Abstract
Plasmalogens (1-O-alk-1′-enyl-2-acyl-sn-glycero-3-phosphocholines and -phosphoethanolamines) are important constituents of spermatozoa membranes and possess significant antioxidative properties. This particularly holds as plasmalogens from spermatozoa also possess a very high content of highly unsaturated fatty acyl residues (especially 22:6). The organic spermatozoa extracts of two different ruminants (cattle and roe deer) were analyzed for their contents of characteristic choline plasmalogen oxidation products by matrix-assisted laser desorption and ionization time-of-flight (MALDI-TOF) mass spectrometry. It will be shown that 1-hydroxy-2-docosahexaenoyl-sn-glycero-3-phosphocholine (LPC 22:6) and formyl-LPC 22:6 are reliable measures of lipid oxidation of spermatozoa and allow, accordingly, conclusions about the storage conditions. All data on spermatozoa were also confirmed by the investigation of the oxidation behavior of selected reference compounds. It will be shown that, equally if plasmalogens or diacyl PC species are used, oxidation takes place primarily at the double bond next to the glycerol backbone. These data were additionally confirmed by recording the corresponding post source decay (PSD) fragment ion spectra.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of natural glycerophospholipids (GPLs) is characterized by acyl linkages, by which the fatty acyl residues are connected with the glycerol backbone. However, in many cells and tissues there are also GPLs with alkyl and alkenyl linkages [1]. Plasmalogens (1-O-alk-1′-enyl-2-acyl-sn-glycero-3-phosphocholines and -ethanolamines) are one particularly important lipid class and it was estimated that the percentage of plasmalogens is about 18% of the total GPL mass in human cells [2].
Animal spermatozoa are also known to contain very large amounts of plasmalogens [3]. As this is often combined with surprisingly high levels of highly unsaturated acyl chains, particularly docosahexaenoyl (22:6) and docosapentaenoyl residues (22:5), plasmalogens are considered as important antioxidants [2].
Surprisingly, however, even under in vitro conditions there is not yet an agreement about the primary oxidation products of plasmalogens. For instance, Thompson et al. [4] found that defined photooxidation of 1-O-alk-1′-enyl-2-palmitoyl-sn-glycero-3-phosphocholines led to the formation of a mixture of lyso-glycerophospholipid and formyl-ly-soglycerophospholipid by cleavage of the alkenyl ether in the sn-1 position, as well as further minor photooxidation products, for instance, an allylic hydroperoxide [4]. Both main oxidation products of 1-O-hexadecyl-1′-enyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine (GPC 16:0plasm/22:6) are shown in Fig. 1.
Oxidation of 1-O-hexadec-1′-enyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine (GPC 16:0plasm/22:6) to 1-hydroxy-2-docosahexaenoyl-sn-glycero-3-phosphocholine (LPC 22:6) and formyl-LPC 22:6. For further mechanistic details concerning oxidation of the docosahexaenoyl residue see [5]
Alternatively, oxidation may occur at the sn-2 position, as demonstrated using arachidonyl and docoahexaenoyl plasmalogens under the influence of Cu(II)/H2O2 or the free radical initiator AAPH (2,2′-azobis-2-methyl-propanimidamide, dihydrochloride) [5, 6]. Whereas the first oxidant induced primarily oxidation at carbon-5 of the arachidonoyl residue [6], the second oxidant gave a more complex mixture of products [5].
In the present paper we investigated the organic extracts of spermatozoa from cattle and roe deer because they are known to possess large amounts of plasmalogens [3]. Matrix-assisted laser desorption and ionization time-of-flight mass spectrometry (MALDI-TOF MS) [7] with post source decay (PSD) fragmentation [8] was used for lipid analysis as a fast and reliable method. This method was already successfully used for the investigation of oxidation products from PCs [9] under the influence of HOCl as well as for the analysis of oxidatively modified human lipoproteins [10].
