Abstract
The Omega-3 Index, a measure of long-chain omega-3 fats in red blood cell membranes, predicts heart disease mortality in adults, but its association with cardiovascular risk factors in younger populations is unknown. We determined the Omega-3 Index in adolescents participating in the Western Australian Pregnancy (Raine) Cohort, assessed associations with diet, lifestyle and socioeconomic factors, and investigated independent associations with cardiovascular and metabolic risk factors. Red blood cell fatty acid analysis was determined for 1,301 adolescents aged 13–15 years. Risk factors examined were blood pressure, fasting blood insulin and glucose concentrations, and fasting blood lipids including ratios. The mean Omega-3 Index was 4.90 ± 1.04% (range 1.41–8.42%). When compared with categories identified in adults, 15.6% of adolescents were in the high risk category (Index < 4%). Age (P < 0.01), maternal education (P < 0.01) and BMI (P = 0.05) were positively associated with the Omega-3 Index. The Index was positively associated with dietary intakes of eicosapentaenoic and docosahexaenoic acid (P < 0.01), protein (P < 0.01), omega-3 fats (P < 0.04), and food groups of fish and wholegrains (both P < 0.01), and negatively associated with intakes of soft drinks and crisps (both P < 0.01). In boys, the Omega-3 Index was independently associated with total (β = 0.06, P = 0.01) and HDL-cholesterol (β = 0.03, P = 0.01), and diastolic blood pressure (β = −0.68, P = 0.04). The predictability of the Index for the risk of cardiovascular disease later in life warrants further investigation in the adolescent population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Omega-3 Index has been suggested as a physiologically relevant, modifiable, and independent risk factor for cardiovascular disease [1]. The Index is equal to the content of long-chain omega-3 fatty acids—eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3)—in red blood cell (RBC) membranes, as a percentage of the total fatty acids. The omega-3 fatty acid content of RBC membranes reflects the fatty acid content of cardiac membranes [2]. Dietary intake of fats is thought to modify the Index [3], and supplementation with long-chain omega-3 fatty acids has been shown to increase the Omega-3 Index in a randomised trial [4]. Other factors such as age and smoking habits may also affect the Index [3].
Long-chain omega-3 fatty acids in RBC membranes exert beneficial metabolic effects, in part, by altering membrane characteristics and the activity of membrane-bound proteins [5]. They reduce the risk of cardiovascular disease through their strong anti-inflammatory effects, their capacity to reduce platelet adhesiveness, and by benefitting blood pressure and vascular reactivity, cardiac function, lipid metabolism, platelet and leukocyte function, cytokine production and oxidative stress [6–9]. Harris and von Schacky have shown, through analyses of epidemiological and randomised controlled trials, that an Omega-3 Index of <4% is associated with a high risk, 4–8% an intermediate risk and >8% a low risk of coronary heart disease mortality in adults [5].
Existing literature on investigations of the Omega-3 Index is predominantly focussed on adults [10–12]. To our knowledge, there have been no reports describing associations between the Omega-3 Index and cardiovascular and metabolic risk, dietary, lifestyle and socioeconomic factors in an adolescent population. To assess its potential value as an early predictor of adult cardiovascular and metabolic disease, we have investigated cross-sectional associations between cardiometabolic risk factors and the Omega-3 Index in a large population-based adolescent cohort in Western Australia.
Materials and Methods
Subjects
As a longitudinal observational study, the Raine Study recruited 2,900 pregnant women from May 1989 to November 1991 through the public antenatal clinic at the King Edward Memorial Hospital and private clinics in Perth, Western Australia. Further details on the Raine Study have been previously published [13]. Of the initial cohort of 2,868 live births, assessments occurred at birth and at ages one, two, three, five, eight, ten and 14 years. This study utilises data collected at the 14-year follow-up when assessments of RBC fatty acids and dietary intake were conducted. The ethics committees of King Edward Memorial Hospital and Princess Margaret Hospital approved the protocol for all aspects of the study. Each adolescent, as well as their parent or guardian, provided written consent for participation in the study.
