Abstract
Dietary levels of n-3 PUFA are believed to influence the immune system. The importance of the source of n-3 PUFA is debated. This study addressed how the content and source of n-3 PUFA in the maternal diet influenced tissue FA composition and the immune response to ovalbumin (OVA) in mice pups. From the day of conception and throughout lactation, dams were fed diets containing 4% fat from linseed oil (LSO), fish oil (FO) or a n-3 PUFA-deficient diet (DEF). Pups were injected with OVA within 24 h of birth and sacrificed at weaning (day 21). Overall, the content of n-3 PUFA in milk, liver and spleen reflected the source and only minor differences were observed in brain phospholipid 22:6n-3. The source had only limited influence on the n-3 PUFA accretion in peripheral tissue, with most pronounced differences in the spleen. The marine PUFA-group had reduced levels of total OVA-specific antibodies and OVA-IgG1 titers in the pup blood, while the response in the LSO-group did not differ from that in the DEF-group. There were no statistical differences in the cytokine responses to OVA-stimulated splenocytes, but the decrease in IgG1 was paralleled by an increase in IFNγ-production and a decrease in IL-6-production. Our results indicate that maternal intake of FO, but not of LSO, changes the offspring’s antigen-specific response and potentially increases Th1-polarization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An adequate supply of essential fatty acids during pregnancy and lactation is crucial for optimal fetal and postnatal development. It is well known that n-3 PUFA are required for growth and development of the central nervous system of the fetus and infant [1]. Long-chain n-3 PUFA (n-3 LCPUFA) have in studies with adults been shown to be immunosuppressive and to exert beneficial effects in a variety of inflammatory disorders [2]. Dietary n-3 LCPUFA have, in both human and animal experiments, been shown to affect a wide range of immune functions, including lymphocyte proliferation, cytokine production, NK-cell activity, adhesion molecules expression, and antigen presentation in adults [3]. However, the effect of maternal n-3 PUFA intake on immune function in the offspring is less well characterized and only few experimental studies have addressed this topic [4].
The immune system of the newborn has not fully matured and is believed to be more sensitive to environmental conditions, including diet. Such conditions have been hypothesized to influence the polarization of T-helper lymphocytes (Th1/Th2) of the immune system and accordingly the risk of allergy development [5]. The processes of importance for shaping the immune system take place already in utero and in the early postnatal period, and the immune system is therefore particularly susceptible to changes in diet and external stimuli to the fetus/newborn [6]. To this end, human studies with fish oil-supplementation of pregnant women have been shown to affect immune function in the neonate [7, 8]. We have previously shown that fish oil-supplementation during lactation resulted in a higher predisposition of the blood from the children to produce Interferon-γ (IFNγ) upon polyclonal stimulation [9, 10], indicating a higher intake of n-3 LCPUFA is associated with faster immune maturation and Th1-polarization. An association between a high maternal n-3 PUFA intake and a decreased risk of infant allergy is supported by recent randomized trials [11, 12]. In mice, maternal fish oil has been shown to lead to lower levels of prostaglandin E2 in pups’ lung and increased survival to infection compared to offspring of dams on a corn oil diet [13] and in another study manipulation of the ratio of n-6 to n-3 PUFA in the maternal diet showed better induction of oral tolerance in neonatal mice with a high relative intake of n-3 PUFA [14].
Even though mammals can convert α-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3), it is controversial whether a dietary intake of 18:3n-3 is as efficient as fish oil as a means to affect disease risk. Fish oil [11–13] as well as α-linolenic acid [14] have been used in the various human and animal studies, but since different doses and experimental designs have been used, the effect of the two sources is difficult to compare. In the present study, we hypothesized that maternal n-3 PUFA intake from fish oil or plant oil during gestation and lactation affects the Th1/Th2-polarization early in life and that this is reflected in the antibody and cytokine production against food allergens encountered postnatally. We fed groups of female mice fish oil or linseed oil from mating until weaning of their offspring, and tested the antibody response to ovalbumin (OVA) injected in the offspring on day 1.
