Abstract
Abnormal distribution of plasma fatty acids and increased inflammation are prominent features of metabolic syndrome. We tested whether these components of metabolic syndrome, like dyslipidemia and glycemia, are responsive to carbohydrate restriction. Overweight men and women with atherogenic dyslipidemia consumed ad libitum diets very low in carbohydrate (VLCKD) (1504 kcal:%CHO:fat:protein = 12:59:28) or low in fat (LFD) (1478 kcal:%CHO:fat:protein = 56:24:20) for 12 weeks. In comparison to the LFD, the VLCKD resulted in an increased proportion of serum total n-6 PUFA, mainly attributed to a marked increase in arachidonate (20:4n-6), while its biosynthetic metabolic intermediates were decreased. The n-6/n-3 and arachidonic/eicosapentaenoic acid ratio also increased sharply. Total saturated fatty acids and 16:1n-7 were consistently decreased following the VLCKD. Both diets significantly decreased the concentration of several serum inflammatory markers, but there was an overall greater anti-inflammatory effect associated with the VLCKD, as evidenced by greater decreases in TNF-α, IL-6, IL-8, MCP-1, E-selectin, I-CAM, and PAI-1. Increased 20:4n-6 and the ratios of 20:4n-6/20:5n-3 and n-6/n-3 are commonly viewed as pro-inflammatory, but unexpectedly were consistently inversely associated with responses in inflammatory proteins. In summary, a very low carbohydrate diet resulted in profound alterations in fatty acid composition and reduced inflammation compared to a low fat diet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Development of metabolic syndrome (insulin resistance syndrome) is associated with altered composition of circulating fatty acids characterized by higher saturated fatty acids (14:0, 16:0), higher palmitoleic acid (16:1n-7, the MUFA product derived from palmitic acid), higher dihomo-γ-linolenic acid (20:3n-6, the precursor of arachidonic acid), and lower levels of linoleic acid (18:2n-6) [1]. The effect of dietary fatty acid composition on circulating fatty acids [2] is not well understood. Two recent studies demonstrated that consumption of a diet higher in saturated fat resulted in lower circulating palmitic acid (16:0) in cholesteryl ester compared to a diet low in saturated fat [3, 4], a paradox likely explained by the level of carbohydrate [5] whose increase is known to be associated with de novo fatty acid synthesis [6] and decreased fatty acid oxidation. We have previously described a comparison between a very low carbohydrate diet (VLCKD) and a low fat diet (LFD) in subjects with features of metabolic syndrome. A notable finding was an inverse relationship between dietary and plasma saturated fatty acids (SFA). The VLCKD, with three-fold greater dietary SFA than the LFD, showed a consistently greater reduction in plasma SFA compared to the LFD [7].
Metabolic syndrome is generally defined by high fasting glucose, triglycerides (TG), blood pressure and waist circumference, and low HDL cholesterol. Resistance to the effects of insulin provides a metabolic basis for changes in these disparate physiologic markers as well an increasing number of associations that extend beyond the original description of the syndrome almost 20 years ago [8]. New features that appear to be associated with metabolic syndrome include disturbed circulating fatty acid composition, perturbed lipid metabolism and increased oxidative stress and inflammation [9]. Fatty acids contribute to overall inflammatory balance by several mechanisms. In macrophages, SFA activate toll-like receptor signaling leading to activation of nuclear factor-kappa B (NF-κB) and expression of cyclooxygenase-2 [10, 11]. NF-κB is a transcription factor that regulates over 100 genes, many with an established role in inflammatory responses and atherosclerosis, and may therefore represent a crucial link between fatty acids, metabolic syndrome and atherogenesis [12]. Arachidonic acid (20:4n-6) in membranes is commonly assumed to have a deleterious effect on overall inflammatory balance because of its enzymatic conversion to proinflammatory, proaggregative, and vasoconstrictive eicosanoids (e.g., prostaglandin E2, thromboxane A2, leukotrienes B4). Arachidonic acid is also capable of non-enzymatic conversion to other proinflammatory bioactive products (F2-isoprostanes) via interaction with molecular oxygen. In contrast, eicosanoids derived from the 20-carbon n-3 PUFA, eicosapentaenoic acid (20:5n-3), have less potent inflammatory effects [13]. A recent report showed a marked increase in the plasma 20:4n-6/20:5n-3 ratio in subjects consuming a VLCKD, while CRP, a marker of constitutive inflammation, decreased slightly [14]. The relations between inflammatory markers and arachidonic acid metabolism are complex [15], and may be further modified by the level of dietary carbohydrate.