We will show that along with 1-hydroxy-2-docosahexaenoyl-sn-glycero-3-phosphocholine (LPC 22:6), formyl-LPC 22:6 is an additional important oxidation product of plasmalogens under conditions that promote lipid oxidation. Particularly formyl-LPC 22:6 may serve as a selective marker of plasmalogen oxidation.
In addition to lipids from animal spermatozoa, selected experiments were also performed with purified phospholipids in order to check whether the results obtained under both conditions agree with each other.
Materials and Methods
Chemicals
All phospholipid standards used as reference compounds were obtained from Avanti Polar Lipids (Alabama, USA) as solutions in CHCl3 and used without further purification. All further chemicals for sample preparation, MALDI-TOF MS (2,5-dihydroxybenzoic acid, DHB) as well as all solvents (chloroform and methanol) were obtained in highest commercially available purity from Fluka Feinchemikalien GmbH (part of Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) and used as supplied. Phospholipase A2 from hog pancreas was also obtained from Fluka. Percoll (L 6143) was purchased from Biochrom (Berlin, Germany).
Semen Samples and Processing
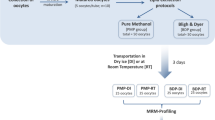
Bull semen was obtained from different fertile animals at a breeding station (Rinderbesamung Berlin-Brandenburg GmbH, Besamungsbullenstation Schmergow, Germany). One ml portions of each ejaculate were centrifuged at room temperature (12,000×g, 2 min) and the seminal plasma was removed by pipette. The sperm cell sediment was carefully re-suspended with 1 ml 0.9% NaCl and the supernatant discarded after centrifugation (12,000×g, 2 min).
After the second washing step in 0.9% NaCl the cell concentrations in the remaining pellets were adjusted with 0.9% NaCl to about 4 × 108 spermatozoa/ml. Those samples were stored for 24 h at 16 °C without further protective agents or frozen at −20 °C until use for lipid extraction. Lipid extraction (according to a modified Bligh and Dyer method [11]) was performed in the following way: 0.3 ml of the thawed cell suspension (i.e., about 1.2 × 108 spermatozoa) were diluted with 0.5 ml 0.9% NaCl solution. Afterwards, 3 ml of a CHCl3/CH3OH (1:2 v/v) mixture were added and the sample was vigorously vortexed for 1 min and incubated for 30 min at RT. One ml CHCl3 was added and the sample again vortexed for 1 min. After addition of 1 ml acetic acid (40 mM) and vortexing, the sample was centrifuged (10 min, 1,000×g, 4 °C) and the organic layer was removed and used for all further experimental procedures. Roe deer spermatozoa were obtained by electroejaculation at the field station of the Leibniz Institute for Zoo and Wildlife Research, and washed and extracted in the same way. Unprotected storage of semen was performed at 5 °C. Spermatozoa from cattle and roe deer were cryopreserved by slow freezing in commercial media (Minitüb, Landshut, Germany) containing egg yolk. After thawing sperm cells were centrifuged (20 min, 600×g, 25 °C) through a Percoll gradient [45, 70, 90% in Hepes-buffered NaCl solution (HBS)] to remove the cryoextender (particularly the egg yolk). The resulting sperm pellets were washed again with 5 ml HBS (10 min, 500×g, 25 °C) and the pellet was re-suspended in HBS with a sperm number of about 1 × 107 cells in 800 μl for lipid extraction.
Autoxidation of Phospholipids
Oxidation of 1-O-octadecyl-1′-enyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine and 1-palmitoyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine was performed as described [12]. Briefly, 0.1 mg of the corresponding phospholipid dissolved in 100 μl of chloroform was transferred to a small glass test tube and evaporated under a stream of nitrogen. The lipid residue was allowed to autoxidize while being exposed to air for 24 h. This simple approach was considered to be sufficient as no quantitative data analysis was planned.