Experimental Procedure
Animals
The experiment was approved by the Danish Committee for Animal Experiments. BalbC mice from Taconic M&B (Ll. Skensved, Denmark) 8–10 weeks of age were housed in plastic cages in a temperature (21 °C) and humidity (50%) controlled environment on a 12 h/12 h light/dark cycle with a standard nonpurified diet (Altromin No. 1324, Chr. Petersen A/S, Ringsted, Denmark) and water freely available. They were acclimatized to the housing conditions for 7 days before conception. The mice were then allocated to individual cages and randomized to the three dietary treatments from the time of mating and throughout the pregnancy and lactation periods. In this feeding experiment, 3 dams on fish oil diet (FO) gave birth to 17 pups, 4 dams on n-3 PUFA-deficient diet (DEF) to 13 pups, and 2 dams on a linseed oil based diet (LSO) to 11 pups. An additional feeding experiment was performed with the same diets, since the first experiment resulted in fewer pregnancies than expected. This resulted in birth of further 12 pups in the FO groups, 11 in the DEF-group, and 10 in the LSO-group, from 2 dams in each of the groups. No differences were found in milk gland TAG fatty acid composition from the two feeding periods, so the results from the two experiments were pooled.
Diets
The overall composition of the three dietary groups was as follows: 56 g/100 g corn starch (Bestfoods Nordic A/S, Skovlunde, Denmark); 20 g/100 g casein (Miprodan milkproteins, Arla Foods amba, Viby, Denmark), 10 g/100 g sucrose (Danisco Sugar, Copenhagen, Denmark), 5 g/100 g salt mixture (including trace elements), 4 g/100 g fat, 4 g/100 g cellulose powder (MN 100, Machery-Nagel GmbH & Co, Düren, Germany), 0.5 g/100 g vitamin mixture, and 0.5 g/100 g choline chloride (Merck, Darmstadt, Germany). The vitamin and salt mixtures were composed as described by Aaes-Jørgensen and Hølmer [15].
One group was given a DEF, in which fat was supplied as a 78:10-mix of coconut fat (Aarhus United A/S) and safflower oil (Urtekram A/S, Mariager, Denmark). The other four groups were given n-3 PUFA sufficient diets with equal amounts of n-3 PUFA, but in the form of α-linoleic acid (18:3n-3) from linseed oil (Unikem, Copenhagen, Denmark) mixed with safflower oil, olive oil (FDB, Albertslund, Denmark), and coconut fat (43:13:25:19) (LSO) or n-3 LCPUFA from fish oil (Aarhus United A/S, Aarhus, Denmark) mixed with safflower oil (79:21) (FO). All diets had an equal content of n-6 PUFA (Table 1). All diets were supplied ad libitum. The diets were powdered, stored at –20 °C and provided fresh every day.
Immunization and Organ Collection
All neonatal mice were immunized both intraperitoneally and subcutaneously with 20 μL 10 mg/mL OVA within the first 24 h of life. 3 weeks after birth, blood from the offspring was drawn from retro-orbital venous plexus using calibrated 25 μL heparinized micropipettes. Blood was immediately diluted in 375 μL PBS and stored at −20 °C until antibody determination.
Dams and pups were weighed weekly and when pups were 3 weeks of age, all mice were anesthetized with 0.06 mL/10 g body weight of a mixture composed of Hypnorm (Janssen Pharmaceutica, Beerse, Belgium):Dormicum (Hoffmann-La Roche AG, Basel, Switzerland):sterile water (1:1:2), thereafter death was assured by cervical dislocation. Spleens were removed, and leucocytes were immediately after isolation and used for determination of the fatty acid composition and for OVA-specific cell proliferation and cytokine production. Brains, eyes, and livers were also removed from pups and milk glands from the dams and all organs were immediately frozen in liquid nitrogen. The organs were stored at −80 °C until analysis.