Carbohydrate restriction is generally effective at ameliorating those physiologic markers associated with metabolic syndrome: high fasting glucose and insulin, and particularly the atherogenic dyslipidemia characterized by high TG and low HDL [16–19]. The effects are presumed to be attributed to better regulation of plasma glucose and insulin levels and improvement in the hyperinsulinemia/insulin resistance that are fundamental features of metabolic syndrome. Here we evaluated circulating fatty acid composition in three lipid fractions as well as a large number of inflammatory makers and show that a VLCKD results in profound alterations in fatty acid composition and reduced inflammatory markers to a greater extent than a low fat diet.
Materials and Methods
Study Design and Subjects
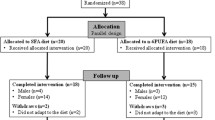
Details of this investigation have been described previously [7]. In brief, 40 overweight men and women aged 18–55 year with a BMI >25 kg/m2 participated in this 12 week randomized, controlled, dietary intervention trial comparing a VLCKD to a LFD. All participants were required to have atherogenic dyslipidemia defined by moderately elevated TG (150 to 500 mg/dl) and low HDL [<40 (men) or <50 (women) mg/dl]. The two dietary groups were balanced for gender, age and BMI. Exclusion criteria were any metabolic and endocrine disorders, use of glucose-lowering, lipid-lowering or vasoactive prescriptions or supplements, consumption of a VLCKD, or weight loss >5.0 kg in the past 3 months. Habitual physical activity was maintained throughout the study intervention and was documented daily. Blood was drawn at baseline and after 12 week of diet intervention in the morning after a 12 h overnight fast and a 24 h abstinence from alcohol and strenuous exercise. All procedures were approved by the Institutional Review Board of the University of Connecticut, and all participants provided written informed consent.
Dietary Intervention
Subjects received individual and personalized dietary counseling from Registered Dietitians during the dietary intervention. No explicit instructions were provided regarding caloric intake for either diet to allow expression of any non-cognitive aspects on food intake. Subjects received weekly follow-up counseling during which body mass was measured, compliance was assessed, and further dietetic education was provided. Dietary intake and compliance was assessed with detailed and weighed 7-day food records at baseline, during weeks one, 6, and 12, and was analyzed for energy and macro/micronutrient content using NUTRITIONIST PRO™ (Version 1.5, First Databank Inc, The Hearst Corporation, San Bruno, CA, USA). The nutrient analysis program had no missing values for the nutrients reported and the database was extensively updated with new foods and individualized recipes. All subjects were given a multi-vitamin/mineral complex that provided micronutrients at levels ≤100% of the RDA.
The main goal of the VLCKD was to restrict carbohydrate to a level that induced a low level of ketosis. Subjects monitored their level of ketosis daily using urine reagent strips. In this diet there were no restrictions on the intake of fat from saturated and unsaturated sources or the intake of cholesterol. Examples of foods consumed by the subjects included unlimited amounts of beef, poultry, fish, eggs, oils and heavy cream; moderate amounts of hard cheeses, low carbohydrate vegetables and salad dressings; and small amounts of nuts, nut butters and seeds. Subjects restricted fruit and fruit juices, dairy products (with the exception of heavy cream and hard cheese), breads, grains, pasta, cereal, high carbohydrate vegetables, and desserts. Subjects were instructed to avoid all low carbohydrate breads and cereal products, and were limited to a maximum of one sugar alcohol-containing, low carbohydrate snack per day.
The LFD was designed to provide <10% of total calories from saturated fat and <300 mg cholesterol. Foods encouraged included whole grains (breads, cereals, and pastas), fruit/fruit juices, vegetables, vegetable oils, low-fat dairy and lean meat products. Standard diabetic exchange lists were used to ensure a macronutrient balance of protein (~20% energy), fat (~25% energy), and carbohydrate (~55% of energy).
