Abstract
An open-labeled randomized trial with parallel groups was carried out to study the effects of Dif1stat® (Monascus purpureus–Linear aliphatic alcohols–Niacin) in the treatment of primary moderate hypercholesterolemia. The trial lasted 8 months. The patients, males and females, were assigned to two groups: A (#130), treated with diet, and B (#110) submitted to diet + Dif1stat®. After 4 months, group A did not show significant changes in Total cholesterol (TC), LDL-cholesterol (LDLC), HDL-cholesterol (HDLC) or non-HDL-cholesterol (non-HDLC). The same group, showed a reduction in TC (–22%), LDLC (–30%) and non-HDLC (–27%) after 8 months (P ≤ 0.001). After 4 months, TC (–21.3%), LDLC (–29%), and non-HDLC (–26%) were significantly lowered in group B (P ≤ 0.001). In group B, TC, LDLC and non-HDLC showed a further reduction after 8 months: –29.4, –38 and –37%, respectively (P ≤ 0.001). Even triglycerides (TG) decreased significantly (–33%) (P ≤ 0.001). After 8 months, group B showed a significant reduction of TG (–33%) (P ≤ 0.001), when compared to group A. Some safety parameters were significantly reduced in both groups: AST and γ-GT in group A after 4 and 8 months, as well as ALT, AST and γ-GT in group B after 8 months (P ≤ 0.001). Dif1stat®, given with a suitable diet, was well tolerated in the long-term and induced an anti-atherogenic plasma lipid and lipoprotein profile, in patients with moderate hypercholesterolemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Monascus purpureus (MP) is a fungus traditionally used in China to produce rice wine. It induces the fermentation of cellulose, maltose, fructose and glucose but not of sugar cane. On the other hand, fermentation is due to two microorganisms: MP and a kind of mold. The first one changes starch into molecules of sugar while the second makes it possible to transform such molecules into alcohol. MP is used in China in the treatment of lipid disorders. In Western countries, the use is mainly limited to the pigmentation of meat, fish, cheese, alcoholic drinks and cured meats [1–4]. The hypocholesterolemic efficacy of MP was evaluated through experimental [5–8] and clinical [9–13] trials. By acting through the direct inhibition of 3-hydroxy-3-methylglutaryl coenzymeA reductase, MP could partially have the same effect as statins [14]. In order to enhance agent safety by giving the lowest dose without losing its hypocholesterolemic effect, MP was combined with Linear aliphatic alcohols (LAAs). LAAs show a synergic effect with MP as they induce the down-regulation of 3-hydroxy-3-methylglutaryl coenzymeA reductase [15]. MP and LAAs seem to have lowering effect on cholesterol, but not on TG. Niacin (N) was added because of its well-known hypotriglyceridemic effect. If given in high doses, N also induces an increase in HDLC [16, 17]. In this study Dif1stat® effects have been evaluated on a long-term basis in patients with primary moderate hypercholesterolemia.
Aim of the Study
Experimental Design
The target of this randomized trial for parallel groups was to evaluate the efficacy of adding MP–LAAs–N (Dif1stat®), 1 capsule/day [Composition: MP, dry extract, 200 mg (corresponding to 3 mg of mevinolin) + LAAs, 10 mg + N, 27 mg] to the treatment of patients with primary moderate hypercholesterolemia (lipoprotein phenotype IIa). One group (A) was treated with hypolipidemic diet according to adult treatment panel III (ATPIII) criteria [18]. In the second group (B) diet was supplemented with Dif1stat®. The study lasted 8 months.
Patients
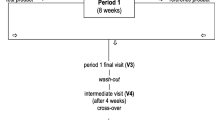
The trial included 240 patients randomly assigned into two groups. The first group (group A # 130) was treated with diet only. In the second group (group B # 110) a capsule of Dif1stat® a day was added to the diet (Fig. 1). The average patient age was 56.5 ± 9 years. The average BMI was 22.3 ± 8.5 kg/cm² (Table 1). All the patients were under primary prevention with an overall coronary risk lower than 20% according to the Framingham algorithm [19]. All the patients were given an hypocholesterolemic diet in accordance with ATPIII guidelines (30% of total calories represented by lipids, less than 10% of them being saturated fats, 19% of proteins, 52% of carbohydrates for a total amount of 1,500/1,800 Kcal a day). The compliance to the diet was assessed every 2 months by two dieticians by way of 24-h dietary recall.