Analysis of Diets and Tissue Lipids
Lipid from diets and tissues were extracted with chloroform and methanol according to Folch et al. [16]. Fatty acid composition of the dietary fat and the structure of the TAG, represented by the fatty acid composition in sn-2 MAG, were measured as described previously [17]. TAG from milk glands were isolated by TLC with heptane–isopropanol–acetic acid (95:5:1, v/v/v) and methylated with a modified BF3 procedure [18], as were the lipid extracts from the pup livers. Phospholipid classes in pup brain, eyes and spleen (from brain: phosphatidylcholine (PC), phosphatidylethanolamine (PE) and phosphatidylserine and PC and PE from eyes and spleen) were separated by TLC with chloroform–methanol–hexane–acetic acid–boric acid (40:20:30:10:1.8, v/v/v/v/w), visualized with 2,7-dichlorofluorescein (0.2% in ethanol), scraped off and methylated with BF3 [19]. The resulting fatty acid methyl esters were analyzed by GLC in a Hewlett–Packard 5890 chromatograph equipped with a split/splitless injector, SP2380 capillary column (60 m, ID. 0.25 mm; Supelco Inc., Bellefonte, PA), flame-ionization detection and helium as carrier gas. The GLC conditions were as follows: injector temperature 270 °C, flame-ionization detector 270 °C, helium carrier gas at 1.2 mL/min, injector split ratio 1:11, initial oven temperature 70 °C, which was held for 5 min and the increased by 15 °C/min until 160 °C, 1.5 °C/min until 200 °C that was then maintained for 15 min followed by a finally temperature rise to 225 °C maintained for 10 min. The initial oven temperature for the fatty acid determinations in milk gland TAG was 50 °C. Peak areas were calculated using a Hewlett–Packard integrator and the fatty acids were identified by comparing the retention time with standards of known fatty acid composition (Nu-Chek-Prep, Elysian, MN).
Spleen Cell Preparation and Ex-Vivo-Stimulated Cytokine Production
Single-cell suspensions were prepared from spleens pooled from 2 to 3 offspring within a litter in DMEM (Gibco, Life Technologies, Scotland). Red blood cells were lyzed with ammonium chloride buffer (0.83% NH4Cl). For the measurement of cytokine production, cells were washed and resuspended in X-VIVO 10 (serum free medium, Bio Whitaker, USA) at a final concentration of 2 × 106 cells/ mL and were cultured with 550 μL/well in flat-bottom 48-well tissue culture plates (Nunc, Denmark). Cells were stimulated with 0.5 mg OVA/well (0.9 mg/mL) or with medium in triplicates, and supernatants were collected after 72 h of culture. Supernatants were stored at −80 °C until determination of IFNγ, tumor necrosis factor-α (TNFα), interleukin (IL)-6, IL-10, IL-5 and IL-12 by commercial ELISA kits from R&D systems Europe (Abingdon, UK) according to manufactures instructions (Duosets DY 485, DY 410, DY 406, DY 417, DY 405, DY 419). Cytokine concentrations were quantified relative to standard curves representing a range of dilutions of recombinant cytokine using 4-parameter curve fit analysis (Kineticalc software, version KC4 Rev 29, Biotek instruments). Limits of detection for these assays were 31 pg/mL (IFNγ), 16 pg/mL (TNFα), 16 pg/mL (IL-6), 31 pg/mL (IL-10), 16 pg/mL (IL-5) and 31 pg/mL (IL-12).
