Abstract
Excessive formation of advanced glycation end products (AGE) and lipid accumulation in macrophages play a pivotal role in the progression of atherosclerosis in diabetes mellitus. This study aimed to determine the molecular link between AGE-induced fatty acid binding protein 4 (FABP4) expression and macrophage lipid accumulation. AGE–BSA markedly increased macrophage FABP4 expression via engagement of RAGE, a 35-kDa transmembrane receptor that is able to bind extracellular AGE and responsible for the corresponding signal transduction, whereas knockdown of RAGE significantly reversed the FABP4 up-regulation. This effect was further paralleled with elevated intracellular total cholesterol and triacylglycerol levels. Finally, administration of FABP4 inhibitor totally abolished the increased lipid contents in response to AGE–BSA. These results indicate that FABP4 up-regulation is responsible for the enhanced macrophage lipid accumulation by AGE, which may underlie the accelerated formation of foam cells and development of atherosclerotic cardiovascular diseases in diabetic patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accelerated atherosclerosis accounts for about 80% of morbidity and mortality in patients with diabetes [1, 2]. Increased formation of advanced glycation end products (AGE) is recognized as an important player in diabetic vascular complications [3, 4]. After longstanding exposure to hyperglycemic milieu, proteins and lipids in blood and tissues undergo non-enzymatic Maillard reaction, and finally give rise to the irreversible formation of AGE [4]. AGE exert adverse effects largely through engagement of receptor for AGE (RAGE) on the cell membrane of vascular wall [5–7]. Postmortem studies have consistently shown that RAGE expression was mainly localized in macrophages within or around necrotic cores of atherosclerotic plaques, and was substantially increased in diabetic patients [8].
Macrophages actively participate in atherogenic processes, including formation of lipid-laden foam cells and atheromatous lipid cores [9]. FABP4, also known as adipocyte protein 2 (aP2), is highly expressed in macrophages [10]. It functions in cellular lipid metabolism, cholesterol trafficking and other biologic responses [11, 12]. Expression of FABP4 in THP-1 monocytes/macrophages was greatly increased upon treatment with phorbol 12-myristate 13-acetate (PMA) and oxidized low-density lipoprotein (oxLDL), while transformation of THP-1 monocytes into foam cells was significantly suppressed by FABP4 inhibitor, a small synthetic molecule designed to competitively prevent FABP4 from binding fatty acids [13, 14]. Likewise, inhibition of FABP4 expression either by macrophage-specific gene deficiency or administration of FABP4 inhibitor in vivo led to a protection from the development of atherosclerosis [15]. Iwashima et al. [6] observed that macrophage lipoprotein receptors, including CD36, macrophage scavenger receptor (MSR) class A and lectin-like oxLDL receptor 1 (LOX-1) were up-regulated in response to AGE stimulation, whereas key proteins localized on the cell membrane that mediate macrophage cholesterol efflux, including ABCA1 and ABCG1, were down-regulated or destabilized [16, 17].
It is still unclear, however, whether macrophage FABP4 remains, equally or to a greater extent, an active pro-atherogenic participator in the context of diabetes where AGE are greatly enriched. In this study, we tested the hypothesis that AGE-induced FABP4 expression in THP-1 macrophages was potentially associated with an increase in intracellular lipid levels. The assessment of lipid contents after FABP4 inhibitor administration was also performed.
Materials and Methods
Cell Culture
Human monocytic leukemia THP-1 cells were obtained from ATCC and cultured in Gibco RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 50 U/ml penicillin and 50U/ml streptomycin (Gibco, Auckland, New Zealand) at 37 °C in 5% CO2. THP-1 monocytes were differentiated into macrophages by induction of 100nM phorbol 12-myristate 13-acetate (Sigma-Aldrich, MO) for 48 h. Cells were then incubated in the presence or absence of bovine serum albumin (BSA; fatty acid free, catalog no. 126575; Calbiochem, CA) or AGE–BSA (catalog no. 121800; Calbiochem, CA) for the indicated time intervals. FABP4 inhibitor (Calbiochem, CA) dissolved in dimethyl sulfoxide (DMSO) at the indicated concentrations was administrated for study purpose.