Red Blood Cell Analysis
Fatty acids of interest for this study were EPA and DHA. RBC fatty acid analysis was performed as previously described [14]. Briefly, chloroform:methanol (2:1) was used to extract total lipids and fatty acid methyl esters were prepared by treatment of extracts with 4% H2SO4 in methanol at 90 °C for 20 min. Samples were analysed by gas chromatography using an Agilent 7890A gas chromatograph. The column was a BPX70 (25 m × 0.32 mm, 0.25 μm film thickness) (SGE, Ringwood, Victoria, Australia) with programmed temperatures of 150–210 °C at 4 °C/min. N2 was used as the carrier gas at a split ratio of 30:1. Peaks were identified by comparison with a known standard mixture. Reproducibility for duplicate analysis was ~10–20%. Coefficients of variation were 2% for DHA and 4% for EPA. The fatty acids were expressed as a percentage of the total fatty acids measured (C14–C22).
Assessment of Cardiovascular Factors
Blood pressure readings were taken using a Dinamap ProCare 100 automatic oscillometric blood pressure recorder (GE Healthcare Technologies, Rydalmere, Australia) while subjects were seated after a 5-min rest. Over a 10-min period, six blood pressure measurements were taken. The first was disregarded and blood pressure was calculated as the mean of the next five measurements. Trained phlebotomists visited the adolescents at their homes and obtained fasting blood samples prior to breakfast. The biochemistry assays for the study were conducted by PathWest Laboratories at Royal Perth Hospital. Serum triglycerides were measured using the Cobas MIRA analyser (Roche Diagnostics, Basel, Switzerland). Glucose was measured using an automated Technicon Axon Analyzer (Bayer Diagnostics, Sydney, Australia) and insulin was measured on an Immunlite 2000 Insulin Analyzer (Siemens Medical Solutions Diagnostic, LA, USA). Insulin resistance was determined using the homeostasis model assessment of insulin resistance (HOMA-IR), calculated as fasting plasma insulin (mU/L) × plasma glucose (mmol/L)/22.5 [15]. High density lipoprotein (HDL) cholesterol was determined on a heparin–manganese supernatant [16]. Cholesterol ratios were calculated for total/HDL, low density lipoprotein (LDL)/HDL and triglycerides/HDL [17, 18].
Dietary Intake
Dietary intake at this follow-up was assessed from three-day food records in household measures, as previously reported [19]. In brief, subjects were provided with a record booklet with instructions and a set of metric measuring cups and spoons. Food records were individually checked by a dietitian as they were returned in order to clarify any ambiguous or potential omissions [20]. The Australian Food and Nutrient database through the FoodWorks dietary analysis program (Professional Version 5, 2007, Xyris Software, Brisbane) was used to analyse the food record data. Individual nutrients as well as overall diet was considered from the food diaries, with dietary patterns used to investigate overall diet. Using exploratory factor analysis, two major dietary patterns were identified: a ‘healthy’ pattern high in fresh fruit, vegetables, whole grains and grilled or canned fish, and a ‘western’ pattern high in takeaway foods, confectionery, soft drinks, crisps and fried potato [21]. Each adolescent received a z-score for both western and healthy dietary patterns. A positive score indicated a greater intake of foods representative of that pattern. To assess differences in the Omega-3 Index due to early infant feeding, information on breastfeeding cessation was obtained from the year 1, 2 and 3 Raine follow-up questionnaires. At the time of these follow-ups it was not mandatory for infant formulas to be enriched with long-chain omega-3 fatty acids in Australia.
Additional Factors
Anthropometry and Puberty
Adolescents were dressed in running shorts and singlet tops for anthropometric measurements. Height was measured to the nearest 0.1 cm with a Holtain Stadiometer, and body weight was measured to the nearest 100 g using a Wedderburn Digital Chair Scale. The body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. The Tanner stages of pubic hair development [22, 23] was used to assess puberty; adolescents selected their corresponding developmental stage in a questionnaire completed privately. Adolescents were asked to choose from a set of standard drawings depicting the different Tanner stages from two (sparse) to five (adult) (stage one was omitted as this corresponds to pre-pubescent age younger than 10 years).
Sociodemographic and Family Characteristics
Family income, mother’s age at conception and mother’s education level were obtained by parent report. Maternal education was assessed by the highest school year completed. Current family income, defined as the annual income for the household before tax at the time of the follow-up, was determined as ($AUD): <$35,000 pa, $35,000–70,000 pa, or >$70,000 pa. Family history of cardiovascular disease was assessed as either yes or no, depending on whether a biological parent or sibling of the adolescent had been medically diagnosed with diabetes mellitus, hypertension, hypercholesterolemia, or other cardiac condition.