Exclusion Criteria
The subjects involved in the trial did not show clinical and hematological evidence of hepatic, kidney or thyroid disorders. There was no clinical evidence of cardiovascular disease, hypertension, diabetes or obesity. The patients were not taking lipid-lowering agents or drugs affecting lipid metabolism such as: β-blockers, diuretics, and corticosteroids.
Laboratory Methods
Clinical laboratory measurements were performed in a certified central university hospital laboratory. Blood samples for lipid measurements were taken from peripheral veins after overnight fasting (12 h). Plasma TC and TG levels were determined by enzyme assay (Cholesterol and Triglycerides Tests TM Instrumentation Laboratory Company-Lexington, MA, USA). The plasma HDLC level was determined with a combined immune and enzymatic assay (HDL Cholesterol Test TM Instrumentation Laboratory Company-Lexington, MA, USA). The plasma LDLC level was calculated according to the formula of Friedewald: LDLC = TC – (HDLC + TG/5) [20]. The plasma non-HDLC level was calculated according to the following formula: non-HDLC = TC–HDLC. Laboratory safety parameters were determined by usual enzymatic and chemical methods. The blood cell count was determined with a Beckman Coulter ACT Diff. (Beckman Coulter, S.p.A, Milan, Italy-EU).
Ethics
Informed consent was obtained from all patients, according to the recommendations of the declaration of Helsinki guiding physicians in biomedical research involving human subjects. Adopted by the 18th World Medical Assembly, Helsinki, Finland, June 1964, amended by the 29th World Medical Assembly, Tokyo, Japan, October 1975, the 35th World Medical Assembly, Venice, Italy, October 1983, and the 41st World Medical Assembly, Hong Kong, September 1989. The work was approved by the ethical panel of our institution.
Statistical Analysis
Statistical analysis was performed according to parametric tests, depending on parameters under evaluation. All results are expressed as means ± SD. Within group and between groups differences were tested for statistical significance using Student’s t test for paired data. The percent variation (Δ%) of mean lipid and lipoprotein levels in plasma was also estimated. Patients were randomized according to a list created by a random number generator included in the statistical package (SPSS version 15.0—Statistical Product and Services Solutions, SPSS Inc., Chicago, IL, USA).
Results
Plasma TC, LDLC, HDLC, TG, non-HDLC, ALT, AST, γGT, CK, creatinine, urea and the fibrinogen profile at T0, are shown in Table 2. Between groups, statistically significant differences at baseline were not observed. Neither significant changes in lipid nor lipoprotein levels in plasma were observed after 4 months (T1) in group A. After 8 months (T2), significant reductions in plasma TC (Δ%: –22), LDLC (Δ%: –30) and non-HDLC (Δ%: –27) (P ≤ 0.001) levels were observed in the same group; TG (Δ%: –8.4) and HDLC (Δ%: –5.5) were only slightly lower (Table 3; Fig. 2). Table 3 and Fig. 3 show the results obtained after Dif1stat® addition to the diet in group B. After 4 months (T1), the addition of Dif1stat® to the diet had induced a significant reduction in TC (Δ%: –21.3), LDLC (Δ%: –29) and non-HDLC (Δ%: –26) (P ≤ 0.001). Significant differences in TG (Δ% :–6) and HDLC (Δ%: –2) were not observed. After 8 months (T2), the change of TC (Δ%: –29.4), LDLC (Δ% –38), non-HDLC (Δ%: –37%) and TG (Δ%: –33%) in plasma showed a further increase(P ≤ 0.001). HDLC was only slightly increased (Δ%: +2) when compared to T0. The variation of safety parameters in plasma in both groups is shown in Table 4. A change in plasma profile of AST (Δ%: –29.6; Δ%: –59) and γGT (Δ%: 25; Δ%: 33) was observed in group A after 4 (T1), and 8 (T2) months of treatment, respectively. The above mentioned variations were statistically significant when compared to the basal values (P ≤ 0.001). Statistically significant reductions of ALT (Δ%: –21), AST (Δ%: 43) and γGT (Δ%: –33%) (P ≤ 0.001) levels were observed also in group B, but only after 8 months of treatment (T2). The change of lipid and lipoprotein profile between groups (A vs. B) after 4 (T1) and 8 (T2) months, is shown in Table 5, and Fig. 4. After 8 months (T2), the addition of Dif1stat® to the diet in group B had induced a significant reduction in TG: (B) Δ% –33 versus (A) Δ% –8.4 (P ≤ 0.001). Side effects were not observed in either of the groups.