Artificial Modifications of Phospholipids Extracted From Spermatozoa
Commercially available PLs and PLs extracted from spermatozoa were digested by the enzyme phospholipase A2 to obtain the corresponding lysophospholipids (LPLs) [13]. Briefly, aliquots of the organic extracts of spermatozoa (see above) were evaporated to dryness. Lipid vesicles were prepared by dissolving the resulting PL film in 50 mM phosphate buffer, pH 7.4 and vortexing vigorously for 30 s. Vesicles were treated with 0.5 mg/ml phospholipase A2 (ca. 100 U/ml) for 2 h at 37 °C. No further efforts were made to determine the exact PLA2 activity as the only aim was the complete digestion of the PLs of spermatozoa. PLs were subsequently extracted in the same way as described above.
In order to prove the sensitivity of alkenyl–acyl species to acid treatment, a flask with the dried lipids obtained from roe deer spermatozoa was exposed to HCl fumes for 10 min by keeping the inverted flask over an open bottle of concentrated HCl [14].
MALDI-TOF Mass Spectrometry
Total spermatozoa extracts and individual autoxidized PLs were investigated by MALDI-TOF MS using 0.5 mol/l 2,5-dihydroxybenzoic acid (DHB) in methanol as matrix [15]. A recent investigation has shown that this isomer of dihydroxybenzoic acid is most suitable as MALDI matrix [16]. All MALDI-TOF mass spectra were acquired on a Bruker Autoflex mass spectrometer (Bruker Daltonics, Bremen, Germany). The system utilizes a pulsed nitrogen laser, emitting at 337 nm. The extraction voltage was 20 kV and gated matrix suppression was applied to prevent the saturation of the detector by matrix ions [17]. One hundred and twenty-eight single laser shots were averaged for each mass spectrum to minimize shot-to-shot deviations. The laser strength was kept about ten percent above threshold to obtain optimum signal-to-noise (S/N) ratio. In order to enhance the spectral resolution all spectra were acquired in the reflector mode using delayed extraction conditions. A more detailed methodological description of MALDI-TOF MS is given in [7, 18].
In the PSD (post source decay) experiments, the precursor ions of interest were isolated using a timed ion selector. The laser intensities for PSD spectra were maintained the same as in the (reference) reflector mode spectra for the first segment spectrum but were gradually enhanced for all further segment spectra. Further information how to record PSD spectra is available in [19]. The fragment ions were refocused onto the detector by stepping the voltage applied to the reflectron in appropriate increments. This was done automatically by using the “FAST” (fragment analysis and structural TOF) subroutine of the Flex Control Program delivered by Bruker Daltonics [20]. As peaks in PSD spectra are broadened in comparison to standard mass spectra, not even the first decimals are given in that case [19].
Results and Discussion
Although a MALDI-TOF mass spectrometric characterization of bull spermatozoa lipid extracts was already performed [21], corresponding data on the lipid composition of roe deer spermatozoa are not yet available. Therefore, the method how the PL analysis may be performed by MALDI-TOF MS in combination with chemical modifications of the organic spermatozoa extracts shall be shortly introduced.
In Fig. 2 selected positive ion MALDI-TOF mass spectra of an organic extract of roe deer (a–c) and of a selected reference plasmalogen (d–f) are shown. Trace (2a) represents the native roe deer extract, whereas (2b) was recorded subsequent to PLA2 digestion and (2c) subsequent to exposition to HCl fumes. PLA2 cleaves the fatty acyl residue in sn-2 position selectively and HCl affects the vinyl ether linkage in sn-1 position. Therefore, the combination of both modifications provides important structural information.