Blood Analyses
Whole blood was collected into tubes with no preservative or EDTA and centrifuged at 1500×g for 15 min and 4°C, and promptly aliquoted into separate storage tubes which were stored at 75 °C until analyzed for serum fatty acid composition and plasma inflammatory markers. An aliquot of anti-coagulated whole blood (~3 ml) was sent to a certified medical laboratory (Quest Diagnostics, Wallingford, CT, USA) for a white blood cell differential count.
Inflammatory Biomarkers
The Evidence Investigator™ Biochip Array technology (Randox Laboratories Ltd, UK) that uses sandwich chemiluminescent immunoassays to simultaneously detect multiple analytes from a single sample was used to determine the following serum cytokines and adhesion molecules: IL-6, IL-8, vascular endothelial growth factor (VEGF), TNF-α, IFN-γ, epidermal growth factor (EGF), monocyte chemotactic protein-1 (MCP-1), intracellular cellular adhesion molecule-1 (ICAM-1), vascular cellular adhesion molecule-I (VCAM-I), E-selectin, P-selectin and L-selectin. In addition, serum C-reactive protein (CRP) was determined on an IMMULITE Automated Analyzer using the commercially available immulite chemiluminescent enzyme immunometric assay (Immulite®, Diagnostic Products Corp, Los Angeles, CA, USA) and plasma plasminogen-activator inhibitor-1 (PAI-1) was determined utilizing the Luminex 200 analyzer (Luminex Corp, Austin, TX, USA) using an immunoassay kit from LINCO Research.
Fatty Acid Composition
Serum lipids were extracted according to the method of Bligh–Dyer whereby mixtures of plasma, methanol, chloroform and water were prepared such that lipid is recovered in a chloroform layer. The resulting lipid extracts were maintained under an atmosphere of nitrogen following extraction and kept frozen prior to additional processing. Immediately prior to lipid class separation, lipid samples were dried under a gentle stream of nitrogen, rediluted in 50 μl of chloroform and prepared for lipid class separation. Lipid classes including total TAG, PL and CE were separated on commercial silica gel G plates (AnalTech, Newark, DE, USA). The chromatographic plates were developed in a solvent system consisting of distilled petroleum ether (bp 30–60 °C):diethyl ether:acetic acid (80:20:1, by vol). Following development, the silica gel plates were sprayed with a methanolic solution containing 0.5% 2,7-dichlorofluorescein which was then used to visualize lipid classes under ultraviolet light. Desired corresponding lipid bands were then scraped into Teflon line screw cap tubes. The samples were then transesterified with boron trifluoride (10%) in excess methanol (Supelco, Bellefonte, PA, USA) in an 80 °C water bath for 90 min. Resulting fatty acid methyl esters were extracted with water and petroleum ether and stored frozen until gas chromatographic analysis was performed.
Lipid class fatty acid methyl ester composition was determined by capillary gas chromatography. Methyl ester samples were blown to dryness under nitrogen and resuspended in hexane. Resulting fatty acid methyl esters were separated and quantified with a Shimadzu capillary gas chromatograph (GC17) utilizing a 30 m Restek free fatty acid phase (FFAP) coating and EZChrom software. The instrument temperature was programmed from 190 to 240 °C at 7°C/min with a final hold of 10 min, separating and measuring fatty acid methyl esters ranging from 12:0 to 24:1. The detector temperature was 250 °C. Helium carrier gas was used at a flow rate of 1.4 ml/min. and a split ratio of 1:25. Chromatographic data was collected and processed with EZChrom software (Scientific Products, CA, USA). Fatty acids were identified by comparison to authentic fatty acid standards and quantitated with peak area and internal standard. Resulting data are expressed in percent composition. Individual peaks, representing as little as 0.05% of the fatty acid methyl esters, were distinguished.
Statistical Analyses
All statistical analyses were done with Statistica software (StatSoft Inc, Tulsa, OK, USA). A 2 × 2 ANOVA with one between effect (VLCKD vs. LFD) and one within effect (Week 0 vs. Week 12) was used to compare biochemical responses over time in both groups. Significant main or interaction effects were further analyzed using a Fishers LSD post hoc test. Relationships among selected variables were examined using Pearson’s product-moment correlation coefficient. The alpha level for significance was set at 0.05.