Determination of OVA-Specific Antibodies in Pups' Plasma
Presence of total immunoglobulins (Ig) and IgG1 plasma antibodies specific for OVA was tested by ELISA. In brief, micro-titer plates (Nunc, Denmark) were coated with OVA overnight at 4 °C and plasma and controls were serially diluted in the washed plates. Bound OVA-specific Ig was detected with peroxidase-conjugated rabbit anti-mouse Ig (DAKO, Denmark). OVA-specific IgG1 and IgG2a were detected with rabbit anti-mouse IgG1 and IgG2a antibodies (Zymed, CA, USA) followed by peroxidase-conjugated swine anti-rabbit Ig (DAKO, Denmark). Hydrogen peroxide and tetramethylbenzidine (TMB, Merck, Germany) were used as substrates and the reaction was stopped after 10 min by the addition of phosphorous acid (2 M). Plates were measured spectrophotometrically at 450 nm using 690 nm as reference on an ELISA-reader (Bio-kinetics reader, EL 340, Biotek instruments). Controls, monoclonal antibodies against OVA (of IgG1 and IgG2a isotypes) and a pool of plasma from OVA-immunized animals were included on each plate. The antibody titers were expressed as log2 titers and defined as the interpolated dilution (4-parameter analysis, Kineticalc software, KC4 Rev 29, Biotek instruments) of a blood sample leading to an absorbance on 0.2 above background.
Statistics
Results were expressed as means ± standard errors (SEM). Differences in fatty acid compositions between groups were tested by one-way ANOVA (GraphPad PRISM version 3.02, GraphPad software, San Diego, CA). Tukey’s Multiple Comparison post hoc test was used to determine the exact nature of the differences. The level of statistical significance was P < 0.05.
Results
Dams weighed 23 ± 0.5 g (mean ± SEM) at the beginning and 30 ± 0.6 g at the end of the 7 weeks study. During the course of the study all mice gained more weight due to pregnancy, which they lost again at delivery. The pups increased their weight by a factor 1.8–3.4 from birth until sacrifice at 3 weeks of age. The increase in weight was dependent on the number of pups in the litter, but there was no significant difference in pup litter size (3–7 pups/group in FO-group, 2–6 in DEF-groups, and 3–8 in the LSO-group) and weight between the dietary groups (results not shown).
The n-3 PUFA content in the milk gland TAG reflected the fatty acid composition of the maternal diets (Table 2). Milk gland TAG from both of the n-3 PUFA sufficient groups, LSO and FO, had a significantly higher content of n-3 PUFA relative to the DEF-group. In the LSO-group this was due to an increase in the content of 18:3n-3 and 20:5n-3, whereas also 22:6n-3 was increased in the FO-group. It is noteworthy that although approximately 25% of dietary fatty acids in the LSO-diet were 18:3n-3, only 2% of the milk gland TAG fatty acid was 18:3n-3. The n-6 PUFA-content of the milk glands was not affected by an increased intake of n-3 PUFA, whereas that of MUFA was decreased in the FO-group compared to that in the DEF-group. The composition of PUFA in the pup liver lipid in turn reflects that in the milk gland TAG (Table 3). Pups from both the FO- and the LSO-group had pronouncedly increased liver levels of n-3 PUFA, increased levels of PUFA as such, and lower levels of n-6 PUFA. These changes were more pronounced in the FO-group, but the two groups responded similar with respect to changes in the overall n-6 to n-3 PUFA-ratio, the content of 20:5n-3 and 22:5n-3, and the observed deceases in the content of the n-3 PUFA-deficiency markers, 22:4n-6 and 22:5n-6.
The phospholipid PUFA-composition of pup tissues was also affected by the n-3 PUFA-content of the maternal diet. All types of n-3 LCPUFA were increased in both PC and PE in both brain and spleen, whereas the n-6 PUFA-content of the tissue phospholipids decreased. The n-6 PUFA-decrease was most pronounced for 22:5n-6, which is the dominant of the two n-3 PUFA-deficiency markers and which decreased to the same extent in the LSO- and FO-group (Table 4). The dietary n-3 PUFA-induced decrease in the tissue phospholipid n-6 PUFA-content was generally larger in the spleen than in the brain. The phospholipid PUFA-incorporation of the LSO- and FO-group only differed significantly in the PE-pool of the tissues, whereas the two groups were very comparable with respect to the composition in PC in brain but exhibited a minor difference in total n-3 PUFA in spleen PC (Table 4). Brain phosphatidylserine PUFA-incorporation was changed in the same way as that of PC and PE, with only minor differences between the LSO- and FO-group (data not shown). Furthermore, the observed diet-induced changes in eye PC and PE PUFA-composition were as those in the brain, except that no differences were found in the PUFA-composition of the LSO- and FO-group (data not shown).
