Abstract
Modern diets are often deficient in ω-3 fatty acids and additional dietary sources of ω-3 fatty acids are useful. In order to investigate the molecular basis of the high accumulation of the ω-3 fatty acid, α-linolenic acid (18:3), in three different plants, flax (Linum usitatissimum), Dracocephalum moldavica, and Perilla frutescens ω-3 desaturase activity, transcript levels, and 18:3 in-vivo synthesis were examined. The 18:3 content was found to be higher at the later developmental stage of D. moldavica (68%) compared with P. frutescens (59%) and flax (45%) cotyledons. The 18:3 and 18:2 contents in both PC and TAG were determined during various stages of seed development for all three plants in addition to soybean (Glycine max). Northern blot analysis data of three different stages of D. moldavica, flax, and P. frutescens compared with moderately low 18:3 producers, soybean (Glycine max), and Arabidopsis thaliana and Brassica napus, (8–10% 18:3) at a stage of zygotic embryo development of high triglyceride synthesis showed that ω-3 desaturase mRNA levels were higher in all three high 18:3 producers, flax, D. moldavica and P. frutescens. This indicates that the high level of α-linolenic acid in TAG may be largely controlled by the level of ω-3 desaturase gene expression. However, the PC versus TAG fatty acid composition data suggested that along with ω-3 desaturase other enzymes also play a role in 18:3 accumulation in TAG, and the high accumulators have a selective transfer of α-linolenic acid into TAG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The diets of people in some countries come close to the apparent ω-3/ω-6 fatty acid ratio of diet during human evolution: such as the traditional diets of Greece and Japan. In both countries the rate of death due to cardiovascular disease is among the lowest. It is now recognized that there is a need to return ω-3 fatty acids into the food supply, both for normal growth and development and for the prevention and management of chronic diseases [1]. Human beings evolved on a diet in which the ratio of omega-6/omega-3 EFA was about 1, whereas in the western diets the ratio is 15/1 to 16.7/1. Increased use of certain plant oils and modern day agriculture has led to a decrease in omega-3 fatty acids and increases in omega-6 fatty acids [2–4] Linoleic acid and α-linolenic acid are not interconvertible in animals and compete for the rate limiting delta-6 desaturase in the synthesis of long chain PUFA. Among the cardiovascular benefits of increased omega-3 fatty acids in the diet is the prevention of cardiac arrhythmias [4]. Already eggs high in omega-3 fatty acids are available on the market [5]. All these factors increase proportionally with an increase in the omega-3 fatty acid intake, including either ALA or EPA, but especially DHA.
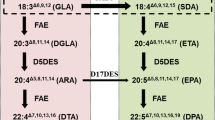
Seed oils are composed primarily of triacylglycerols (TAGs), which are glycerol esters of fatty acids. The primary fatty acids in the TAGs of oilseed crops are 16–18 carbons in length and contain 0–3 double bonds. Palmitic acid (16:0), oleic acid (18:1), linoleic acid (18:2), and linolenic acid (18:3) predominate. In this study we used soybean (G. max) as a moderate linolenic acid producer and three different high linolenic acid producers: flax (Linum usitatissimum) which has a high content of linolenic acid in the triacylglycerol (TAG), normally more than 45% of the total fatty acid content [6–8], Perilla frutescens oil contains 50–64% of 18:3 [9–11], and Dracocephalum moldavica contains 59–68% 18:3 [9, 12, 13].
Flax (L. usitatissimum) oil content ranges from 40 to 52% and 40 to 64% of this oil is linolenic acid depending on the flax variety [6–8]. D. moldavica contains 25–30% oil and 59–68% of it is 18:3 [9, 12, 13]. The oil content of P. frutescens is 35–45%, and contains 50–64% of 18:3 [9–11] It is known that ω-3 fatty acids are synthesized from ω-6 fatty acid precursor membrane lipids in plastids and the ER by ω-3 desaturases [14].
The objective of this study was to investigate the biochemical basis of α-linolenic acid accumulation in developing seeds of flax (L. usitatissimum), D. moldavica and P. frutescens as high linolenate accumulators compared to the moderate accumulators soybeans and canola.