Results
Dietary Intake and MetS Responses
Dietary nutrient intake and responses of MetS biomarkers will be presented elsewhere [7]. In brief, subjects in both groups reduced energy intake to approximately 1500 kcal/day, but the diets had markedly different macronutrient distributions based upon the analysis of individual diet records (VLCKD, %CHO:fat:protein = 12:59:28) and (LFD, %CHO:fat:protein = 56:24:20) (Table 1). Dietary saturated fat and cholesterol intake were significantly higher during the VLCKD than the LFD. The LFD led to improvements in some metabolic markers, but subjects following the VLCKD had consistently greater weight loss, decreased adiposity, improved glycemic control and insulin sensitivity and more favorable TAG, HDL-C and total cholesterol/HDL-C ratio responses. In addition to these markers for MetS, the VLCKD subjects showed more favorable responses in alternative indicators of atherogenic dyslipidemia and cardiovascular risk: postprandial lipemia, apo B, apo A-1, the apo B/Apo A-1 ratio, LDL particle distribution and postabsorptive and postprandial vascular function. Most striking, we reported that despite a three-fold higher intake of dietary saturated fat during the VLCKD compared to the LFD, circulating saturated fatty acids in TAG and CE were significantly decreased, as was 16:1n-7, an endogenous marker of lipogenesis. There were profound changes, as well, in other fatty acids in circulating TG, PL, and CE fractions (Tables 2–4).
Circulating Triglyceride Fatty Acids
Compared to the LFD, consumption of the VLCKD resulted in a significantly greater increase in TG n-6 PUFA, mainly attributed to a marked increase in arachidonic acid: 17 of 20 subjects in VLCKD showed marked increases while only 7 of 20 subjects on LFD showed increases in 20:4n-6 and these were more modest in amplitude (Table 2). In both groups, n-3 PUFA was decreased due largely to lower α-linolenic acid (18:3n-3) and 20:5n-3. The n-6/n-3 and arachidonic/eicosapentaenoic acid ratios were, on average, nearly doubled in response to the VLCKD and virtually unchanged by the LFD: 15 of 20 subjects on VLCKD showed increases in the n-6/n-3 ratios, while only 8 of 20 of the LFD showed increases. In contrast to the response in 20:4n-6, the metabolic intermediates in the biosynthetic pathway, especially 20:3n-6, were decreased after the VLCKD. As previously reported, total MUFA was unchanged but 16:1n-7 and total SFA was significantly decreased in response to the VLCKD.
Circulating Phospholipid Fatty Acids
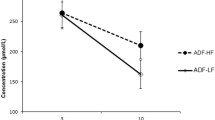
The pattern of fatty acid changes seen in the TAG fraction was also found in circulating phospholipids: consumption of the VLCKD was associated with an increase in n-6 PUFA, again primarily due to a distinct increase in 20:4n-6 (Fig 1a), whereas 18:3n-6 and 20:3n-6 were markedly decreased (Table 3). The VLCKD was associated with a significant reduction in 18:3n-3, 18:4n-3, 20:4n-3, and 20:5n-3. However docosahexaenoic acid (22:6n-3) was increased so that total n-3 PUFA was not significantly changed. Compared to the LFD, ingestion of the VLCKD resulted in a significant increase in total PUFA and the ratio of n-6/n-3 (Fig. 1b) and arachidonic/eicosapentaenoic acid (Fig. 1c). In comparison to the LFD, total MUFA was significantly decreased in response to the VLCKD due to significant decreases in the most abundant MUFA, 18:1n-9, and a consistent reduction in 16:1n-7.
Individual responses in serum phospholipid arachidonic acid (20:4n-6) (a), the n-6/n-3 ratio (b) and the arachidonic/eicosapentaenoic acid ratio (20:4n-6/20:5n-3) (c) in subjects who consumed a very low carbohydrate ketogenic diet (VLCKD) or a low fat diet for 12 weeks. Shaded bars indicate mean responses
Circulating Cholesteryl Ester Fatty Acids
The patterns of CE fatty acid responses to diet resembled that in TG and PL, and in general were more dramatic in this lipid fraction (Table 4). Total n-6 PUFA was significantly increased in response to the VLCKD due to a large increase in 18:2n-6 and a smaller increase in 20:4n-6: most of the VLCKD group showed a significant increase in 20:4n-6 while only five of the LFD group showed an increase. On the other hand total n-3 PUFA was significantly decreased due to a reduced proportion of 18:3n-3 and 20:5n-3 in response to the VLCKD but, similar to other fractions, there was an increase in 22:6n-3. The n-6/n-3 and arachidonic/eicosapentaenoic acid ratios were unchanged in response to the LFD but increased sharply after consumption of the VLCKD. Total MUFA decreased in response to the VLCKD, again due to a reduced proportion of 18:1n-9 and a striking decrease in 16:1n-7.