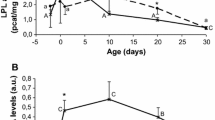
OVA-immunization gave rise to a significant increase in the plasma concentration of OVA-specific antibodies and of OVA-IgG1, which in non-immunized mice of the same age was 6.0 ± 0.07 and 5.5 ± 0.06, respectively. The observed OVA-antibody titers reflect a Th2-polarization of the response, since the IgG1 titers corresponded to the titer measured for all antibody classes. It was not possible to obtain IgG2a titers (representing aTh1-polarization) that were significantly above the background (data not shown), possibly due to the much lower level of antibodies involved in this type of responses. The OVA-Ig titers were significantly reduced in FO group as compared to the DEF-group, whereas the LSO-group did not differ from the DEF-group (P < 0.01, Fig. 1). The results for OVA-IgG1 were similar to those of IgG (data not shown).
Levels of OVA-specific antibodies in plasma of 3 weeks old mouse pups of mothers fed either fish oil (white bars, n = 29), linseed oil (gray bars, n = 21) or the n-3 PUFA-deficient diet (black bars, n = 24) during gestation and lactation. Results are shown as titer values (means ± SEM). Mice were immunized within 24 h after birth with OVA solubilized in PBS
The cytokines produced in spleen cells from the same mice upon stimulation with OVA did not reveal any significant differences between the different groups (Table 5). The responses were for most of the analysed cytokines only slightly over the detection levels and rather variable and it was thus not possible to show any statistically significant differences between the groups. The high variation within groups may be ascribed to the fact that a proportion of cell cultures within each group did not result in cytokine productions above the background (for IFN-γ, 50, 60 and 40% were detectable in the FO, LSO and DEF-group, respectively). However, a trend towards an increasing IFNγ- and TNFα-production and a decreasing production of IL-6 with increasing incorporation of n-3 LCPUFA in spleen cells was observed. The production of IL-12 and IL-5 could not be detected in any of the groups. There were no significant differences in the spleen cell proliferation or in CD69-expression on the cultured spleen cells from the pups in the dietary groups (data not shown).
Discussion
The present study showed a reduction in total OVA-specific antibodies and in the IgG1 titers in the blood in the neonatal mice from dams in the fish oil-fed group. As we were not able to measure OVA-specific IgG2a antibodies in the plasma, we cannot establish whether this reduction is caused by a general reduction in the ability to respond to an antigen or an increased Th1-polarization. However, although our results were not significant, the decrease in the Th2-facilitated IgG1-response was paralleled by an increase in IFNγ-production and a decrease in the production of IL-6 in OVA-stimulated spleen cells ex vivo, indicating an increase in Th1-cells in the spleen in FO-group compared to control. The response in the LSO-group did not differ from that in the DEF-group. This is supported by the study of Rayon et al. [13], who showed that maternal intake of menhaden oil resulted in a higher survival in rat pups towards group B Streptococcal infection. This was correlated with a lower PGE2 production in the lungs as compared to the corn oil-fed group, indicative of conditions favoring a Th1- over Th2-polarization [20]. Korotkova et al. [14] have shown that a high maternal n-3 PUFA intake affected the induction of tolerance against orally administered OVA in neonatal rats, thus also supporting a critical need for n-3 PUFA in the perinatal period. Although the results of these two studies are not comparable, they both point toward a role of n-3 PUFA in directing the immature immune system towards responses which are considered to be beneficial. Furthermore, our results also support our earlier findings in humans, where intake of fish oil in infancy or milk from fish oil-supplemented mother were associated with an increase in IFNγ-production in ex vivo stimulated blood cells also indicative of a Th1-polarization [9, 10]. As regards the effect of fish oil on allergy development in infancy, some studies suggest a protective role of n-3 LCPUFA [21, 22]. Whether a higher ex vivo IFNγ and a reduction in antigen-specific IgG1 antibodies directly can be compared to human studies on infant allergy is, however, purely speculative.