Materials and Methods
Plant Materials
Soybean (G. max) cv. ‘Jack’, Brassica napus cv. ‘Westar’, flax (L. usitatissimum) cv. ‘NorLin’, P. frutescens and D. moldavica plants were grown in the greenhouse at the University of Kentucky, Lexington. Dicot embryogenesis is divided into five general stages: globular, heart, cotyledon, maturation, and dormancy. By the end of the cotyledon stage and during maturation primary storage products including lipids accumulate in preparation for seed germination later [15]. In this study we focused on cotyledon to maturation stages and classified them to six different stages based on seed size, color, and weight. Seed coats were carefully removed; embryo length and fresh weight were measured. Samples were frozen in liquid N2, lyophilized and the dry weight was measured.
Fatty Acid and Lipid Analysis
The overall fatty acid composition of seed tissues of different cotyledon developmental stages of the investigated plants were analyzed by modifications of the procedure of [16]. About 1–10 mg lyophilized seed samples with added heptadecanoic acid (17:0) were placed into 2 mL of 2% (v/v) H2SO4 in methanol. The samples were ground finely, and then heated at 80 °C until the volume was reduced to approximately 0.5 mL (2 h). One and one-half milliliters of hexane containing 0.001% butylated hydroxytoluene (BHT) were added and the mixture was vortexed vigorously. The fatty acid methyl esters in the hexane layer were separated by gas chromatography on a Hewlett-Packard 0.25 mm I.D. × 0.33 mm × 10 m FFAP column and quantified using a flame ionization detector. A Hewlett-Packard 5890A gas chromatograph was programmed for an initial temperature of 140 °C for 1 min followed by increases of 12 °C/min to 210 °C and to 235 °C. The final temperature was maintained for 8 min. The injector and detector temperatures were 220 and 250 °C, respectively. Helium was used as the carrier gas with a flow rate of 10 mL/min. Total lipids were extracted from seed tissue as described by [17]. Individual lipids were separated by one-dimensional thin layer chromatography 20 × 20 cm TLC plate (LK60 silica gel 60 A°, Whatman) by the method of [18]. TLC plates were developed in two solvent systems. Phospholipids and glycolipids were separated in chloroform:methanol:water (65:25:4, v/v). Neutral lipids were separated in hexane:diethyl ether:acetic acid (100:100:2, v/v) [17, 19]. Lipids were located by spraying the plates with a solution of 0.005% primulin in 80% acetone, followed by visualization under UV light. In order to determine the fatty acid composition of individual lipids, ten μg of 19:0 was added to the silica gel of each lipid band before scraping, transferred to a tube containing 2 mL of 2% (v/v) H2SO4 in methanol, and fatty acid methyl esters were prepared and analyzed as described above. Radiolabeled 18:2 CoA was synthesized using 14C linoleic acid and co-enzyme A by the method described by [20].The labeled 14C 18:2-CoA was fed to the embryos on a μL (0.0028 μCi/μL) per gram fresh weight basis and incubated for 90 min in light as described by [8]. After incubation the embryos were washed to remove unabsorbed radioactivity and crushed in chloroform/methanol (2:1) to extract lipids. Reverse phase chromatography was used to separate the 18:2 from 18:3. The free fatty acids were methylated by diazomethane and the esterified fatty acids by sodium methoxide. The samples along with the standards were loaded on to a KV18 silica gel plate and ran in a solvent system consisting of acetonitrile/acetic acid/water (70:10:10). The image was developed in a phosphorimager. The standards were identified and thus used to identify the location of the radioactivity, by placing the plate into an iodine chamber.
RNA Isolation
RNA was isolated using the Trizol Reagent (Life Technologies, GibcoBrl). 50–100 mg seed tissues of different cotyledon developmental stages of tested plants were frozen in liquid N2, homogenized in 1 mL Trizol reagent, transferred into microcentrifuge tubes and incubated for 5 min at room temperature. 0.2 mL chloroform per 1 mL of Trizol was added; tubes were vigorously shaken for 15 s and incubated at room temperature for 3 min. Samples were centrifuged at 12,000×g for 15 min at 2–8 °C. The upper aqueous phase containing RNA was collected in fresh tubes; RNA was precipitated with 0.5 mL of isopropyl alcohol and samples incubated at room temperature for 10 min. Samples were centrifuged at 12,000×g for 10 min, and the RNA pellet was washed once with 1 mL of 75% ethanol. Samples were vortexed and centrifuged at 7,500×g for 5 min. The RNA pellet was air-dried, then dissolved in RNase-free water and incubated for 10 min at 60 °C. The concentration of RNA was estimated by reading the absorbance at 260 nm. The 260/280 ratio of the RNA was 1.9–2.0. The quality of RNA was checked using 1.2% agarose gels. Samples were stored at −80 °C until used.