Fitness
The Physical Working Capacity 170 (PWC 170) test was applied to estimate aerobic fitness [24]. PWC170 was measured using a bicycle ergometer to determine the power output (watts) required at a heart rate of 170 beats per minute. This measure of fitness is highly correlated with self-reported physical activity level in the Raine cohort at the 14-year follow-up [25].
Statistical Analysis
Independent t tests or cross-tabs analysis were used to compare characteristics of Raine Study adolescents between Omega-3 Index risk categories, with low and intermediate categories combined due to the small number of subjects in the low category. Mann–Whitney tests were used to assess significance of dietary intakes of omega-3, the omega-6 to 3 ratio and EPA + DHA due to the skewed distribution of these variables. Associations between continuous variables and the Omega-3 Index were described with Pearson’s or Spearman’s correlations. Linear regression was used to examine associations between Omega-3 Index as a continuous independent variable and cardiovascular risk factors as dependent variables. General Linear Modelling was used to analyse associations with Omega-3 Index categories and cardiovascular risk factors. Log values were used for HOMA, triglycerides, and insulin due to their skewed distribution. To evaluate the relationship between the Omega-3 Index and cardiometabolic risk factors, parsimonious models were created which adjusted for age, sex, BMI, maternal education, total energy intake and family history of cardiovascular disease or diabetes, based on the relationship of the variables with the risk factors and the Omega-3 Index. Other variables considered for the model included puberty, aerobic fitness, physical activity, family income, single parent family, maternal age, but were excluded due to strong correlation between covariates or lack of association with risk factors and the Index. Subjects who were on cardiac or diabetes related medications (n = 4) or diagnosed with diabetes (n = 9) were excluded from analysis investigating cardiometabolic disease risk factors. The Statistical Package for Social Sciences for Windows, Rel.15.0.0. 2006 (Chicago: SPSS Inc) was used for analyses; statistical significance was set at P ≤ 0.05.
Results
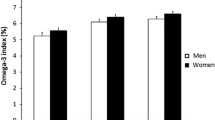
From the initial Raine Study cohort of 2,868 live births, 1,861 subjects completed at least one aspect of the 14-year follow-up (mean age 14.0 ± 0.2 years, range 13.0–15.0 years), with the remainder lost to follow-up, withdrawn or temporarily deferred from the study (n = 975), or deceased (n = 32). RBC fatty acids were determined for 1,301 subjects. The mean ± SD Omega-3 Index was 4.90 ± 1.04% (range 1.41–8.42%). Relative to the published Omega-3 Index risk categories [5], 15.6% of adolescents were in the high risk category (<4%), 84.0% were in the intermediate risk category (4–8%) and 0.4% were in the low risk category (>8%). Omega-3 Index values in the population were normally distributed, while dietary intake of EPA and DHA was not (Fig. 1). Characteristics of this sample across high and low/intermediate Omega-3 Index risk categories are shown in Table 1.
When associations with subject characteristics were examined with the Omega-3 Index as a continuous variable, age at assessment (r = 0.12, P < 0.01) and maternal education (r = 0.09, P < 0.01) showed significant correlations with the Index. BMI showed borderline significance (r = 0.05, P = 0.05). The Omega-3 Index as a continuous variable showed significant positive associations with healthy eating pattern scores (r = 0.14, P < 0.01), energy adjusted dietary intakes of EPA + DHA (r = 0.22, P < 0.01), protein (r = 0.12, P < 0.01) and total omega-3 fats (r = 0.08, P = 0.04), and a significant negative association with western eating pattern scores (r = −0.13, P < 0.01) (data not shown). The significant association with EPA + DHA was also observed in comparison of Omega-3 Index risk categories (P < 0.01), along with a positive association with glycemic index (P = 0.02) (Table 1).
Several food group intakes were correlated with the Omega-3 Index (Fig. 2). Fish and seafood (r = 0.18, P < 0.01), wholegrain foods such as multigrain bread, brown rice and pasta (r = 0.11, P < 0.01) and vegetables (r = 0.10, P < 0.01) showed the strongest positive associations with the Omega-3 Index. Intakes of soft drinks (r = −0.11, P < 0.01) and crisps (r = −0.10, P < 0.01) were negatively correlated with the Omega-3 Index.