Not surprisingly we found that the milk gland TAG fatty acid composition was reflecting that of the diet, the PUFA-composition in the pup liver lipid in turn reflected that of the milk and that the tissue phospholipid PUFA-composition reflected that in the liver. Furthermore, the present study showed that the source of dietary n-3 PUFA, plant oil 18:3n-3 or marine n-3 LCPUFA, had only limited influence on the accretion of n-3 LCPUFA in the peripheral tissue, but with more pronounced differences in spleen than in brain. Intake of both fish oil and linseed oil gave rise to an increased incorporation of 22:6n-3 compared to that in the DEF-group, but the 22:6n-3 incorporation was more specific in the FO-group as intake of linseed oil also gave rise to pronounced increases in both 20:5n-3 and 22:5n-3. The accumulation of these intermediate n-3 LCPUFA is in agreement with many previous results and a key issue in the debate on whether intake of 18:3n-3 is an optimal way to fulfill requirements [23]. The difference in brain phospholipid 22-6n-3 content was however only minor, where as that in spleens was more pronounced. Brain phospholipid has been shown to saturate with 22:6n-3 at lower levels of intake than other tissues [24, 25]. The present study did not examine the dose response relationship but only compared the two sources of n-3 PUFA at levels well over levels of deficiency (2.5E% from n-3 PUFA) with that from a DEF (0.01E%, 2E% from n-6 PUFA). The level of 18:3n-3 and 22:5n-3 has been shown to increase in the brains of suckling rat pups when the mothers were fed diets with high compared to low levels of 18:3n-3 [26, 27]. A previous four-generation study in rats that compared incorporation of n-3 PUFA from marine oils with that from plant oils at an overall fat intake of 20 g/100 g (39 E% from fat, hereof around ¼ from n-3 PUFA) also found better n-3 LCPUFA-incorporation in brain PE and PS [28]. One of the strengths of this study is that we used intake levels that are similar to that in ordinary mouse chow (11E% from fat). We were unfortunately not able to get milk samples from the mice and used the fatty acid composition of mammary gland TAG as a proxy to the milk fatty acid composition. Several studies have determined the fatty acid composition of mouse milk after oxytocin injection and milking [25, 29, 30]. Although different dietary regimes were used in these studies the overall reported fatty acid composition of mice milk, including the relative content of the MCFA that are characteristic for milk, was comparable to the milk gland TAG composition in the present study. Most of the milk lipids are TAG, but there is however some phospholipid in milk and we expect these to be enriched in LCPUFA. It is therefore, possible that our results may be an exact estimate of the differences in milk-LCPUFA between the FO- and LSO-group. The levels of 22:6n-3 that we found in the milk glands of these mice and the range that we induce by the dietary changes are comparable to the variation in human milk. The average 22:6n-3 content in human milk in populations with a low fish intake such as the Australian or American is typically around 0.1–0.2% of the fatty acids [1] and has in some Chinese milk been found to be as high as 3.6% [31].
This study showed that maternal fish oil intake was associated with a decrease in plasma concentrations of OVA-induced IgG1in the pups and tended to show a Th1-polarization also in the ex vivo cytokine response to OVA in spleen cells, whereas no immuno-modulating effects were observed after intake of 18:3n-3 from linseed oil. Both types of n-3 PUFA were associated with an increase in milk and tissue n-3 LCPUFA-incorporation, although a slightly lower incorporation of 22:6n-3 was seen after 18:3n-3. Early growth is associated with a high n-3 LCPUFA accretion in the central nervous system and therefore a high requirement of n-3 PUFA. Other tissues may therefore be more susceptible to differences in the n-3 LCPUFA intake during the perinatal period than there are later in life. Since early processes play an important role in the long-term shaping of the immune system, the immune system may be especially vulnerable towards suboptimal intakes during the perinatal period. The acute and potential programming effects of an increased intake of n-3 LCPUFA early in life is not well characterized and this study indicate a need for more thorough investigation especially with respect to their potential effects on the immunological development.