Northern Blotting
RNA samples (30 μg) were prepared by adding 2.0 μL of 5× formaldehyde gel-running buffer, 3.5 μL 12.3 M formaldehyde, 10 μL formamide, and incubated 15 min at 65 °C. The samples were chilled in ice for 3 min. Samples were immediately loaded on a pre-run denaturing 1% agarose-formaldehyde gel, the electrophoresis was carried out in the presence of 1× formaldehyde gel-running buffer. The gel was washed with 0.05 N NaOH for 20 min, rinsed with RNase-free water, followed by 10× SSC for 45 min. Blotting and transfer of RNA onto the 6× SSC pre-soaked nylon membrane was carried out according to the standard procedure [21]. RNA was fixed to the Zeta-probe nylon membrane by UV cross-linking (UV stratalinker 1800, Stratagene). The B. napus ω-3 desaturase probe was labeled with [α-32P] dCTP using the random priming method. Pre-hybridization and hybridization were carried out at 42 °C following the instructions for using the Zeta-Probe Membrane (Bio-Rad) as outlined by the manufacturer. After hybridization a PhosphorImager system (Molecular Dynamis, 445 SI) was used to visualize the bands obtained. The radioactive probes were stripped by washing the blots three times with 0.1% SDS in 2× SSC at 95 °C, 10 min each.
Results
Fatty Acid Composition in Different Oil Seeds of Moderate and High Linolenate Accumulators During Maturation
The percentage of total fatty acids in flax seeds at different cotyledon developmental stages were analyzed (Fig. 1). Linolenic acid (18:3) accumulated during seed development and reached 46% by the end of maturation, oleic acid increased during maturation until stage IV at 40% and then become steady until the end of the maturation. 18:2 was found to be 45% at the first stage and dropped dramatically during the rest of the developmental stages to reach 10% by the end of the maturation process. Also 16:0 dropped from 25% at the beginning of the cotyledon maturation stage to 10% by the end of seed maturation.
The synthesis of 18:3 takes place rapidly at the beginning of cotyledon development in P. frutescens. The level of 18:2 declines with the increase in 18:3 while the 18:1 content slightly increased during seed maturation but its level remained relatively lower than the case of flax seed. This suggests that Δ-12 desaturase is more efficient in desaturating 18:1–18:2 in P. frutescens than in flax. P. frutescens seed has a high content of α-linolenate (57%) [10, 22, 23] and other fatty acid contents were also similar to reported values. The percentage ratio for both 18:2 and 18:3 in P. frutescens seed during maturation indicates that ω-3 desaturase is very active at the beginning of cotyledon development. However, the 18:3 content reached a steady state at the third stage and remained constant thereafter until the end of seed maturation (Fig. 1). The amount of 18:3 (μmol/mg dry weight) increased during cotyledon developmental stages to reach 1.15 μmol/mg dry weight at the maturation stage.
The accumulation of 18:3 in D. moldavica increased gradually to 68% by the end of seed maturation (Fig. 1). The 18:2 and 18:1 levels are lower at maturity. This suggests that both Δ12 and Δ15 (ω-3) desaturases are very active in desaturation of 18:1 and 18:2 and are largely responsible for the high level of 18:3 in D. moldavica. The accumulation of other FA including 18:1 is minimal as in P. frutescens unlike the accumulation of 18:1 in flax during seed maturation. In all high 18:3 accumulators, the linolenic acid content starts at a moderate level. The percentage of 18:3 is higher at a very early cotyledon developmental stage (first stage) of P. frutescens (35%) compared with flax (25%) and D. moldavica (15%). α-Linolenic acid gradually accumulated in developing cotyledons of all three plants. However, 18:3 content was found to be higher at the late mature developmental stage of D. moldavica (68%) compared with P. frutescens (59%) and flax (45%) cotyledons under our greenhouse growth conditions (Fig. 1).