Bivariate correlations between food group intakes (g/day) and the Omega-3 Index in Raine Study adolescents. **P < 0.01, *P < 0.05. Wholegrains includes wholegrain crackers and bread, oats, wholegrain breakfast cereal, brown rice and pasta. Refined grains includes white bread, crackers, rice and pasta, savory crackers. Confectionery includes ice and chocolate confection. Takeaway foods includes hamburgers, pizzas, fried chicken, savory pastries. Sweet baked goods includes cakes, biscuits, sweet pastries. Crisps includes popcorn, corn chips, extruded cheese snacks
After adjustment for potential confounding factors in a linear regression model, the Omega-3 Index was positively associated with total cholesterol (β = 0.064, P = 0.01) and HDL-cholesterol (β = 0.029, P = 0.01) in boys and girls (Table 2). There were no significant associations with other cardiovascular or metabolic risk factors. When analysed according to gender, significant associations were observed with boys for total cholesterol (β = 0.074, P = 0.03), HDL-cholesterol (β = 0.036, P = 0.01) and diastolic blood pressure (β = −0.684, P = 0.04); insulin (β = −0.019, P = 0.05) and HOMA-IR (β = −0.019, P = 0.07) showed borderline significance. No significant associations were observed with girls. Associations with risk factors were also examined according to Omega-3 Index risk categories [5]. General linear modelling suggested boys and girls in the low/intermediate risk category were more likely to have lower HDL-cholesterol levels, with borderline significance (β = −0.057, P = 0.07); no other risk factors were significant when boys and girls were analysed together or separately (data not shown).
Discussion
Due to variation in the methods used for red blood cell analysis in different laboratories, direct comparison of results is not possible. However, we found that the mean Omega-3 Index in the Raine Study adolescents (4.9 ± 1.0%) was consistent with the value of 4.7 ± 0.4% previously reported in a smaller control sample of healthy Australian children and adolescents aged 9–18 years [26]. Our value is also similar in value to that reported in US adults (4.9 ± 2.1% [27] and 3.5 ± 1.2% [28]), but lower than that in young overweight and obese adults from Iceland, Spain and Ireland (7.0 ± 1.9% [29] and Korean pre-school chidren (9.1 ± 0.8%) [30]. Although results are not directly comparable, dietary variations between cultures, particularly in regards to fish intake, may contribute to the variation in the Omega-3 Index observed in different populations.
Dietary fish intake was the strongest food group predictor of the Omega-3 Index in the Raine adolescents (P < 0.01), and has previously been shown to be a determinant of the Index in older US adults [3]. Fish is a good source of dietary EPA and DHA, and this finding is consistent with trials showing increased dietary EPA and DHA leads to higher concentrations of RBC omega-3 fatty acids [4, 31]. Further, our results showed daily intake of EPA + DHA positively correlated with the Omega-3 Index (P < 0.01). Fish is also a good source of protein, along with legumes, nuts and eggs which may have contributed to the positive association observed with protein and Omega-3 Index. The wholegrain food group showed the next strongest positive association with the Omega-3 Index (P < 0.01). Wholegrains contain omega-3 fats in the form of alpha-linolenic acid (18:3n-3) in the bran outer layer of the grain. Interestingly, refined grain products that are missing the bran layer were also observed to have a significant positive association with the Omega-3 Index (P < 0.05). This may be partially due to the consumption of DHA enriched white bread observed in the Raine Study cohort at this follow up [19]. However, given the additional positive associations with vegetables and fruit, it is more likely that these associations with the Omega-3 Index are related to an overall ‘healthy’ pattern of eating. Similarly, a high intake of soft drinks and crisps, along with a high dietary glycemic index, may reflect a less ‘healthy’ eating pattern. This is reflected in the associations observed with the dietary pattern scores identified in this cohort: the Index showed a positive association with the healthy pattern score and a negative association with the western pattern score. Food groups loading on the healthy pattern included fresh fruit, vegetables, wholegrains and grilled or canned fish, while the ‘western’ pattern score represented a higher intake of takeaway foods, crisps and soft drinks [21]. Although soft drinks do not contain fat, their consumption has been shown to result in a rapid increase in glucose and insulin concentrations in adolescents [32], and the fructose component of sucrose in soft drinks may lead to enhanced fatty acid synthesis, contributing to higher circulating triglycerides [33]. Higher GI diets, as observed in subjects in the high risk Omega-3 Index category, may also contribute to higher plasma triglycerides [34]. Together, these dietary factors may play a role in alteration of the fatty acid content of RBC membranes.
