E, Michaud D (2007) The role of obesity and related metabolic disturbances in cancers of the colon prostate, and pancreas. Gastroenterology 132(6):2208–2225
Punjabi NM (2008) The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 5(2):136–143
Larsson B, Bjorntorp P, Tibblin G (1981) The health consequences of moderate obesity. Int J Obes 5(2):97–116
Bray GA (2003) Risks of obesity. Endocrinol Metab Clin North Am 32(4):787–804 viii
Scimeca JA, Miller GD (2000) Potential health benefits of conjugated linoleic acid. J Am Coll Nutr 19(4):470S–471S
Wang YW, Jones PJ (2004) Conjugated linoleic acid and obesity control: efficacy and mechanisms. Int J Obes Relat Metab Disord 28(8):941–955
Belury MA (2002) Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Annu Rev Nutr 22:505–531
Brown JM, McIntosh MK (2003) Conjugated linoleic acid in humans: regulation of adiposity and insulin sensitivity. J Nutr 133(10):3041–3046
Churruca I, Fernandez-Quintela A, Portillo MP (2009) Conjugated linoleic acid isomers: differences in metabolism and biological effects. Biofactors 35(1):105–111
Evans M, Brown J, McIntosh M (2002) Isomer-specific effects of conjugated linoleic acid (CLA) on adiposity and lipid metabolism. J Nutr Biochem 13(9):508
Brown JM et al (2003) Isomer-specific regulation of metabolism and PPARgamma signaling by CLA in human preadipocytes. J Lipid Res 44(7):1287–1300
Ma DW et al (1999) Conjugated linoleic acid in Canadian dairy and beef products. J Agric Food Chem 47(5):1956–1960
Whigham LD, Watras AC, Schoeller DA (2007) Efficacy of conjugated linoleic acid for reducing fat mass: a meta-analysis in humans. Am J Clin Nutr 85(5):1203–1211
Ip C et al (1994) Conjugated linoleic acid suppresses mammary carcinogenesis and proliferative activity of the mammary gland in the rat. Cancer Res 54(5):1212–1215
Jain VP, Proctor A, Lall R (2008) Pilot-scale production of conjugated linoleic acid-rich soy oil by photoirradiation. J Food Sci 73(4):E183–E192
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123(11):1939–1951
Christie WW, Dobson G, Adlof RO (2007) A practical guide to the isolation analysis and identification of conjugated linoleic acid. Lipids 42(12):1073–1084
Folch J, Lees M, Sloane Stanley GH et al (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226(1):497–509
Searcy RL, Bergquist LM (1960) A new color reaction for the quantitation of serum cholesterol. Clin Chim Acta 5:192–199
Benjamin S, Spener F (2009) Conjugated linoleic acids as functional food: an insight into their health benefits. Nutr Metab (Lond) 6:36
Jain VP, Proctor A (2006) Photocatalytic production and processing of conjugated linoleic acid-rich soy oil. J Agric Food Chem 54(15):5590–5596
Sanders SR et al (2004) Effects of specific conjugated linoleic acid isomers on growth characteristics in obese Zucker rats. Lipids 39(6):537–543
Sisk MB et al (2001) Dietary conjugated linoleic acid reduces adiposity in lean but not obese Zucker rats. J Nutr 131(6):1668–1674
Pariza MW, Park Y, Cook ME (2001) The biologically active isomers of conjugated linoleic acid. Prog Lipid Res 40(4):283–298
Whigham LD, Cook ME, Atkinson RL (2000) Conjugated linoleic acid: implications for human health. Pharmacol Res 42(6):503–510
Poulos SP et al (2001) Pre- and postnatal dietary conjugated linoleic acid alters adipose development, body weight gain and body composition in Sprague-Dawley rats. J Nutr 131(10):2722–2731
Noto A et al (2006) Conjugated linoleic acid reduces hepatic steatosis, improves liver function, and favorably modifies lipid metabolism in obese insulin-resistant rats. Lipids 41(2):179–188
Fish R et al (2008) Anesthesia and Analgesia in Laboratory Animals. Academic Press, San Diego, CA
Sinert R et al (2005) The effect of non-insulin dependent diabetes mellitus on uncontrolled hemorrhage in a rodent model. Resuscitation 66(1):83–90
Saha JK et al (2005) Acute hyperglycemia induced by ketamine/xylazine anesthesia in rats: mechanisms and implications for preclinical models. Exp Biol Med (Maywood) 230(10):777–784
Houseknecht KL et al (1998) Dietary conjugated linoleic acid normalizes impaired glucose tolerance in the Zucker diabetic fatty fa/fa rat. Biochem Biophys Res Commun 244(3):678–682
Aminot-Gilchrist DV, Anderson HD (2004) Insulin resistance-associated cardiovascular disease: potential benefits of conjugated linoleic acid. Am J Clin Nutr 79(6 Suppl):1159S–1163S
Duan SZ et al (2008) PPAR-gamma in the cardiovascular system. PPAR Res 2008:745804
Miranda J et al (2009) A comparison between CLNA and CLA effects on body fat, serum parameters and liver composition. J Physiol Biochem 65(1):25–32
Ringseis R et al (2006) LDL receptor gene transcription is selectively induced by t10c12-CLA but not by c9t11-CLA in the human hepatoma cell line HepG2. Biochim Biophys Acta 1761(10):1235–1243
Yeung C, Yang L, Huang Y, Wang J, Chen Z (2000) Dietary conjugated linoleic acid mixture affects the activity of intestinal acyl coenzyme A: cholesterol acyltransferase in hamsters. Br J Nutr 84:935–941
Yotsumoto H, Hare E, Naka S, Adlof RO, Emken EA, Yanagita T et al (1999) Trans-10, cis-12-linoleic acid reduces apolipoprotein B secretion in HepG2 cells. Food Res Int 31:403–409
Peters JM et al (2001) Influence of conjugated linoleic acid on body composition and target gene expression in peroxisome proliferator-activated receptor alpha-null mice. Biochim Biophys Acta 1533(3):233–242
Moya-Camarena SY et al (1999) Conjugated linoleic acid is a potent naturally occurring ligand and activator of PPARalpha. J Lipid Res 40(8):1426–1433
Ide T, Ontko JA (1981) Increased secretion of very low density lipoprotein triglyceride following inhibition of long chain fatty acid oxidation in isolated rat liver. J Biol Chem 256(20):10247–10255
Yahagi N et al (1999) A crucial role of sterol regulatory element-binding protein-1 in the regulation of lipogenic gene expression by polyunsaturated fatty acids. J Biol Chem 274(50):35840–35844
Fukuda H, Katsurada A, Iritani N (1992) Nutritional and hormonal regulation of mRNA levels of lipogenic enzymes in primary cultures of rat hepatocytes. J Biochem 111(1):25–30
Ide T et al (2000) Comparative effects of perilla and fish oils on the activity and gene expression of fatty acid oxidation enzymes in rat liver. Biochim Biophys Acta 1485(1):23–35
Takahashi Y et al (2003) Activity and mRNA levels of enzymes involved in hepatic fatty acid synthesis and oxidation in mice fed conjugated linoleic acid. Biochim Biophys Acta 1631(3):265–273
Acknowledgments
This study was funded by the Arkansas Biosciences Institute.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Gilbert, W., Gadang, V., Proctor, A. et al. Trans–trans Conjugated Linoleic Acid Enriched Soybean Oil Reduces Fatty Liver and Lowers Serum Cholesterol in Obese Zucker Rats. Lipids 46, 961–968 (2011). https://doi.org/10.1007/s11745-011-3585-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-011-3585-6