The ratio of 18:2 and 18:3 in TAG in developing soybean seeds is the opposite to that in the three high 18:3 accumulators previously mentioned, which suggests that the level of desaturation of 18:2 into 18:3 decreases in soybean as the seeds mature (Fig. 2). The unsaturated fatty acid levels in PC remain almost constant throughout seed maturation. The increase in lipid content in soybean seed is accompanied by a shift in lipid composition from PC (membrane lipids) to TAG accumulation (storage lipids) during seed maturation [24, 25]. Figure 2 shows the amount of 18:3 in flax seed increases during cotyledon maturation stages in TAG in comparison to the 18:3 content in PC.
The level of 18:3 in P. frutescens TAG is much higher than in PC, also compared with flax (Fig. 2) the 18:2 levels in P. frutescens is lower in PC, which agree with the fact that 18:3 level in TAG of P. frutescens seed (Fig. 2) is much higher.
A slightly higher 18:3 amount was found in PC of flax seed in the first three stages of cotyledon development compared to the amount of 18:3 in PC of P. frutescens seeds (Fig. 2). However, the percentage of 18:3 in P. frutescens PC was slightly higher (6%) in the first two stages compared to the first two stages in flax PC (0.9–3%). Unlike TAG, the 18:3 content was low in PC throughout seed development in flax and P. frutescens (Fig. 2). An increase in 18:3 content was observed in TAG of developing seeds in both flax and P. frutescens, however, flax seeds showed a gradual increase of 18:3 in TAG throughout all stages of development, while on the other hand a dramatic increase of 18:3 in TAG of P. frutescens seeds was observed during the later stages of development (from 0.15 μmol/mg seed at the second stage up to 0.9 μmol/mg seed at seed maturation). It was found that the second stage of cotyledon development is the optimum stage for monitoring ω-3 desaturase activity so this stage was used for northern blots.
The 18:3 levels are much higher in TAG compared to its level in PC in D. moldavica developing cotyledons while the level of 18:2 is lower in TAG but in PC the amount of 18:2 (μmol/mg seed) is relatively higher during second to third stages of cotyledon development (data not shown). The 18:2 level in PC dropped by the end of maturation of the seed.
ω-3 Desaturase Activity
To investigate whether higher ω-3 desaturase activity is responsible for the higher synthesis of 18:3 fatty acid, which in turn is incorporated in to TAG in higher amounts, the ω-3 desaturase of flax and two moderate accumulators Arabidopsis and soybean were assayed. Feeding studies with 14C labeled 18:2-CoA showed that developing embryos of flax have higher ω-3 desaturase activity compared to Arabidopsis and soybean (Fig. 3). Stage II flax seeds have much higher activity compared to the stage V flax seeds. During stage II 18:3 accumulation is linear and in stage V embryos 18:3 reaches a plateau (Fig. 1).
Microsomal ω-3 Desaturase Transcript Levels
The ω-3 desaturase transcripts levels in stage II flax embryos are much higher compared to soybean embryos of both stages and stage V flax embryos (Fig. 4). These results were also in line with 18:3 fatty acid accumulation in flax seeds (Fig. 1) and also indicated by the ω-3 desaturase activity assay (Fig. 3). The activity assay results show that stage II flax embryos had much higher activity than both stages of soybean embryos and stage V flax. Northern blot analysis data also show developing embryos of P. frutescens have much higher ω-3 desaturase transcript levels than soybean and Arabidopsis (Fig. 5). When three different stages of D. moldavica, P. frutescens and flax, were compared with the moderately low 18:3 producers (8–10%), soybean and Arabidopsis, and B. napus, ω-3 desaturase mRNA levels were also higher in all three high 18:3 producers, flax, D. moldavica and P. frutescens compared with the moderately low 18:3 producers (figure not shown for D. moldavica). However among high 18:3 producer plants, the ω-3 desaturase transcript level was found to be much higher in D. moldavica than in P. frutescens, which in turn has a higher transcription level than flax (Fig. 5).