Inflammatory Markers
Both diets led to a similar significant reduction in the acute phase reactant C-reactive protein (−23%), VEGF (−21%), P-selectin (−11%), and a trend for EGF (−38%), and V-CAM (−6%); however, there was an overall greater anti-inflammatory effect associated with the VLCKD as evidenced by significantly greater decreases in the proinflammatory cytokine TNF-α (−32 vs. −12%), the chemokines IL-8 (−33 vs. 4%) and MCP-1 (−24 vs. −5%), and the adhesion molecules E-selectin (−34 vs. −14%) and I-CAM (−17 vs. −3%) (Table 5). There was also a trend for a greater reduction in IL-6 (−35%) in response to the VLCKD (P = 0.07). Plasminogen-activator inhibitor-1 (PAI-1) has antifibrinolytic functions, and was also reduced more in subjects consuming the VLCKD compared to LFD (−34 vs. −8%). There was no effect of the interventions on leukocyte subpopulations.
Correlations
The results described above are surprising in that consumption of the VLCKD showed substantially greater increases in arachidonic acid and the arachidonic/eicosapentaenoic acid and n-6/n-3 ratios that are commonly viewed as contributing to an overall proinflammatory state, while simultaneously there was a significant decrease in many inflammatory markers. An analysis of these data bears out the idea that changes in the fatty acid proxies were consistently inversely associated with responses in most of the inflammatory markers we measured (Fig. 2). The fatty acid with the most consistent positive association with changes in inflammatory markers was palmitoleic acid (16:1n-7). The correlations between weight loss and changes in inflammatory markers were generally small and not significant. As shown in Fig. 3 for two of the more important markers TNF-α and IL-8, there is essentially no correlation.
Discussion
Because of the continued emphasis on dietary recommendations for cardiovascular disease and general health, the relation between dietary fat intake and plasma fatty acids and inflammatory markers is of great importance. The findings presented here support our hypothesis that the components of metabolic syndrome are distinctly those that respond favorably to reduction in dietary carbohydrate [19]. Responses in fatty acid composition to the VLCKD in this study were exactly opposite to the fatty acid profile recently shown to be associated with development of metabolic syndrome over a 20 year period in previously healthy men (i.e., higher circulating 14:0, 16:0, 16:1n-7, 18:1n-9, 18:3n-6, and 20:3n-6, and lower levels of 18:2n-6) [1]. Abnormal fatty acid composition and inflammatory status are now recognized as prominent features of metabolic syndrome, and are reliably improved in subjects consuming a low carbohydrate diet compared to a low fat diet.
Acute ingestion of carbohydrate clearly induces an increase in reactive oxygen species and activation of pro-inflammatory pathways [9], and isocaloric high carbohydrate [20] and high glycemic [21] diets are associated with increased biomarkers of inflammation. In the context of hypocaloric diets, we showed that reducing dietary total and saturated fat only had a small effect on circulating inflammatory markers whereas reducing carbohydrate led to considerably greater reductions in a number of proinflammatory cytokines, chemokines, and adhesion molecules. These data implicate dietary carbohydrate rather than dietary fat as a more significant nutritional factor contributing to inflammatory processes; although increased fat in the presence of high carbohydrate may be particularly deleterious. Dietary carbohydrate also has a fundamental role in determining fatty acid composition of lipids and membranes, and it is the endogenous fatty acids (as opposed to the exogenous dietary fatty acids) that influence inflammation by acting as ligands for receptors or transcription factors that regulate inflammatory signaling cascades or serving as substrates for proinflammatory bioactive products.