Results of our study also extend the findings of previous research in adults [3, 31] by confirming that higher dietary intake of EPA and DHA through consumption of fish is associated with a higher Omega-3 Index in adolescents. However, the difference in distributions of dietary EPA and DHA compared to Omega-3 Index values (as shown in Fig. 1) highlight the contribution that non-dietary factors may also make in determination of the Omega-3 Index.
The Omega-3 Index has been proposed to predict coronary heart disease mortality in adults [5]. Our data show it is also associated with total and HDL-cholesterol concentrations and diastolic blood pressure in boys in our adolescent cohort. The positive relationship between increasing Omega-3 Index and higher HDL-cholesterol supports Australia’s National Heart Foundation’s position statement that marine omega-3 PUFA supplementation increases HDL-cholesterol levels [35]. This result is also consistent with previous research in adult populations [36]. In contrast, the Omega-3 Index was not significantly associated with HDL-cholesterol or other cardiovascular risk factors in a study of overweight and obese European adults [29], although borderline significance was observed with lower LDL. The findings, however, may have been influenced by weight status, as our results suggest that the BMI is positively associated with the Omega-3 Index (P = 0.05). Consistent with previous research showing a positive association between age and the Index [3], the adults in the European study had a noticeably higher Omega-3 Index than we observed in our adolescent population (7.0 ± 1.9% vs. 4.9 ± 1.0%), which may have affected associations with disease risk. Although high total cholesterol is commonly considered a risk factor for cardiovascular disease, high HDL-cholesterol is beneficial [37], and the two measures are related as HDL-cholesterol contributes to total cholesterol. Ratios may therefore be considered more useful as indicators of cardiovascular disease risk [17], however we found no significant associations between the Index and ratios of total/HDL-cholesterol, LDL/HDL-cholesterol or triglycerides/HDL-cholesterol.
The significant negative association observed with diastolic blood pressure in boys in our cohort supports a role for omega-3 fatty acids in optimisation of blood pressure. Omega-3 fatty acids have been shown to have a variety of actions that can lead to improved vasodilation and arterial compliance, including increased membrane fluidity, suppression of vasoconstrictors, and changes in mobilisation of intracellular calcium [9, 38, 39].
In our adolescent cohort, we observed a gender difference in the relationship between Omega-3 Index and risk factors for cardiovascular and metabolic disease. Analysis with boys showed significant or borderline associations with measures of cholesterol, blood pressure, and insulin resistance, whereas no significant associations were observed for measures in the girls analysis. Boys were more likely to be in the high risk category of Omega-3 Index (<4%) compared to the low risk (≥4%, P = 0.10), and similar gender differences have previously been identified in the long-chain omega-3 fatty acid composition of RBC membranes in rats, with a link between ovarian hormones and DHA composition proposed [40]. Sex hormones may play a role in modification of the omega-3 content of tissues, possibly by altering expression of enzymes in the liver [41], while also contributing to gender specific pathophysiological differences in cardiovascular and metabolic disease [42].
To our knowledge, this is the first study to evaluate the Omega-3 Index with cardiovascular and metabolic risk factors in a large cohort of adolescents. Interpretation of our study results is limited by the cross-sectional study design, however an important strength of our study is that it represents a large, population based cohort and has data on a wide range of cardiometabolic, socioeconomic and dietary variables. Our results demonstrate a significant and independent association between the Omega-3 Index and total and HDL-cholesterol, as well as blood pressure, in Australian adolescent boys. Although the Omega-3 Index did not demonstrate a statistically significant association with the cholesterol ratios, it is considered to be a risk factor in its own right and has not been shown to mediate through effects on traditional risk factors. Therefore the Index may still be useful in the prediction of cardiovascular disease later in life, and our results support further long term investigation of this concept.