Abbreviations
- DEF:
-
The n-3 PUFA-deficient group
- 20:5n-3:
-
Eicosapentaenoic acid (individual fatty acids are named by number of carbon atoms: number of double bonds followed by the position of the last double bond)
- FO:
-
The fish oil group
- Ig:
-
Immunoglobulin
- IL:
-
Interleukin
- IFNγ:
-
Interferon-γ
- LCPUFA:
-
Long-chain PUFA
- LSO:
-
The linseed oil-group
- OVA:
-
Ovalbumin
- PC:
-
Phosphatidylcholine
- PE:
-
Phosphatidylethanolamine
- TAG:
-
Triacylglycerol
- Th:
-
T-helper lymphocytes
- TNFα:
-
Tumor necrosis factor-α
References
Lauritzen L, Hansen HS, Jørgensen MH, Michaelsen KF (2001) The essentiality of long-chain n-3 fatty acids in relation to development and function of the brain and retina. Prog Lipid Res 40:1–94
Calder PC (2010) The 2008 ESPEN Sir David Cuthbertson lecture: fatty acids and inflammation–from the membrane to the nucleus and from the laboratory bench to the clinic. Clin Nutr 29:5–12
Calder PC, Yaqoob P, Thies F, Wallace FA, Miles EA (2001) Fatty acids and lymphocyte functions. Br J Nutr 87(Suppl 1):S31–S48
Kremmyda LS, Vlachava M, Noakes PS, Diaper ND, Miles EA, Calder PC (2009) Atopy risk in infants and children in relation to early exposure to fish, oily fish, or long-chain omega-3 fatty acids: a systematic review. Clin Rev Allergy Immunol. doi: 10.1007/s12016-009-8186-2
Hanson LA, Korotkova M, Haversen L et al (2002) Breast-feeding, a complex support system for the offspring. Pediatrics Int 44:347–352
Garn H, Renz H (2007) Epidemiological and immunological evidence for the hygiene hypothesis. Immunobiol 212:441–452
Dunstan JA, Mori TA, Barden A et al (2003) Maternal fish oil supplementation in pregnancy reduces interleukin-13 levels in cord blood of infants at high risk of atopy. Clin Exp Allergy 33:442–448
Dunstan JA, Mori TA, Barden A et al (2003) Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol 112:1178–1184
Lauritzen L, Kjær TMR, Fruekilde MB, Michaelsen KF, Frøkiær H (2005) Fish oil supplementation of lactating mothers affects cytokine production in 2½-year-old children. Lipids 40:669–676
Damsgaard CT, Lauritzen L, Kjær TMR et al (2007) Fish oil supplementation modulates immune function in healthy infants. J Nutr 137:1031–1036
Furuhjelm C, Warstedt K, Larsson J et al (2009) Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatr 98:1461–1467
Mihrshahi S, Peat JK, Webb K, Oddy W, Marks GB, Mellis CM (2004) Effect of omega-3 fatty acid concentrations in plasma on symptoms of asthma at 18 months of age. Pediatr Allergy Immunol 15:517–522
Rayon JI, Carver JD, Wyble LE et al. (1997) The fatty acid composition of maternal diet affects lung prostaglandin E2 levels and survival from group B streptococcal sepsis in neonatal rat pups. J Nutr 127:1989–1992
Korotkova M, Telemo E, Yamashiro Y, Hanson LA, Strandvik B (2004) The ratio of n-6 to n-3 fatty acids in maternal diet influences the induction of neonatal immunological tolerance to ovalbumin. Clin Exp Immunol 137:237–244
Aaes-Jørgensen E, Hølmer G (1969) Essential fatty acid-deficient rats. I. growth and testes development. Lipids 4:501–506
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Porsgaard T, Høy CE (2000) Lymphatic transport in rats of several dietary fats differing in fatty acid profile and triacylglycerol structure. J Nutr 130:1619–1624