Despite the two diet groups consuming roughly the same caloric intake and all losing at least some weight, there were larger reductions in the VLCKD group in TNF-α, IL-8, MCP-1, PAI-1, E-selectin and I-CAM, while these markers showed little change on low fat suggesting that it is the macronutrient composition not weight loss or caloric reduction that is key. Most of the inflammatory markers did not correlate with weight loss. A correlation would not have proved that weight loss caused change in inflammatory markers but the lack of correlation makes it extremely unlikely. As shown in Fig. 3, there is essentially no correlation and if anything the associations tend to go in the opposite direction of what is expected if weight loss caused change in markers. In both cases, individuals with the largest reductions in inflammatory markers tended to be in the middle of the weight-loss range. The question of weight loss as a stimulus versus a response has been raised before with regard to other effects of carbohydrate restriction. Our group [22] and others [16, 18, 23] have consistently shown that there is a benefit to atherogenic dyslipidemia, glycemic control, and insulin from reduction in carbohydrate independent of weight loss. We therefore suggest that reduction in carbohydrate is primary, and weight loss (more precisely caloric restriction) is not the controlling variable.
One of the most striking responses in fatty acid composition was the increase in arachidonic acid and total n-6 PUFA in subjects consuming a VLCKD. Rather than being a negative factor within lipid membranes, increased arachidonic acid appears to be a beneficial outcome of weight-reducing diets associated with greater lipolysis [24]. The increase in plasma arachidonic acid only in response to the low carbohydrate diet is best explained by decreased degradation presumably due to less interaction with reactive oxygen species [25]. Increased production from 18:2n-6 was unlikely since the metabolic intermediates 18:3n-6 and 20:3n-6 were reduced in all three circulating fractions and there was no increase in 20:3n-9, which typically occurs in cases where PUFA anabolism is increased [13, 25]. Since arachidonic acid was elevated in all circulating fractions, a shift from other pools is unlikely. This is supported by the fact that fat loss was only moderately greater on the VLCKD compared to the LFD (−5.6 vs. −3.7 kg), and by week 12, the rate of weight loss on both diets was low. Given that daily dietary contribution of arachidonic acid is on the order of 0.5% of the total body pool [26] also suggests reduced degradation as the major explanation. Thus, an increase in the proportion of arachidonic acid resulting from a diet that restricts carbohydrate may be due to lower catabolism (i.e. better preservation) and therefore reduced formation of proinflammatory products. The consistent inverse associations between changes in arachidonic acid and responses in inflammatory markers indicate that the adverse effects of arachidonic acid are due to metabolites produced subsequent to its release from membranes rather than the proportion of the intact fatty acid.
The 19% rise in PL arachidonic acid in the low carbohydrate group (from 11.54 to 13.70 wt%) is consistent with the change seen after 12 weeks of a very low calorie diet in a more obese population (e.g,, from 9.16 to 11.77 wt%) [24]. Given the regulatory role of arachidonic acid as a ligand for PPAR and in gene expression (e.g., fatty acid synthase) [27], this degree of rise in 20:4n-6 has the potential to influence fuel partitioning. In the obese Zucker rat, the increase in liver PL arachidonic acid from 22.68 to 25.23 wt% (an 11% change) induced by feeding 18:3n-6 was associated with significant reductions in food intake and body fat content [28].
Scenarios associated with less oxidative stress should result in better preservation of the substrate arachidonic acid, due to the interaction of free radicals with several steps in its metabolism. Inflammatory cytokines are known to increase production of hydroxyl radicals which in turn initiate arachidonic acid release and breakdown. The VLCKD in this study resulted in significantly greater reductions in several proinflammatory markers including TNF-α, E-selectin, ICAM-1, and IL-8, that were related to the increase in arachidonic acid. The significantly greater reduction in TNF-α in subjects following the VLCKD is of interest in that it is one of the agents known to activate NF-κB a major transcription factor regulating cytokines, chemokines and adhesion molecules (TNF-α, MCP-1, IL-8, E-selectin, and ICAM-1) [12, 29]. The reduction in all of these agents by the VLCKD suggests that the antiinflammatory effects of carbohydrate restriction may be mediated via down regulation of NF-κB expression [30]. We have previously found that guinea pigs fed high-cholesterol atherogenic diets demonstrated significant increases in aortic TNF-α, an effect that was attenuated by reduction in dietary carbohydrate [31].