Abbreviations
- EPA:
-
Eicosapentaenoic acid
- DHA:
-
Docosahexaenoic acid
- RBC:
-
Red blood cell
- HDL:
-
High density lipoprotein
- LDL:
-
Low density lipoprotein
References
Harris WS, von Schacky C (2004) The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med 39:212–220
Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, Porter CB, Borkon AM (2004) Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation 110:1645–1649
Block RC, Harris WS, Pottala JV (2008) Determinants of blood cell omega-3 fatty acid content. Open Biomark J 1:1–6
Geppert J, Kraft V, Demmelmair H, Koletzko B (2005) Docosahexaenoic acid supplementation in vegetarians effectively increases Omega-3 Index: a randomized trial. Lipids 40:807–814
Harris WS (2008) The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr 87:1997–2002
Mori TA, Watts GF, Burke V, Hilme E, Puddey IB, Beilin LJ (2000) Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation 102:1264–1269
Simopoulos AP (2008) The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med 233:674–688
Dyerberg J, Bang HO, Stoffersen E, Moncada S, Vane JR (1978) Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet 2:117–119
Mori TA, Beilin LJ (2001) Long-chain omega 3 fatty acids, blood lipids and cardiovascular risk reduction. Curr Opin Lipidol 12:11–17
Yongsoon P, Seonhye P, Hyeongjoong Y, Hyun Young K, Seok-Jae K, Juhan K, Hongyup A (2009) Low level of n-3 polyunsaturated fatty acids in erythrocytes is a risk factor for both acute ischemic and hemorrhagic stroke in Koreans. Nutr Res 29:825–830
Friedman AN, Saha C, Watkins BA (2008) Feasibility study of erythrocyte long-chain omega-3 polyunsaturated fatty acid content and mortality risk in hemodialysis patients. J Ren Nutr 18:509–512
Cohen BE, Garg SK, Ali S, Harris WS, Whooley MA (2008) Red blood cell docosahexaenoic acid and eicosapentaenoic acid concentrations are positively associated with socioeconomic status in patients with established coronary artery disease: data from the Heart and Soul Study. J Nutr 138:1135–1140
Newnham JP, Evans SF, Michael CA, Stanley FJ, Landau LI (1993) Effects of frequent ultrasound during pregnancy: a randomised controlled trial. Lancet 342:887–891
Mori T, Burke V, Puddey I, Watts G, O′Neal D, Best J, Beilin L (2000) Purified eicosapentaenoic acid and docosahexaenoic acid have differential effects on serum lipids and lipoproteins, LDL—particle size, glucose and insulin, in mildly hyperlipidaemic men. Am J Clin Nutr 71:1085–1094
Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Warnick GR, Albers JJ (1978) A comprehensive evaluation of the heparin–manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res 19:65–76
Takahashi O, Glasziou PP, Perera R, Shimbo T, Suwa J, Hiramatsu S, Fukui T (2010) Lipid re-screening: what is the best measure and interval? Heart 96:448–452
da Luz PL, Favarato D, Faria-Neto JR Jr, Lemos P, Chagas ACP (2008) High ratio of triglycerides to HDL-cholesterol predicts extensive coronary disease. Clinics 63:427–432
O’Sullivan TA, Ambrosini GL, Beilin LJ, Mori TA, Oddy WH (2010) Dietary intake and food sources of fatty acids in Australian adolescents. Nutrition (in press) [Epub ahead of print]. doi:10.1016/j.nut.2009.1011.1019
Di Candilo KG, Oddy WH, Miller M, Sloan N, Kendall GE, de Klerk NH (2007) Follow-up phone-calls increase nutrient intake estimated by three-day food diaries in 13 year old participants of the Raine study. Nutr Diet 64:165–171
Ambrosini G, O’Sullivan T, de Klerk N, Beilin LJ, Oddy WH (2010) Relative validity of adolescent dietary patterns: a comparison of a FFQ and 3 d food record. Brit J Nutr (in press)
Tanner J (1962) Growth at adolescence: with a general consideration of the effects of hereditary and environmental factors upon growth and maturation from birth to maturity. Blackwell, Oxford
Duke PM, Litt IF, Gross RT (1980) Adolescents’ self-assessment of sexual maturation. Pediatrics 66:918–920
Rowland T, Rambusch J, Staab J, Unnithan V, Siconolfi S (1993) Accuracy of physical working capacity (PWC170) in estimating aerobic fitness in children. J Sports Med Phys Fitness 33:184–188
Hands B, Larkin D, Parker H, Straker L, Perry M (2009) The relationship among physical activity, motor competence and health-related fitness in 14-year-old adolescents. Scand J Med Sci Sports 19:655–663
Clayton E, Hanstock T, Hirneth S, Kable C, Garg M, Hazell P (2008) Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids 43:1031–1038