Plasma concentrations of total cholesterol (TC), triglycerides (TG), low-density lipoprotein-cholesterol (LDLC), high-density lipoprotein-cholesterol (HDLC) and non-HDLC in patients with mild hypercholesterolemia values at baseline (T0), after 4 (T1) and 8 (T2) months treatment with Diet plus Dif1Stat. *P ≤ 0.001
Discussion
Available studies have highlighted that statins have a good lowering effect on C-reactive protein but not on lipoprotein (a) [Lp(a)] [21–24]. The extract of MP seems to be able to reduce the level of Lp(a) [25]. However, the effects of Dif1stat® on the above mentioned inflammatory and lipid parameters were not taken into account in our study. A trial with 60 patients affected by coronary heart disease assigned to two groups (extract of MP 1,200 mg/day vs. placebo for 6 weeks) showed that the extract of MP can significantly inhibit the postprandial increase of TG in blood [26]. The effect of MP on postprandial TG is more evident than the presence of LAAs could suggest. MP seems to be able to reduce TG by 32, 38, and 43%, after 2, 4, and 6 h, respectively. Some authors suggested that the effect of MP is not related only to the presence of LAAs but probably to a synergy between the various therapeutic agents. Some studies have demonstrated that the combination of MP, LAAs and N induces a significant decrease of TC, LDLC and TG. Castaño et al. [10] have carried out a randomized, double blind, controlled trial on patients affected by hypercholesterolemia with lipoprotein phenotype IIa. Two different doses of policosanol were given: 20 mg/day (# 29 patients) and 40 mg/day (# 30 patients). After 24 weeks, the two groups showed a reduction in LDLC of 27.1 and 28.1%, respectively. HDLC showed a statistically significant increase of 17.6 and 17.0%, respectively. The LDLC/HDLC ratio changed by 37.2 and 36.5%. Lastly, TG decreased by 12.7 and 15.6%, respectively. The authors concluded that their trial failed to highlight any difference possibly related to the dosages, at least for the cholesterol-lowering effect of policosanol. In other words, the 40 mg/day dose given, did not demonstrate any further significant cholesterol-lowering effect. In the trial carried out by Cicero et al. [12] 111 patients with low cardiovascular risk profile (<20% acc. to the Framingham algorithm) treated with Dif1stat, were compared to 20 patients taking pravastatin at low doses. The first group showed a significant reduction in TC, LDLC and TG, without side effects. In particular, the reduction of LDLC was 20%; the same observed in the group with moderate hypercholesterolemia treated with pravastatin. An interesting meta-analysis by Chen JT highlighted the efficacy and safety of vegetable sterols and stanols versus policosanol in terms of taking advantage of both lipid-lowering and anti-atherogenic effects [11]. The impact on LDLC was evaluated as a primary endpoint in 4,596 patients belonging to 52 randomized and controlled trials. The weighed average of the percentage differences underlined the following variations: –11% for the vegetable sterols and stanols given at a dosage of 3.4 g/day (range 2–9 g/day; # 893 patients) in 23 trials, versus –23.7% for policosanol given at a dosage of 12 mg/day (range 5–40 mg/day; # 1,528 patients). The differences in both groups versus the placebo group were statistically significant. The absolute decrease in LDLC was higher in the group treated with policosanol: –24 versus –10% (P ≤ 0.0001). The activity of policosanol was also more pronounced on TC, HDLC and TG, inducing a significant improvement in the LDL/HDL ratio. The authors concluded that not only policosanol, but also vegetable sterols and stanols were effective and safe. However, policosanol showed a greater LDLC-lowering effect. A multicenter, randomized, double-blind controlled trial versus placebo carried out by Berthold HK on 143 patients with hypercholesterolemia, was reported in 2006 [13]. The patients had levels of LDLC ≥ 150 mg/dL with no one or only 1 cardiovascular risk factor, or with a documented coronary heart disease, or with baseline levels of LDLC between 150 and 189 mg/dL and two or more cardiovascular risk factors. The patients were treated with policosanol at dosages of up to 80 mg/day. They were assigned to five groups, and the figures analyzed according to the intention-to-treat statistical model. The study failed to demonstrate a decrease of LDLC greater than 10% compared to the values at the baseline. Moreover, TC, HDLC, TG, Lp(a) and the LDLC/HDLC ratio did not demonstrate statistically significant change. The authors concluded that policosanol given at a usual or higher dose, failed to show any substantial effect in terms of reduction of lipids and lipoproteins in plasma compared to the patients on placebo. In our study, the administration of Dif1stat® with a controlled diet induced a significant decrease in plasma of TC, LDLC, non-HDLC and TG, in patients with moderate hypercholesterolemia compared to those treated only with the diet. Interestingly, the diet had positive effects on both groups (A and B). The result regarding the reduction in cholesterolemia in group A after 4 months, goes beyond every possible expectation considering that the control group underwent only dietetic treatment. Nevertheless, it is necessary to observe that the patients were extremely motivated by the investigators and the dieticians who administered the 24-h dietary recall, strongly stimulating the connection with diet. There was much insistence on the modification of life-style and dietary habits. A preventive operation on the consumption of alcoholic beverages was also carried out. The above mentioned recommendations and a hypocaloric diet—1,500/1,800 kcal/day—containing cholesterol equal to 200 mg/day, achieved not only the reduction in the plasmatic concentration of cholesterolemia, but also a discreet ponderal reduction, although not statistically significant. The reduction in weight also contributed to the hypolipidemic effects of the diet. The patients did not have any input concerning the consumption of functional foods, neither from doctors nor dieticians. Moreover, the good result obtained is likely attributed to the detailed operation put into direct action to modify life-style and above all, dietary habits. As reasonably expected, Dif1stat® had better results in terms of reduction of lipids and lipoproteins, and particularly of TG, when both groups were compared after 8 months of treatment. Dif1stat®, in association with a suitable diet, was well tolerated in the long-term and induced an anti-atherogenic plasma profile of lipids and lipoproteins in patients with moderate hyperlipidemia.