Transfection of RAGE-Specific siRNA
Transient transfection of THP-1 macrophages with RAGE-specific and negative control siRNA (Ambion CA) was scheduled as follows: Silencing RAGE expression was performed using human RAGE-specific siRNA and siRNA transfection reagent (Lipofectamine 2000, Invitrogen, CA) to macrophages. Macrophages grown in 6-well plates were transfected with various concentrations of siRNA according to the manufacturer’s protocol. The RAGE-specific siRNA (100 pM) for reducing RAGE expression was added 48 h before AGE stimulation; macrophages were then exposed to BSA or AGE–BSA (Calbiochem CA) for 24 or 48 h for RNA or protein analysis and ORO-staining, respectively.
Assessment of Intracellular Lipid Contents
Cultured and transfected macrophages were washed twice in phosphate-buffered saline (PBS, Gibco, Auckland, New Zealand) and then fixed in 4% paraformaldehyde for 30 min. After rinsing with ddH2O, macrophages were incubated with filtered Oil Red O solution (0.6 g/L ORO in 60% isopropanol) (ORO, Sigma-Aldrich, MO) for 10 min and with 1 mL of Mayer’s hemalum (Merck, Darmstadt) for 1 min. After rinsing with PBS four times, intracellular lipid droplets were evaluated under Olympus DP-71 microscope. Spectrophotometric quantification of the staining was performed as described previously [18]. Briefly, stained oil droplets were dissolved in 100% isopropanol for 10 min. Then optical density was measured at 500 nm and equalized with the cell numbers estimated by microscope. Quantifications of intracellular total cholesterol and triacylglycerol levels were performed following the protocols from the manufacture (Biovision, CA) and analyzed with a micro-titer plate reader (Ex/Em = 538/587 nm). Total cholesterol and triacylglycerol concentrations were expressed as micrograms per milligram protein and nanomoles per milligram protein sample, respectively.
Quantitative Real-Time PCR Analysis
Total RNA was isolated using Trizol reagent (Invitrogen, CA). For reverse transcription, 1 μg of the total RNA was converted to first strand complementary DNA in 20-μl reactions using a reverse transcription kit (Promega, WI). Quantitative real-time PCR analysis was performed (StepOne, Applied Biosystems) using SYBR Green (Takara, Dalian, China). The thermal cycling program was 10 s at 95 °C for enzyme activation and 40 cycles of denaturation for 5 s at 95 °C, 31 s 60 °C for annealing and extension. The comparative cycle threshold (CT) method was used to determine relative mRNA expression of genes as normalized by β-actin housekeeping gene. Primers used are as follows: 5′-TGGGATGGAAAATCAACC-3′ for FABP4-F, 5′-TCTCTCATAAACTCTCGTGG-3′ for FABP4-R, 5′-GGCAGAAAAACTCAGAC-3′ for FABP5-F, 5′-GACACACTCCACCACTAA-3′ for FABP5-R, 5′-GCTGGTGTTCCCAATAA-3′ for AGER-F, 5′-AGTGTGAAGAGCCCTGT-3′ for AGER-R, 5′-CGTGGACATCCGCAAAG-3′ for β-actin-F, and 5′-TGGAAGGTGGACAGCGA-3′ for β-actin-R.