Positive ion MALDI-TOF mass spectra of an organic extract of roe deer (a–c) and one commercially available plasmalogen 1-O-1′-(Z)-octadecenyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine (d–f). Spectra were recorded from the native samples (a, d) and subsequent to phospholipase A2 digestion (b, e) or exposition to HCl fumes (c, f). Peaks are labeled according to their m/z values and peaks stemming from the applied DHB matrix (0.5 mol/l in CH3OH) are marked with asterisks [16]. Please note that the contribution of matrix signals is more pronounced in the spermatozoa extracts because these are much less concentrated and contain higher amounts of salts than the commercially available plasmalogen sample
The peaks at m/z = 790.6 and 812.6 (2a) can be assigned to the H+ and Na+ adducts of 1-O-hexadec-1′-en-yl-2-docosahexaenoyl-sn-glycero-3-phosphocholine (GPC 16:0plasm/22:6), whereas the peaks at m/z = 792.6 and 814.6 are stemming from 1-O-hexadec-1′-enyl-2-docosapentaenoyl-sn-glycero-3-phosphocholine (GPC 16:0plasm/22:5) and the corresponding alkyl ether, 1-O-palmityl-2-docosahexaenoyl-sn-glycero-3-phosphocholine (GPC 16:0alkyl/22:6). The reasons leading to these assignments will be outlined below in more detail. This composition is similar to that of bull [21] and boar [22] spermatozoa. It is well known that the content of ether-linked PLs and particularly plasmalogens is characteristic of bull and boar spermatozoa [21]. In contrast, spermatozoa from man consist nearly exclusively of regular PCs but with high contents of highly unsaturated fatty acyl residues [13]. As the contribution of further PLs in spermatozoa is relatively low and PCs are most sensitively detected in mixtures under MALDI conditions [17], PCs will be exclusively discussed in this paper.
Of course, the peak assignment given above is based exclusively on the m/z value and, therefore, rather ambiguous. The most simple and traditional method of confirming peak assignments is to alter the compound of interest chemically in a defined way and to check whether the observed changes are in agreement with the putative compound [21]. Subsequent to the digestion of the roe deer spermatozoa lipid extract with the enzyme PLA2 (2b) that cleaves the acyl residue in sn-2 position selectively, it is evident that the peaks in the original extract are replaced completely by the corresponding lyso compounds at m/z = 480.3 and 482.3 (H+ adducts) as well as 502.3 and 504.3 (Na+ adducts). This is in agreement with the previous assumption of the alkenyl (about 75%) and the alkyl ether (about 25%) but does not allow clear conclusions about the acyl residues in sn-2 position. However, this information can also be easily obtained. Subsequent to HCl-induced hydrolysis (2c), there are significant amounts of LPC 22:6 (m/z = 568.3 and 590.3), but only relatively small amounts of LPC 22:5 (m/z = 570.3 and 592.3). Please note that there are still small peaks at m/z = 792.6 and 814.6. These signals represent the corresponding alkyl ether that is––in contrast to the plasmalogens––not affected by the HCl fumes. It was not the primary aim of this paper to investigate the lipid composition of roe deer spermatozoa in more detail but it is evident from these data that ether-linked PLs (1-O-hexadecyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine (GPC 16:0alkyl/22:6) and particularly plasmalogens (1-O-hexadec-1′-enyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine (GPC 16:0plasm/22:6) and 1-O-hexadec-1′-enyl-2-docosapentaenoyl-sn-glycero-3-phosphocholine (GPC 16:0plasm/22:5) are their most abundant constituents. Please also note that the peaks at m/z = 551.0, 545.0 and 511.0 are stemming from the applied DHB matrix [16].
The reliability of the applied method can be easily verified by using a commercially available plasmalogen species and treating this compound in the same way as the spermatozoa extract: 1-O-1′-(Z)-octadecenyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine was used because the most relevant plasmalogen of spermatozoa (with a hexadecenyl instead a octadecenyl residue) is not commercially available. The only difference between this reference compound and the plasmalogen of spermatozoa is one bismethylene group and, therefore, all masses differ for 28 Da. Accordingly, the pure plasmalogen gives two peaks at m/z = 818.6 and 840.6 (2d), after cleavage of the docosahexaenoyl residue by PLA2 digestion at m/z = 508. 3 and 530.3 (2e) and after cleavage of the alkenyl ether by HCl treatment at m/z = 568.3 and 590.3 (2f). This is in perfect agreement with the data obtained with the spermatozoa extract. Please note that the much higher contribution of matrix peaks [8] in the spermatozoa extracts (marked with asterisks) is a clear indication of the lower lipid concentration in the case of the spermatozoa. Finally, spermatozoa extracts also give signals at m/z = 469.3 and 535.3 (2c). Unfortunately, the origin of these peaks could not be clarified so far. However, it is most likely that these compounds represent PLs as they disappear completely subsequent to digestion with PLA2 (cf. 2b).