Hypercaloric high carbohydrate feeding stimulates the production of several fatty acids including 16:0, the major lipogenic product, and palmitoleic acid (16:1n-7), the product of Δ9 desaturase. Palmitoleic acid is a minor constituent in dietary fat and its increase is a marker of lipogenesis [32] and its presence has been linked to higher levels of adiposity [33, 34]. In this study, the VLCKD resulted in concurrent reductions in both 16:0 and 16:1n-7 in both TG and CE lipid fractions despite an increase in dietary saturated fat load. The significant reduction in dietary saturated fat in the LFD led to little decrease in total saturates and essentially no change in 16:1n-7, with one subject actually showing a drastic increase. The greater decrease in circulating SFA in response to carbohydrate restriction may have contributed to the larger decline in several inflammatory markers that are regulated by NF-κB [10, 11]. The decrease in circulating saturated fatty acids on the VLCKD is likely due to greater oxidation of the saturated fat from both diet and endogenous lipolysis, and a reduction in de novo lipogenesis.
In summary, carbohydrate restricted diets that are proportionately high in saturated fatty acids show very different results from what might be expected [7, 18]. A VLCKD significantly increases arachidonic acid levels, presumably due to a better preservation as a result of reduced oxidative stress and decreased inflammation. Sparing of arachidonic acid (by reducing its degradation to oxy-lipids) may provide a signaling mechanism by which dietary carbohydrate restriction favorably alters lipid metabolism and inflammatory processes [27].
Abbreviations
- VLCKD:
-
Very low carbohydrate ketogenic diet
- LFD:
-
Low fat diet
- PL:
-
Phospholipid
- CE:
-
Cholesteryl ester
- CVD:
-
Cardiovascular disease
- RDA:
-
Recommended daily allowance
- BMI:
-
Body mass index
- IL:
-
Interleukin
- TNF-α:
-
Tumor necrosis factor-α
- VEGF:
-
Vascular endothelial growth factor
- IFN-γ:
-
Interferon-γ
- EGF:
-
Epidermal growth factor
- MCP-1:
-
Monocyte chemotactic protein-1
- ICAM-1:
-
Intracellular cellular adhesion molecule-1
- VCAM-I:
-
Vascular cellular adhesion molecule-I
- NF-κB:
-
Nuclear factor-kappa B
- CRP:
-
C-reactive protein
- TAG:
-
Triglycerides
References
Warensjo E, Riserus U, Vessby B (2005) Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia 48:1999–2005
Dougherty RM, Galli C, Ferro-Luzzi A, Iacono JM (1987) Lipid and phospholipid fatty acid composition of plasma, red blood cells, and platelets and how they are affected by dietary lipids: a study of normal subjects from Italy, Finland, and the USA. Am J Clin Nutr 45:443–455
King IB, Lemaitre RN, Kestin M (2006) Effect of a low-fat diet on fatty acid composition in red cells, plasma phospholipids, and cholesterol esters: investigation of a biomarker of total fat intake. Am J Clin Nutr 83:227–236
Raatz SK, Bibus D, Thomas W, Kris-Etherton P (2001) Total fat intake modifies plasma fatty acid composition in humans. J Nutr 131:231–234
Phinney SD (2006) The low fat paradox––do dietary carbohydrates increase circulating saturated fatty acids? Am J Clin Nutr 84:461–462
Parks EJ (2002) Changes in fat synthesis influenced by dietary macronutrient content. Proc Nutr Soc 61:281–286
Volek JS, Feinman RD, Phinney SD, Forsythe CE, Silvestre R, Judelson D, Quann EE, Wood RJ, Puglisi MJ, LaBonte CC, Kraemer WJ, Bibus DM, Contois JH, Fernandez ML (2007) Comparative effects of dietary restriction of carbohydrate or fat on circulating saturated fatty acids and atherogenic dyslipidemia. (Submitted)
Reaven GM (1988) Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 37:1595–1607
Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R (2005) Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 111:1448–1454
Lee JY, Hwang DH (2006) The modulation of inflammatory gene expression by lipids: mediation through Toll-like receptors. Mol Cells 21:174–185
Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, Lee WH, Fitzgerald KA, Hwang DH (2004) Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem 279:16971–16979