Western Blot
Total proteins were prepared by standard procedures and assessed by microplate protein assay (BSA; Pierce, IL). Thirty micrograms of protein per sample and known molecular weight markers were loaded onto a 12% SDS-polyacrylamide gel. After SDS-polyacrylamide gel electrophoresis, proteins were transferred to PVDF membranes (Millipore, MA). The blocked membranes (5% nonfat milk in TBS buffer containing 0.1% Tween 20) were incubated with anti-FABP4 (1:1000; Santa Cruz Biotechnology, CA), anti-RAGE (1:1000; Abcam, MA) and anti-β-actin antibody (1:1000; Cell Signaling Technology, MA) for overnight at 4 °C. The membranes were then treated with horseradish peroxidase-conjugated rabbit anti-goat, goat anti-rabbit or goat anti-mouse antibodies (1:5000; Bio-Rad, Hercules, CA). After washing, immunodetection analysis was accomplished using the Immobilon Western Chemiluminescent HRP Substrate (Millipore, MA).
Statistical Analysis
Data are expressed as means ± SEM. The mean values for biochemical measurements from each group were compared using Student’s t test. A P < 0.05 was considered statistically significant.
Results
AGE–BSA-Induced Up-Regulation of FABP4 Expression
After incubation with AGE–BSA for 24 h at various concentrations (from 25 to 200 μg/ml), FABP4 expression was substantially up-regulated, and reached the maximum level at 100 μg/ml (3.59 ± 0.53-fold vs. con; P = 0.001), whereas BSA did not affect FABP4 expression significantly (0.95 ± 0.14-fold vs. con, P = 0.577; Fig. 1a). A 3.9-fold increase in FABP4 expression was observed by 48 h of incubation (P = 0.0003; Fig. 1b). FABP5, another fatty acid binding protein expressed in macrophages, was not affected by AGE (Fig. 1c).
Up-regulation of FABP4 mRNA expression by AGE–BSA. a, c THP-1 macrophages were treated with indicated doses of BSA or AGE–BSA for 24 h. b Macrophages were either untreated or treated with 100 μg/ml AGE–BSA for various time intervals. FABP4 and FABP5 mRNA levels were detected by quantitative real-time PCR; data are expressed relatively to untreated cells, arbitrarily set at the level of 1, and are the means ± SEM of at least three independent experiments. *P < 0.05 and **P < 0.01 when compared with untreated cells
RAGE Mediates AGE–BSA-Induced FABP4 Expression
AGE–BSA moderately increased RAGE expression by 1.2-fold in cells transfected with non-specific negative control siRNA (P = 0.010, Fig. 2a). After gene silencing of RAGE, AGE exposure no longer affected FAPB4 expression both at mRNA (1.38 ± 0.24-fold vs. BSA, P = 0.069; Fig. 2b) and protein (Fig. 2c) levels when compared with negative control-treated groups (2.78 ± 0.36-fold vs. BSA, P = 0.001).
Effect of RAGE knockdown by siRNA transfection on FABP4 expression. Macrophages were transfected with negative control or RAGE specific siRNA for 48 h and exposed to BSA or AGE–BSA, respectively, at the concentration of 100 μg/ml. Then mRNA expression levels of RAGE (a) and FABP4 (b) were determined using quantitative real-time PCR; data are expressed relatively to untreated cells, arbitrarily set at the level of 1, and are the means ± SEM of at least three independent experiments. c Protein expression levels of FABP4 and RAGE in each group were analyzed by Western blot. β-actin demonstrated equal loading. The right panel shows the average densitometric analysis of three independent experiments. Data are expressed in arbitrary units. *P < 0.05 and **P < 0.01 compared with BSA-treated cells in the same group. # P < 0.01 versus cells with the same BSA or AGE–BSA treatment in negative control group
AGE–BSA-Induced Macrophage Lipid Accumulation
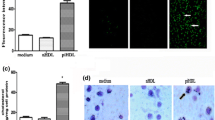
Oil red O (ORO) staining of lipid droplets was most evident at 100 and 200 μg/ml of AGE–BSA (Fig. 3a, b). A similar trend was also obtained for intracellular TC (100 μg/ml AGE–BSA: 37.20 ± 2.52 μg/mg, P = 0.0002; 200 μg/ml AGE–BSA: 29.89 ± 4.67 μg/mg, P = 0.0066 vs. con: 15.10 ± 1.61 μg/mg; Fig. 3c) and TAG levels (100 μg/ml AGE–BSA: 4.35 ± 1.35 nmol/mg, P = 0.0252; 200 μg/ml AGE–BSA: 2.89 ± 0.75 nmol/mg, P = 0.0463 vs. con: 1.64 ± 0.08 nmol/mg; Fig. 3d). RAGE knockdown markedly reduced lipid contents in response to AGE exposure (TC, 21.92 ± 3.09 μg/mg vs. BSA: 17.23 ± 2.20 μg/mg, P = 0.0980; TAG, 5.64 ± 1.43 nmol/mg vs. BSA: 1.85 ± 1.10 nmol/mg, P = 0.0220) when compared with negative control-treated cells, as demonstrated by ORO staining (Fig. 3e, f), TC (Fig. 3g) and TAG (Fig. 3h) quantification.