All MALDI-TOF mass spectrometers equipped with a reflector detector are basically capable of recording MS/MS spectra and this technique is called post source decay (PSD). PSD enables the identification of significant fragment ions that may help to confirm the identity of putative lipids, but has also the disadvantage that higher sample amounts in comparison to conventional MALDI-TOF MS are required [20].
The post source decay mass spectra of the most prominent peaks of spermatozoa extracts are shown in Fig. 3. The quality of the PSD spectra derived from the precursor ions with m/z = 812.6 (3a) and 814.6 (3b) is rather poor. Nevertheless both spectra are, besides the mass shift of 2 Da, virtually identical and confirm the proposed structures. The only difference is one fragment ion at m/z = 279 that is exclusively derived from the plasmalogen but not from the ether lipid.
Post source decay (PSD) MALDI-TOF fragment ion mass spectra of the spermatozoa lipids with m/z = 812.6 (a) and 814.6 (b) as parent ions. The same sample as shown in trace 1a was used. The derived fragment ions were refocused onto the detector by stepping the applied reflector voltage in appropriate increments. This is done automatically by using the “FAST” (“fragment analysis and structural TOF”) subroutine of the Flex Analysis Program provided by Bruker Daltonics. Please note that the reflector voltage was gradually increased from higher to lower masses. Therefore, in the same order the noise level increases. Structural assignments of the observed fragments are also provided (according to data given in [23]). Please note that under the applied PSD conditions no cleavage of C–C bonds occurs [20]
Unfortunately, it is very difficult to obtain information on acyl compositions of PCs by PSD because PCs are under conditions of MALDI-TOF MS only detectable as positive but not as negative ions [7]. In contrast, the negative ion PSD mass spectra would be most useful to obtain information about the released fatty acids. Nevertheless, the PSD spectra clearly confirm the presence of PC species [23].
One aim of this paper is the evaluation of the lipid composition of spermatozoa and the generation of characteristic lipid oxidation products in dependence of storage conditions. In Fig. 4 characteristic positive ion MALDI-TOF mass spectra of organic extracts of cattle (4a, c) and roe deer spermatozoa (4b, d) are shown. Fresh ejaculates were cryopreserved and lipids were extracted after thawing and washing (4a, 4b). Fresh ejaculates were washed and stored in physiological NaCl solution (without any preserving agent) for 24 h at 16 °C (c) or 5 °C (d) before lipid extraction. Both data sets (4a, b and 4c, d) differ significantly as the cryomedium contains a significant amount of egg yolk PC (m/z = 782.6, for instance, is attributable to the Na+ adduct of PC 16:0/18:1) that––even after centrifugation through a Percoll gradient––sticks to the spermatozoa membrane [24] and cannot be simply removed from the spermatozoa extracts by the washing process. Although the egg yolk PC interferes with the spermatozoa membrane lipids, it is obvious that the preserved spermatozoa do not give significant signals in the low mass region (the peak at m/z = 551.0 in trace 4a and 4b is a matrix peak [17]).