Northern blot analyses of ω-3 desaturase expression in developing embryos of flax and soybean. RNA was extracted from: B. napus (Br), the second and fifth developmental stage of flax cotyledons (F1, F5) and the second and fifth developmental stage of soybean cotyledons (S2, S5). (Above) B. napus ω-3 desaturase (FAD3) cDNA was used as a probe to check the expression of FAD3 gene (Below) to confirm the amount of RNAs loaded were equal 18S ribosomal DNA was used as probe to measure 18S rRNA expression. Equal RNA loading was also confirmed by measuring RNA concentration spectroscopically at 260 nm
Northern blot analyses of ω-3 desaturase gene expression in developing embryos of B. napus, Arabidopsis, soybean and P. frutescens. RNA was extracted from B. napus (Br), Arabidopsis seed (Ar), soybean seed cotyledon developmental stages 5–6 mm (S1), 7–9 mm (S2), 9–11 mm (S3), Pr1, Pr2, Pr3, are P. frutescens cotyledon at developmental stages (based on size, color, and seed weight) 1, 2 and 3?. (Above) RNAs were blotted on a nylon membrane and probed with B. napus ω-3 desaturase cDNA to check the ω-3 desaturase gene expression (Below) To confirm the amount of RNAs loaded were equal 18S ribosomal DNA was used as probe to measure 18S rRNA expression. Equal RNA loading was also confirmed by measuring RNA concentration spectroscopically at 260 nm
The lipid content and compositions of soybean seeds changes considerably as the embryo progress from the early stages of development to the maturation stage [26]. In soybean the level of 18:3 is higher at the beginning of the cotyledon maturation stage and begins to decline toward the late maturation stages while the 18:2 concentration increases. The opposite is found in all high 18:3 accumulators, with levels of 18:3 increasing during the maturation stages while the 18:2 levels decreased .
In flax (Fig. 3), ω-3 desaturase is highly active in converting 18:2-CoA to 18:3 compared to Arabidopsis and soybean.
Discussion
During the first three stages of cotyledon development, in all high 18:3 producers, linear increases in 18:3 levels were found. This suggests that these stages have the highest ω-3 desaturase activity. The 18:1 levels in both flax and P. frutescens (Fig. 1 and 2) suggests that Δ-12 desaturase is more active in desaturating 18:1–18:2 in P. frutescens than in flax. In D. moldavica 18:3 levels was very high compared with other fatty acids levels suggesting that lipid metabolism in this plant is optimally adjusted for 18:3 accumulation. The level of 18:3 in P. frutescens TAG is much higher than in PC, also compared with flax (Fig. 2) the 18:2 levels in P. frutescens is lower in PC, which is consistent with the high 18:3 level in TAG of P. frutescens seed (Fig. 2). This suggests that desaturation of 18:2– 18:3 by ω-3 desaturase is more efficient in P. frutescens than in flax, which in turn is much higher than in soybean.
The changes in lipid concentration are accompanied by a shift in lipid composition from polar lipids to storage lipids [24]. In soybean the 18:2 levels in TG increase while 18:3 levels decrease. Acyltransferase enzymes catalyze the incorporation of fatty acids into the glycerol backbone to produce different glycerolipids classes including PC and TG [25, 27–29], so that from our data (e.g., Fig. 2) and the selective accumulation of 18:2 in TG of soybeans we can suggest that acyltransferases are selective with 18:2 over 18:3 in soybean seed. However, the absolute amount (μ mol/mg seed) of 18:3 in high producing plants suggests that the incorporation rate into TG is very high which in turn indicates that acyltransferases may selectively incorporate 18:3 into storage lipids.