Up-regulation of lipid contents by AGE–BSA via RAGE. a–d Macrophages were either untreated or treated with indicated doses of BSA and AGE–BSA for 48 h. e–h Macrophages were transfected with negative control or RAGE specific siRNA for 48 h and then exposed to BSA or AGE–BSA, respectively, at the concentration of 100 μg/ml. a, e Intracellular lipid droplets were observed through light microscope after ORO staining; pictures acquired at the magnification of 400× and processed using identical conditions are representative of three independent experiments. b, f Spectrophotometric quantifications of ORO staining by optical density at 500 nm were shown. Intracellular total cholesterol (c, g) and triacylglycerol (d, h) concentrations were determined using a fluorometric method; data are presented as micrograms of total cholesterol per milligrams of protein or nanomoles of triacylglycerol per milligrams of protein ± SEM of three independent experiments. *P < 0.05 versus untreated cells (b, c, d) or BSA-treated cells in the same group (f, g, h). **P < 0.01 versus untreated cells
Changes After Incubation with FABP4 Inhibitor
After incubation with FABP4 inhibitor for 48 h, AGE-induced lipid accumulation in macrophages was substantially suppressed and reached the maximum effect at 40 μM as demonstrated by ORO staining (Fig. 4a, b), TC (14.92 ± 1.54 μg/mg vs. BSA: 14.56 ± 0.81ug/mg, P = 0.7356; Fig. 4c) and TAG (0.79 ± 0.35 nmol/mg vs. BSA: 0.52 ± 0.24 nmol/mg, P = 0.2656; Fig. 4d) quantification when compared with macrophages without FABP4 treatment. AGE–BSA up-regulated FABP4 protein levels irrespective of FABP4 inhibitor at each concentration (Fig. 4e). FABP5 expression was not affected by FABP4 inhibitor (Fig. 4f).
Effect of FABP4 inhibitor on AGE–BSA-induced lipid accumulation. Macrophages were treated with 100 μg/ml BSA or AGE–BSA in the absence or presence of indicated doses of FABP4 inhibitor for 48 h. a Intracellular cellular lipid droplets were stained with ORO and quantified by optical density at 500 nm or b observed through light microscope; pictures acquired at the magnification of 400× and processed using identical conditions are representative of three independent experiments. Intracellular total cholesterol (c) and triacylglycerol (d) concentrations were determined using a fluorometric method; data are presented as micrograms of total cholesterol per milligrams of protein or nanomoles of triacylglycerol per milligrams of protein ± SEM of three independent experiments. e FABP4 protein expression levels in each group were analyzed by Western blot. β-actin demonstrated equal loading. f THP-1 macrophages were treated with or without 40 μM FABP4 inhibitor for 48 h. FABP5 mRNA levels were detected by quantitative real-time PCR; data are expressed relatively to untreated cells, arbitrarily set at the level of 1, and are the means ± SEM of at least three independent experiments. *P < 0.05; **P < 0.01 versus BSA-treated cells in the same group
Discussion
In THP-1 macrophages, AGE-induced FABP4 expression via a RAGE-dependent signaling pathway resulted in increased intracellular lipid levels, which could be attenuated by FABP4 inhibitor.