Sands SA, Reid KJ, Windsor SL, Harris WS (2005) The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids 40:343–347
Friedman AN, Moe SM, Perkins SM, Li Y, Watkins BA (2006) Fish consumption and omega-3 fatty acid status and determinants in long-term hemodialysis. Am J Kidney Dis 47:1064–1071
Ramel A, Pumberger C, Martinéz JA, Kiely M, Bandarra NM, Thorsdottir I (2009) Cardiovascular risk factors in young, overweight, and obese European adults and associations with physical activity and omega-3 index. Nutr Res 29:305–312
Hwang I, Cha A, Lee H, Yoon H, Cho B, Lee S, Park Y (2007) N-3 polyunsaturated fatty acids and atopy in Korean preschoolers. Lipids 42:345–349
De Groote G, De Laporte A, Dhondt G, Christophe A (2008) Improvement in the plasma omega-3 index by the use of a fish oil-enriched spread. Ann Nutr Metab 53:23–28
Janssens JP, Shapira N, Debeuf P, Michiels L, Putman R, Bruckers L, Renard D, Molenberghs G (1999) Effects of soft drink and table beer consumption on insulin response in normal teenagers and carbohydrate drink in youngsters. Eur J Cancer Prev 8:289–296
Assy N, Nasser G, Kamayse I, Nseir W, Beniashvili Z, Djibre A, Grosovski M (2008) Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can J Gastroenterol 22:811–816
Liu S, Manson JE, Stampfer MJ, Holmes MD, Hu F, Hankinson S, Willett WC (2001) Dietary glycemic load assessed by food-frequency questionnaire in relation to plasma high-density-lipoprotein cholesterol and fasting plasma triacylglycerols in postmenopausal women. Am J Clin Nutr 73:560–566
National Heart Foundation (2008) Position statement: fish, fish oils, n-3 polyunsaturated fatty acids and cardiovascular health
Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA (2008) EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis 197:821–828
Cooney MT, Dudina A, De Bacquer D, Wilhelmsen L, Sans S, Menotti A, De Backer G, Jousilahti P, Keil U, Thomsen T, Whincup P, Graham IM (2009) HDL cholesterol protects against cardiovascular disease in both genders, at all ages and at all levels of risk. Atherosclerosis 206:611–616
Abeywardena MY, Head RJ (2001) Longchain n-3 polyunsaturated fatty acids and blood vessel function. Cardiovasc Res 52:361–371
Cicero AFG, Ertek S, Borghi C (2009) Omega-3 polyunsaturated fatty acids: their potential role in blood pressure prevention and management. Curr Vasc Pharmacol 7:330–337
McNamara RK, Able J, Jandacek R, Rider T, Tso P (2009) Gender differences in rat erythrocyte and brain docosahexaenoic acid composition: role of ovarian hormones and dietary omega-3 fatty acid composition. Psychoneuroendocrinology 34:532–539
Childs CE, Romeu-Nadal M, Burdge GC, Calder PC (2008) Gender differences in the n-3 fatty acid content of tissues. Proc Nutr Soc 67:19–27
Regitz-Zagrosek V, Lehmkuhl E, Weickert M (2006) Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol 95:136–147
Acknowledgments
We would like to express our gratitude to all the Raine Study participants and their families, and the Raine Study Team for cohort co-ordination and data collection. We thank Royal Perth Hospital laboratories for conducting biochemistry assays and Mr. Peter Jacoby for statistical assistance. We acknowledge the NH&MRC for their long term contribution to funding the study over the last 20 years, and the University of Western Australia (UWA), Raine Medical Research Foundation, UWA Faculty of Medicine, Dentistry and Health Sciences, The Telethon Institute for Child Health Research and the Women and Infants Research Foundation for providing funding for Core Management of the Raine Study. We also acknowledge support from the Telstra Research Foundation, the Western Australian Health Promotion Foundation, the Australian Rotary Health Research Fund and the National Heart Foundation of Australia and Beyond Blue. Biological specimens were funded by NH&MRC (Beilin et al., ID 403981) and RBC fatty acid analysis was funded from the NH&MRC Program Grant (Stanley et al., ID 003209).
Conflict of interest
The authors have no conflict of interests to disclose.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
O’Sullivan, T.A., Ambrosini, G.L., Mori, T.A. et al. Omega-3 Index Correlates with Healthier Food Consumption in Adolescents and with Reduced Cardiovascular Disease Risk Factors in Adolescent Boys. Lipids 46, 59–67 (2011). https://doi.org/10.1007/s11745-010-3499-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-010-3499-8