Northern blot data shows that the ω-3 desaturase gene expression level is higher in high 18:3 producing plants compared to low producers. This indicates that the level of ω-3 desaturase gene expression may largely control the level of α-linolenic acid in TAG, although as mentioned above acyltransferases also appear to be involved. High accumulation of 18:3 correlates with ω-3 desaturase mRNA levels which suggests transcriptional control of fatty acid desaturase genes [30, 31]. However, the PC versus TAG fatty acid composition data suggest that ω-3 desaturase is not the only factor in 18:3 accumulation in TAG, and the high accumulators appear to have a selective transfer of α-linolenic acid into TAG which in turn suggested that certain acyltransferases or transacylases may play an important role in 18:3 accumulation in triacylglycerols. Another possible factor is the speed of flux from phospholipid to TAG as this can compete with 18:2–18:3 desaturation. It was shown in flax cultivars AC Emerson and Vimy that diacylglycerol acyltransferase (DGAT) prefers 18:3-CoA as substrate [32]. The data from both lipid analysis and Northern blot highly suggest that the reduced linolenic acid content in soybean compared with high 18:3 accumulators is mainly due to the lower transcript level of ω-3 desaturase. Byrum et al. [33] found that the reduced 18:3 concentration in the soybean genotype A5 was at least partially the result of a microsomal ω-3 desaturase gene with a reduced activity. Collectively, these results indicate that one can obtain B. napus, soybeans or other oilseed crops that accumulate high ω-3 fatty acid levels simply by increasing expression of the ω-3 desaturase gene by use of a strong promoter or trans-activation. Acyltransferases with specificity for 18:3 are likely to be needed for very high ω-3 fatty acid accumulation, for example, >80%.
Abbreviations
- ACP:
-
acyl carrier protein
- ALA:
-
α-linolenic acid
- DAG:
-
diacylglycerol
- DGAT:
-
diacylglycerol acyl transferase
- DHA:
-
docosohexaenoic acid
- EFA, EPA:
-
eicosapentaenoic acid
- ER:
-
endoplasmic reticulum
- FFAP, PC:
-
phosphatidylcholine
- PUFA:
-
polyunsaturated fatty acid
- TAG:
-
triacylglycerol
References
de Lorgeril M, Salen P (2003) Dietary Prevention of coronary heart disease: Focus on omega-6/omega-3 essential fatty acid balance. In: Simpolus A, Cleland L (eds) Omega-6/omega-3 essential fatty acid ratio: the scientific evidence. Basel, Karger, pp 37–56
Trautwein EA (2001) n-3 Fatty acids—physiological and technical aspects for their use. Eur J Lipid Sci Technol 103:45–55
Sprecher H (2002) The roles of anabolic and catabolic reactions in the synthesis and recycling of polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids 67:79–83
de Lorgeril M, Salen P (2004) Alpha-linolenic acid and coronary heart disease. Nutr Metab Cardiovasc Dis 14(3):162–169
Lewis NM, Seburg S, Flanagan NL (2000) Enriched eggs as a source of N-3 polyunsaturated fatty acids for humans. Poult Sci 79(7):971–974
Kuznetsova NV (1978) Variability of fatty acid contents in seed oil of fiber flax cultivars of different origin. Byulleten Vsesoyuznogo Ordana 59:30–36
Yarosh NP, Megorskaya OM, Ivanova ON (1980) Dynamics of fatty acids in the free and bound lipids in ripening seeds of linseed and flax. Trudy po Prikladnoi Botanike, Genetike i Selektsii 66(3):28–36
Stymne S, Tonnet ML, Green AG (1992) Biosynthesis of linolenate in developing embryos and cell-free preparations of high-linolenate linseed (Linum usitatissimum) and low-linolenate mutants. Arch Biochem Biophys 294(2):557–563
Hagemann JM, Earle FR, Wolff IA (1967) Search for new industrial oils. XIV. Seed oils of labiatae. Lipids 2(5):371–380
Hwang SZ, Ko YS (1982) Studies on the constituents of Korean edible oils and fats, 5: analysis of fatty acids in sesame and perilla oil by high performance liquid chromatography. Korean J Nutr 15(1):15–21
Lee JI et al (1986) Study on the evaluation of oil quality and the differences of fatty acids composition between varieties in perilla (Perilla frutescens Britton var. japonica Hara). Korean J Breed (Korean R.) 18(3):228–233
Domokos J, Peredi J, Halasz-Zelnik K (1994) Characterization of seed oils of dragonhead (Dracocephalum moldavica L.) and catnip (Nepeta cataria var. citriodora Balb). Ind Crops Prod 3(1–2):91–94