It is to be emphasized that up to now, only one patient treated with cyclosporine after a kidney transplantation developed a modest asymptomatic rhabdomyolysis while taking MP [27]. However, it is well known that cyclosporine enhances the cytotoxicity of many drugs by strongly inhibiting the liver CYP3A4 enzymatic system. It will be interesting to study the effect of Dif1stat® with a longer administration (>1–2 years) to definitely assess if it may be considered as a valuable lipid-lowering agent for primary prevention of cardiovascular risk, or if it may be preferably used as a therapeutic alternative for statin-intolerant patients with moderate hypercholesterolemia, [28], or not.
Abbreviations
- LAAs:
-
Linear aliphatic alcohols
- MP:
-
Monascus purpureus
- N:
-
Niacin
- TC:
-
Total cholesterol
- LDLC:
-
LDL-cholesterol
- HDLC:
-
HDL-cholesterol
- non-HDLC:
-
non-HDL-cholesterol
- TG:
-
Triglycerides
- ATPIII:
-
Adult treatment panel III
- BMI:
-
Body mass index
- Lp(a):
-
lipoprotein (a)
- CYP3A4:
-
Cytochrome P450 3A4
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- γGT:
-
Gamma-glutamyl-transpeptidase
- CK:
-
Creatine kinase
References
Wang TH, Lin TF (2007) Monascus rice products. Adv Food Nutr Res 53:123–159 Review
Juzlova P, Rezanka T, Martinova L, Kren V (1996) Long-chain fatty acids from Monascus purpureus. Phytochemistry 43(1):151–153
Heber D, Yip I, Ashley JM, Elashoff DA, Elashoff RM, Go VL (1999) Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am J Clin Nutr 69(2):231–236
Caron MF, White CM (2001) Evaluation of the antihyperlipidemic properties of dietary supplements. Pharmacotherapy 21:481–487 2
Li C, Zhu Y, Wang Y, Zhu JS, Chang J, Kritchevsky D (1997) Monascus purpureus-fermented rice (red yeast): a natural food product that lowers blood cholesterol in animal models of hypercholesterolemia. Nutr Res 18:71–81
Wang IK, Lin-Shiau SY, Chen PC, Lin JK (2000) Hypotriglyceridemic effect of anka (a fermented rice product of Monascus sp.) in rats. J Agric Food Chem 48:3183–3189
Wei W, Li C, Wang Y, Su H, Zhu J, Kritchevsky D (2003) Hypolipidemic and anti-atherogenic effects of long-term Cholestin (Monascus purpureus-fermented rice, red yeast rice) in cholesterol fed rabbits. J Nutr Biochem 14(6):314–318
Lee CL, Tsai TY, Wang JJ, Pan TM (2006) In vivo hypolipidemic effects and safety of low dosage Monascus powder in a hamster model of hyperlipidemia. Appl Microbiol Biotechnol 70(5):533–540
Wang J, Lu Z, Chi J, Wang W, Su M, Kou W, Yu P, Yu L, Chen L, Zhu JS, Chang J (1997) Multicenter clinical trial of the serum lipid-lowering effects of a Monascus purpureus (red yeast) rice preparation from traditional Chinese medicine. Curr Ther Res 58(12):964–978
Castaño, Mas R, Fernández L, Illnait J, Gámez R, Alvarez E (2001) Effects of policosanol 20 versus 40 mg/day in the treatment of patients with type II hypercholesterolemia: a 6-month double-blind study. Int J Clin Pharmacol Res 21(1):43–57
Chen JT, Wesley R, Shamburek RD, Pucino F, Csako G (2005) Meta-analysis of natural therapies for hyperlipidemia: plant sterols and stanols versus policosanol. Pharmacotherapy 25(2):171–183
Cicero AF, Brancaleoni M, Laghi L, Donati F, Mino M (2005) Antihyperlipidaemic effect of a Monascus purpureus brand dietary supplement on a large sample of subjects at low risk for cardiovascular disease: a pilot study. Complement Ther Med 13(4):273–278