de Winther MP, Kanters E, Kraal G, Hofker MH (2005) Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol 25:904–914
Calder PC (2006) Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids 75(3):197–202
Johnston CS, Tjonn SL, Swan PD, White A, Hutchins H, Sears B (2006) Ketogenic low-carbohydrate diets have no metabolic advantage over nonketogenic low-carbohydrate diets. Am J Clin Nutr 83:1055–1061
Peplow PV (1996) Actions of cytokines in relation to arachidonic acid metabolism and eicosanoid production. Prostaglandins Leukot Essent Fatty Acids 54:303–317
Feinman RD, Volek JS (2006) Low carbohydrate diets improve atherogenic dyslipidemia even in the absence of weight loss. Nutr Metab (Lond) 3:24
Feinman RD, Westman EC, Volek JS (2006) Low carbohydrate and low fat diets in diabetes, cardiovascular disease and metabolic syndrome. Cellscience Reviews 3, no. 1 (http://www.cellscience.com/reviews9/Low_carbohydrate_low_fat_diets_in_disease. html)
Krauss RM, Blanche PJ, Rawlings RS, Fernstrom HS, Williams PT (2006) Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am J Clin Nutr 83:1025–1031
Volek JS, Feinman RD (2005) Carbohydrate restriction improves the features of metabolic syndrome. Metabolic syndrome may be defined by the response to carbohydrate restriction. Nutr Metab (Lond) 2:31
Kasim-Karakas SE, Tsodikov A, Singh U, Jialal I (2006) Responses of inflammatory markers to a low-fat, high-carbohydrate diet: effects of energy intake. Am J Clin Nutr 83:774–779
Liu S, Manson JE, Buring JE, Stampfer MJ, Willett WC, Ridker PM (2002) Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr 75:492–498
Volek JS, Sharman MJ, Gomez AL, Scheett TP, Kraemer WJ (2003) An isoenergetic very low carbohydrate diet improves serum HDL cholesterol and triacylglycerol concentrations, the total cholesterol to HDL cholesterol ratio and postprandial pipemic responses compared with a low fat diet in normal weight, normolipidemic women. J Nutr 133:2756–2761
Gannon MC, Nuttall FQ (2006) Control of blood glucose in type 2 diabetes without weight loss by modification of diet composition. Nutr Metab (Lond) 3:16
Phinney SD, Davis PG, Johnson SB, Holman RT (1991) Obesity and weight loss alter serum polyunsaturated lipids in humans. Am J Clin Nutr 53:831–838
Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM (2004) The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol 55:576–580
Zhou L, Nilsson A (2001) Sources of eicosanoid precursor fatty acid pools in tissues. J Lipid Res 42:1521–1542
Brash AR (2001) Arachidonic acid as a bioactive molecule. J Clin Invest 107:1339–1345
Phinney SD, Tang AB, Thurmond DC, Nakamura MT, Stern JS (1993) Abnormal polyunsaturated lipid metabolism in the obese Zucker rat, with partial metabolic correction by gamma-linolenic acid administration. Metabolism 42:1127–1140
Monaco C, Paleolog E (2004) Nuclear factor kappaB: a potential therapeutic target in atherosclerosis and thrombosis. Cardiovasc Res 61:671–682
Shin WS, Szuba A, Rockson SG (2002) The role of chemokines in human cardiovascular pathology: enhanced biological insights. Atherosclerosis 160:91–102
Fernandez ML, Volek JS (2006) Guinea pigs: a suitable animal model to study lipoprotein metabolism, atherosclerosis and inflammation. Nutr Metab (Lond) 3:17
Aarsland A, Wolfe RR (1998) Hepatic secretion of VLDL fatty acids during stimulated lipogenesis in men. J Lipid Res 39:1280–1286
Okada T, Furuhashi N, Kuromori Y, Miyashita M, Iwata F, Harada K (2005) Plasma palmitoleic acid content and obesity in children. Am J Clin Nutr 82:747–750
Kunesova M, Hainer V, Tvrzicka E, Phinney SD, Stich V, Parizkova J, Zak A, Stunkard AJ (2002) Assessment of dietary and genetic factors influencing serum and adipose fatty acid composition in obese female identical twins. Lipids 37:27–32
Acknowledgments
This work was supported by the Dr. Robert C. Atkins Foundation.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Forsythe, C.E., Phinney, S.D., Fernandez, M.L. et al. Comparison of Low Fat and Low Carbohydrate Diets on Circulating Fatty Acid Composition and Markers of Inflammation. Lipids 43, 65–77 (2008). https://doi.org/10.1007/s11745-007-3132-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-007-3132-7