AGE are increasingly regarded as an indispensable factor for severity and progression of atherosclerosis in patients with diabetes [8]. AGE-albumin, a further glycation modified protein derived from Amadori adduct exerts more severe pathogenic influence. Elevated serum AGE-albumin and decreased serum endogenous secretary RAGE (esRAGE) levels highly correlated with the extent of angiographic severity in patients with type 2 diabetes [19, 20]. In contrast, in vivo studies showed that administration of soluble RAGE (sRAGE), another truncated form of RAGE acting as a decoy for AGE, completely suppressed diabetic atherosclerosis in glycemia- and lipid-independent manners [21]. Other approaches utilizing inhibitors for AGE formation, including aminoguanidine and an AGE cross-link breaker ALT-711, also led to a striking reduction in atherosclerotic lesions in diabetic apoE-deficient mice [22].
Despite the important contribution of AGE to the accelerated atherosclerosis in diabetes, the specific molecular mechanisms in response to AGE within a macrophage (a central player in atherogenesis) remain unclear. In this study, we discovered that exposure of THP-1 macrophages to AGE was time-dependently associated with a significant elevation of FABP4 expression, reaching its maximum level when 100 μg/ml AGE–BSA was used. At the same time, the enhanced expression of FABP4 was paralleled with increases in intracellular TC and TAG levels. RAGE-specific gene silencing led to reversions both in FABP4 expression and lipid contents, suggesting that AGE-elicited atherogenic effects in macrophages were, at least partly, RAGE-dependent. Finally, this increase in lipid levels was totally abolished by simultaneous administration of FABP4 inhibitor.
Our study is the first to show that macrophage FABP4 expression, a critical participant in atherogenesis, is further enhanced in the context of diabetes. In an animal experiment, Gerrity et al. [2] observed that FABP4 deficiency protected against the development of insulin resistance, diabetes, and atherosclerotic cardiovascular disease. Recent population studies revealed that genetic variations at the FABP4 locus in humans led to lowered serum triglyceride levels, and a markedly reduced risk of coronary heart disease in type 2 diabetes [23]. These anti-atherosclerotic effects were mainly attributed to the improvement of glucose control, insulin resistance and dyslipidemia [24, 25]. In this study, we demonstrated that exposure of macrophages to AGE was capable of increasing intracellular FAPB4 levels and lipid contents, similar to that when transforming macrophages to foam cells in the presence of oxLDL stimulation [14]. These observations suggest that excessive formation of AGE could render diabetic patients under high risk of developing atherosclerosis like those with dyslipidemia. [26–28].
Furthermore, our results imply that FABP4 might be a potential therapeutic target for the treatment of diabetic patients with atherosclerosis. In this study, mild doses of FABP4 inhibitor (10–20 μM) greatly attenuated AGE–BSA elicited macrophage lipid accumulation, whereas lipid levels in the absence of AGE were not affected. These findings indicate that FABP4 inhibitor may not disturb physiological lipid metabolism in macrophages, but, on the contrary, suppresses exclusively pathological excessive lipid accumulation.
This study does not specify the mechanisms by which lipid contents and trafficking are affected by AGE besides the elevated FAPB4 level. Deficiency of FABP4 enhanced CD36-mediated lipoprotein entry and, at the same time, activated ABCA1-dependent lipid efflux to a greater extent, thereby lowering intracellular lipid contents [29]. Likewise, AGE stimulation on macrophages could increase SRA-1 and CD36 and decrease ABCG1 protein levels [6, 16, 17]. Thus, enhanced expression of FABP4 by AGE might extensively affect lipid influx and efflux, directly or indirectly, at the same time to increase lipid accumulation. Further studies including analysis of lipid trafficking in macrophages from FABP4-deficient mice and blocking lipoprotein transporters by their specific siRNA or antibodies, are needed to elucidate the mechanisms.