Berthold HK, Unverdorben S, Degenhardt R et al (2006) Effect of policosanol on lipid levels among patients with hypercholesterolemia or combined hyperlipidemia: a randomized controlled trial JAMA 295(19):2262–2269
Endo A, Monakolin K (1980) A new hypocholesterolemic agent that specifically inhibits 3-hydroxy-3-methylglutaryl coenzymeA reductase. J Antibiot 33(3):334–336
McCarthy MF, Hung M, Sikorska M, Borowy-Borowski H (2002) Policosanol safely down-regulates HMG-CoA reductase—potential as a component of the Esselstyn regimen. Med Hypotheses 59:268–279
Malik S, Kashyap ML (2003) Niacin, lipids, and heart disease. Curr Cardiol Rep 5:470–476
Ganji SH, Kamanna VS, Kashyap ML (2003) Niacin and cholesterol: role in cardiovascular disease. J Nutr Biochem 6:298–305
(2001) Executive Summary of the Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 285(19):2486–2497
Anderson KM, Wilson PWF, Odell PM, Kannel WB (1991) An updated coronary risk profile. A statement for health professionals. Circulation 83:356–362
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Plenge JK, Hernandez TL, Weil KM, Poirier P, Grunwald GK, Marcovina SM, Eckel RH (2002) Simvastatin lowers C-reactive protein within 14 days: an effect independent of low-density lipoprotein cholesterol reduction. Circulation 106(12):1447–1452
Cobbaert C, Jukema JW Zwinderman AH, Withagen AJ, Lindemans J, Bruschke AV (1997) Modulation of lipoprotein(a) atherogenicity by high density lipoprotein cholesterol levels in middle-aged men with symptomatic coronary artery disease and normal to moderately elevated serum cholesterol. Regression Growth Evaluation Statin Study (REGRESS) Study Group. J Am Coll Cardiol 30:1491–1499
Gonbert S, Malinsky S, Sposito AC, Laouenan H, Doucet C, Chapman MJ et al (2002) Atorvastatin lowers lipoprotein(a) but not apolipoprotein(a) fragment levels in hypercholesterolemic subjects at high cardiovascular risk. Atherosclerosis 48:1454–1459
Marcovina SM, Koschinsky ML, Albers JJ, Skarlatos S (2003) Report of the National Heart, Lung, and Blood Institute Workshop on Lipoprotein (a) and Cardiovascular Disease: recent advances and future directions. Clin Chem 49(11):1785–1796
Liu L, Zhao SP, Cheng YC, Li YL (2003) Xuezhikang decreases serum Lipoprotein(a) and C-Reactive Protein concentrations in patients with coronary heart disease. Clin Chem 49(8):1347–1352
Zhao SP, Liu L, Cheng YC, Li YL (2003) Effect of Xuezhiang, a cholestin extract, on reflecting postprandial triglyceridemia after a high-fat meal in patients with coronary heart disease. Atherosclerosis 168:375–380
Prasad GV, Wong T, Meliton G, Bhaloo S (2002) Rhabdomyolysis due to red yeast rice (Monascus purpureus) in a renal transplant recipient Transplantation 74(8):1200–1201
Becker DJ, Gordon RY, Steven C, Halbert SC, French B, Patti B, Morris PB, Daniel J, Rader DJ (2009) Red yeast rice for dyslipidemia in statin-intolerant patients. Ann Intern Med 150(12):830–839
Conflict of interest statement
The author(s) certify that they have no affiliation with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in this manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Stefanutti, C., Mazza, F., Vivenzio, A. et al. Combined Treatment with Dif1stat® and Diet Reduce Plasma Lipid Indicators of Moderate Hypercholesterolemia More Effectively than Diet Alone: A Randomized Trial in Parallel Groups. Lipids 44, 1141–1148 (2009). https://doi.org/10.1007/s11745-009-3368-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-009-3368-5