Matthaus B (1997) Antinutritive compounds in different oilseeds. Fett-Lipid 99(5):170–174
Toyoaki A, Tomoko Yamada, Takehito Kinoshita, Shaikh M. Rahman, Yutaka Takagi (2005) Identification of corresponding genes for three low-α-linolenic acid mutants and elucidation of their contribution to fatty acid biosynthesis in soybean seed. Plant Sci 168:1615–1623
Norby SW, Adams CA, Rinne RW (1984) An ultrastructural study of soybean seed development. in American Soy Association Monograph. University of Ilinois Press, Champagne Urbana, IL
Dahmer ML et al (1989) A rapid screening technique for determining the lipid composition of soybean seeds. J Am Oil Chem Soc 66(4):543–548
Miquel M, Browse JA (1992) Arabidopsis mutants deficient in polyunsaturated fatty acid synthesis. Biochemical and genetic characterization of a plant oleoyl-phosphatidylcholine desaturase. J Biol Chem 267(3):1502–1509
Khan MU, Williams JP (1977) Improved thin-layer chromatographic method for the separation of major phospholipids and glycerolipids from plant lipid extracts and phosphatidylglycerol and bis (monoacylglyceryl) phosphate from animal lipid extracts. J Chromatogr 140:179–185
Lightner J, Wu J, Browse J (1994) A mutant of Arabidopsis with increased levels of stearic acid. Plant Physiol 106:1443–1451
Taylor DC, Weber N, Hogge LR, Underhill EW (1990) A simple enzymatic method for the preparation of radiolabeled erucoyl-CoA and other long-chain fatty acyl-CoAs and their characterization by mass spectrometry. Anal Biochem 184(2):311–316
Stymne S, Lorraine Tonnet M, Green AG (1992) Biosynthesis of linolenate in developing embryos and cell-free preparations of high-linolenate linseed (Linum usitatissimum) and low-linolenate mutants. Arch Biochem Biophys 294(2):557–563
Karcher SJ (1995) Molecular biology: a project approach. Academic Press, Inc., San Diego, CA
Longvah T, Deosthale YG (1991) Chemical a nutritional studies on Hanshi (Perilla frutescens) a traditional oil seed from northeast India. J Am Oil Chem Soc 68:781–784
Longvah T, Deosthale YG, Kumar PU (2000) Nutritional and short term toxicological evaluation of perilla seed oil. Food Chem 70(1):13–16
Privett OS et al (1973) Studies on the lipid composition of developing soybean seeds. J Am Oil Chem Soc 50:516–520
Suh MC, Schultz DJ, Ohlrogge TB (2002) What limits production of unusual monoenoic fatty acids in transgenic plants? Planta 215:584–595
Wilson RF (1987) Soybean: improvement, production and uses. In: Wilcox JR (ed) Seed metabolism. American Society of Agronomy, Madison, WI, pp 643–686
Ohlrogge JB, Browse J (1995) Lipid biosynthesis. Plant Cell 7:957–970
Topfer R, Martini N, Schell J (1995) Modification of plant lipid synthesis. Science 268:681–686
Thomaeus S, Carlsson AS, Stymne S (2001) Distribution of fatty acids in polar and neutral lipids during seed development in Arabidopsis thaliana genetically engineered to produce acetylenic, epoxy and hydroxyl fatty acids. Plant Sci 161(5):997–1003
Horiguchi G, Kodama H, Iba K (2001) Characterization of Arabidopsis mutants that are associated with altered C18 unsaturated fatty acid metabolism. Plant Sci 161:1117–1123
O’Hara P, Slabas AR, Fawectt T (2002) Fatty acid and lipid biosynthetic genes are expressed at constant molar ratios but different absolute levels during embryogenesis. Plant Physiol 129:310–320
Sorensen B, Furukawa-Stoffer TL, Marshall KS, Page EK, Mir Z, Forster RJ, Weselake RJ (2005) Storage lipid accumulation and acyltransferase action in developing flax seed. Lipids 40:1043–1049
Byrum JR et al (1997) Alteration of the ω-3 fatty acid desaturase gene is associated with reduced linolenic acid in the A5 soybean genotype. Theor Appl Genet 94(3–4):356–359
Acknowledgments
This work was supported by a graduate fellowship from Ain Shams University in Cairo Egypt to Mohammed Abdel-Reheem, the United Soybean Board and the KY Agricultural Experiment Station.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Rao, S., Abdel-Reheem, M., Bhella, R. et al. Characteristics of High α-Linolenic Acid Accumulation in Seed Oils. Lipids 43, 749–755 (2008). https://doi.org/10.1007/s11745-008-3207-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-008-3207-0