In conclusion, this study demonstrates a causal molecular link between AGE and FABP4 in macrophage lipid accumulation. FABP4 inhibitor may be useful for suppressing the atherosclerotic process in patients with diabetes.
Abbreviations
- AGE:
-
Advanced glycation end products
- aP2:
-
Adipocyte protein 2
- BSA:
-
Bovine serum albumin
- DMSO:
-
Dimethyl sulfoxide
- esRAGE:
-
Endogenous secretary RAGE
- FABP4:
-
Fatty acid binding protein 4
- FABP5:
-
Fatty acid binding protein 5
- FBS:
-
Fetal bovine serum
- LOX-1:
-
Lectin-like oxidized low-density lipoprotein receptor 1
- MSR:
-
Macrophage scavenger receptor
- OD:
-
Optical density
- ORO:
-
Oil red O
- oxLDL:
-
Oxidized low-density lipoprotein
- PBS:
-
Phosphate-buffered saline
- PMA:
-
Phorbol 12-myristate 13-acetate
- RAGE:
-
Receptor for advanced glycation end products
- sRAGE:
-
Secretary RAGE
- TAG:
-
Triacylglycerol
- TC:
-
Total cholesterol
References
Kannel WB, McGee DL (1979) Diabetes and cardiovascular disease. The Framingham Study. JAMA 241:2035–2038
Gerrity RG, Natarajan R, Nadler JL, Kimsey T (2001) Diabetes-induced accelerated atherosclerosis in swine. Diabetes 50:1654–1665
Basta G, Schmidt AM, De Caterina R (2004) Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res 63:582–592
Goldin A, Beckman JA, Schmidt AM, Creager MA (2006) Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 114:597–605
Morigi M, Angioletti S, Imberti B, Donadelli R, Micheletti G, Figliuzzi M, Remuzzi A, Zoja C, Remuzzi G (1998) Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-kB-dependent fashion. J Clin Invest 101:1905–1915
Iwashima Y, Eto M, Hata A, Kaku K, Horiuchi S, Ushikubi F, Sano H (2000) Advanced glycation end products-induced gene expression of scavenger receptors in cultured human monocyte-derived macrophages. Biochem Biophys Res Commun 277:368–380
Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM (1997) Activation of the receptor for advanced glycation end products triggers a p21(ras)-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. J Biol Chem 272:17810–17814
Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, Farb A, Virmani R (2004) Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol 24:1266–1271
Luliano L (2001) Inflammation, atherosclerosis, and coronary artery disease. Lipids 352:S41–S44
Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM (1996) Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science 274:1377–1379
Boord JB, Maeda K, Makowski L, Babaev VR, Fazio S, Linton MF, Hotamisligil GS (2002) Adipocyte fatty acid-binding protein, aP2, alters late atherosclerotic lesion formation in severe hypercholesterolemia. Arterioscler Thromb Vasc Biol 22:1686–1691
Fu Y, Luo N, Lopes-Virella MF, Garvey WT (2002) The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis 165:259–269
Pelton PD, Zhou L, Demarest KT, Burris TP (1999) PPARgamma activation induces the expression of the adipocyte fatty acid binding protein gene in human monocytes. Biochem Biophys Res Commun 261:456–458
Fu Y, Luo N, Lopes-Virella MF (2000) Oxidized LDL induces the expression of ALBP/aP2 mRNA and protein in human THP-1 macrophages. J Lipid Res 41:2017–2023
Furuhashi M, Tuncman G, Gorgun CZ, Makowski L, Atsumi G, Vaillancourt E, Kono K, Babaev VR, Fazio S, Linton MF, Sulsky R, Robl JA, Parker RA, Hotamisligil GS (2007) Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature 447:959–965