Porsgaard T, Xu XB, Gottsche J, Mu HL (2005) Differences in the intramolecular structure of structured oils do not affect pancreatic lipase activity in vitro or the absorption by rats of (n-3) fatty acids. J Nutr 135:1705–1711
Morrison WR, Smith LM (1964) Preparation of fatty acid methyl esters+dimethylacetals from lipids with boron fluoride-methanol. J Lipid Res 5:600–608
van der Pouw Krann TCTM, Boeije LCM, Smeenk RJT, Wijdenes J, Aarden LA (1995) Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med 181:775–779
Lowe AJ, Thien FCK, Stoney RM et al (2008) Associations between fatty acids in colostrum and breast milk and risk of allergic disease. Clin Exp Allergy 38:1745–1751
Giwercman C, Halkjær LB, Jensen SM, Bønnelykke K, Lauritzen L, Bisgaard H (2010) Increased risk of eczema but reduced risk of early wheezy disorder from exclusive breast-feeding in high-risk infants. J Allergy Clin Immunol 125:866–871
Gerster H (1998) Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Int J Vitam Nutr Res 68:159–173
Ward GR, Huang YS, Bobik E et al (1998) Long-chain polyunsaturated fatty acid levels in formulae influence deposition of docosahexaenoic acid and arachidonic acid in brain and red blood cells of artificially reared neonatal rats. J Nutr 128:2473–2487
Wainwright PE, Huang YS, Bulmanfleming B et al (1992) The effects of dietary n-3/n-6 ratio on brain-development in the mouse–a dose-response study with long-chain n-3 fatty-acids. Lipids 27:98–103
Bowen RAR, Clandinin MT (2005) Maternal dietary 22:6n-3 is more effective than 18:3n-3 in increasing the 22:6n-3 content in phospholipids of glial cells from neonatal rat brain. Br J Nutr 93:601–611
Bowen RAR, Clandinin MT (2000) High dietary 18:3n–3 increases the 18:3n–3 but not the 22:6n–3 content in the whole body, brain, skin, epididymal fat pads, and muscles of suckling rat pups. Lipids 35:389–394
Jensen MM, Skarsfeldt T, Høy CE (1996) Correlation between level of (n-3) polyunsaturated fatty acids in brain phospholipids and learning ability in rats. A multiple generation study. Biochim Biophys Acta 1300(3):203–209
Silverman J, Stone DW, Powers JD (1992) The lipid-composition of milk from mice fed high or low fat diets. Lab Animals 26:127–131
Singh K, Hartley DG, McFadden TB, Mackenzie DDS (2004) Dietary fat regulates mammary stearoyl CoA desaturase expression and activity in lactating mice. J Dairy Res 71:1–6
Xiang M, Lei S, Li T, Zetterstrom R (1999) Composition of long-chain polyunsaturated fatty acids in human milk and growth of young infants in rural areas of northern China. Acta Pediatr 88:126–131
Acknowledgments
We acknowledge the late CE Høy, who was involved in the funding and planning of this study. We greatly appreciate the technical help of Karen Jensen, Lillian Vile, Charlotte Vajhøj, and Anni Mehlsen. The study was supported by The Danish Research and Development Program for Food and Technology (FELFO) and Center for Advanced Food Studies (LMC). None of the authors have any conflict of interest to disclose in connection with the submitted material.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lauritzen, L., Kjær, T.M.R., Porsgaard, T. et al. Maternal Intake of Fish Oil but not of Linseed Oil Reduces the Antibody Response in Neonatal Mice. Lipids 46, 171–178 (2011). https://doi.org/10.1007/s11745-010-3519-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-010-3519-8