Passarelli M, Tang C, McDonald TO, O’Brien KD, Gerrity RG, Heinecke JW, Oram JF (2005) Advanced glycation end product precursors impair ABCA1-dependent cholesterol removal from cells. Diabetes 54:2198–2205
Isoda K, Folco EJ, Shimizu K, Libby P (2007) AGE–BSA decreases ABCG1 expression and reduces macrophage cholesterol efflux to HDL. Atherosclerosis 192:298–304
Kim KA, Kim JH, Wang Y, Sul HS (2007) Pref-1 (preadipocyte factor 1) activates the MEK/extracellular signal-regulated kinase pathway to inhibit adipocyte differentiation. Mol Cell Biol 27:2294–2308
Lu L, Pu LJ, Zhang Q, Wang LJ, Kang S, Zhang RY, Chen QJ, Wang JG, De Caterina R, Shen WF (2009) Increased glycated albumin and decreased esRAGE levels are related to angiographic severity and extent of coronary artery disease in patients with type 2 diabetes. Atherosclerosis 206:540–545
Pu LJ, Lu L, Shen WF, Zhang Q, Zhang RY, Zhang JS, Hu J, Yang ZK, Ding FH, Chen QJ, Shen J, Fang DH, Lou S (2007) Increased serum glycated albumin level is associated with the presence and severity of coronary artery disease in type 2 diabetic patients. Circ J 71:1067–1073
Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, Goova MT, Moser B, Kislinger T, Lee DC, Kashyap Y, Stern DM, Schmidt AM (2002) RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation 106:2827–2835
Forbes JM, Yee LT, Thallas V, Lassila M, Candido R, Jandeleit-Dahm KA, Thomas MC, Burns WC, Deemer EK, Thorpe SR, Cooper ME, Allen TJ (2004) Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes 53:1813–1823
Tuncman G, Erbay E, Hom X, De Vivo I, Campos H, Rimm EB, Hotamisligil GS (2006) A genetic variant at the fatty acid-binding protein aP2 locus reduces the risk for hypertriglyceridemia, type 2 diabetes, and cardiovascular disease. Proc Natl Acad Sci USA 103:6970–6975
Furuhashi M, Fucho R, Görgün CZ, Tuncman G, Cao H, Hotamisligil GS (2008) Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J Clin Invest 118:2640–2650
Shaughnessy S, Smith ER, Kodukula S, Storch J, Fried SK (2000) Adipocyte metabolism in adipocyte fatty acid binding protein knockout mice (aP2-/-) after short-term high-fat feeding: functional compensation by the keratinocyte fatty acid binding protein. Diabetes 49:904–911
Bahramian N, Ostergren-Lundén G, Bondjers G, Olsson U (2004) Fatty acids induce increased granulocyte macrophage-colony stimulating factor secretion through protein kinase C-activation in THP-1 macrophages. Lipids 39:243–249
Goff DC Jr, Bertoni AG, Kramer H, Bonds D, Blumenthal RS, Tsai MY, Psaty BM (2006) Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation 113:647–656
Nakhjavani M, Khalilzadeh O, Khajeali L, Esteghamati A, Morteza A, Jamali A, Dadkhahipour S (2010) Serum oxidized-LDL is associated with diabetes duration independent of maintaining optimized levels of LDL-cholesterol. Lipids 45:321–327
Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS (2005) The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J Biol Chem 280:12888–12895
Acknowledgments
This work was supported by grant from Chinese National Nature Science Foundation (No. 30871084).
Conflict of interest
All authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Wang, X.Q., Yang, K., He, Y.S. et al. Receptor Mediated Elevation in FABP4 Levels by Advanced Glycation End Products Induces Cholesterol and Triacylglycerol Accumulation in THP-1 Macrophages. Lipids 46, 479–486 (2011). https://doi.org/10.1007/s11745-011-3542-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-011-3542-4