Positive ion MALDI-TOF mass spectra of organic extracts (obtained according to Bligh and Dyer) of spermatozoa from cattle (a, c) and roe deer (b, d). Samples shown in a and b were cryopreserved [21]. Samples in c and d were stored in NaCl solution for 24 h at 16 and 5 °C, respectively. All samples were mixed 1:1 (v/v) with a 0.5 mol/l DHB solution in CH3OH. Peaks marked with asterisks are caused by the DHB matrix. A more detailed methodological description of MALDI-TOF MS is available in [8] and typical matrix peak patterns are discussed in [16]
Compared to cryopreservation, unprotected storage of spermatozoa from both ruminants leads to plasmalogen oxidation (4c, 4d). The LPC content is most pronounced in the roe deer sample after storage at 5 °C (4d). In addition to LPC 22:6 at m/z = 568.4 and 590.4 there are also intense signals of formyl-LPC 22:6 (m/z = 596.4 and 618.4), but only very small signals indicating a cleavage of the double bonds along the docosahexaenoyl residue. Although a variety of such oxidation products was previously detected [5], their generation obviously does not play any major role in ruminantia spermatozoa. Additionally, the LPC 22:6 and formyl-LPC 22:6 ratio indicates that the generation of the latter species is slightly preferred under these conditions (cf. the intensities of the signals at m/z = 590.4 and 618.4). Accompanying investigations of isolated plasmalogen and PC species gave very similar results and will be discussed below in more detail. Please note that the peaks at m/z = 531.4 and 553.4 are not caused by oxidation but by the cleavage of the quaternary amine group of LPC 22:6 [23].
Further confirmation of peak assignments was obtained by PSD mass spectrometry. In Fig. 5 two typical PSD spectra of putative LPC 22:6 (5a) and formyl-LPC 22:6 (5b) are shown. The Na+ adducts (m/z = 590.4 and 618.4) were exclusively chosen as parent ions because Na+ adducts give a more intense fragmentation pattern than the corresponding H+ adducts [23]. The structural assignments of all observed fragments are also provided in Fig. 5. It is obvious that all detected fragment ions agree perfectly with the previous peak assignments and, accordingly, LPC 22:6 as well as formyl LPC 22:6 can be assumed as the prime products of plasmalogen oxidation.
PSD MALDI-TOF mass spectra of m/z = 590.4 (a) and 618.4 (b) as parent ions. Structural assignments of the observed fragments are also provided. Please note that it was not the aim of this paper to assign details of fragment ion structures. Therefore, the positions of charges and ions were only made by comparison with reference data [23]
Spermatozoa are known to contain different types of phospholipases [25] that might be released if the spermatozoa membrane gets damaged upon storage. We cannot rule out the contribution of phospholipases completely. However, formyl-LPC 22:6 may be only generated in the presence of oxygen. As the formyl-LPC 22:6 peaks possess a higher intensity than the LPC 22:6 peaks, we suggest the dominating role of oxidation processes but only a minor contribution of phospholipases.
In order to exclude that PCs (e.g., from the applied media or the spermatozoa) potentially interfere with the observed oxidation products, one selected PC (1-palmitoyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine, PDPC) was also investigated by positive ion MALDI-TOF MS prior (6a) and subsequent to air oxidation (6b). These results were compared with 1-O-1′-(Z)-octadecenyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine prior (6c) and subsequent to air oxidation (6d). It is obvious from (6a) that pure PDPC does not provide any other peaks than the H+ and the Na+ adducts at m/z = 806.6 and 828.6, respectively. However, subsequent to air oxidation (6b), many peaks with lower masses are obvious. These peaks can be assigned to LPC 16:0 (m/z = 496.3 and 518.3) that indicates the cleavage of the complete docosahexaenoyl residue and an oxidation product that corresponds to the cleavage of the double bond next to the ester linkage under generation of an aldehyde (cf. structure in the Fig) [7]. The two peaks with highest intensities (m/z = 580.4 and 602.4) can be easily assigned to the corresponding aldehyde, whereas the minor peaks at m/z = 596.4 and 618.4 are assumed to correspond to the organic acid resulting from the oxidation of the initially generated aldehyde. There are no products indicating an oxidative cleavage along the docosahexaenoyl residue in other positions than the position next to the ester linkage. In contrast, the artificial oxidation of the plasmalogen (6d) gives nearly exclusively LPC 22:6 and formyl-LPC 22:6. Therefore, in both cases the preferred oxidation site is the double bond next to the glycerol backbone and the number of the remaining olefinic residues along the acyl chain is less important.
From these results we conclude that (a) formyl-LPC 22:6 is an intrinsic marker of spermatozoa lipid oxidation and (b) this compound as well as LPC 22:6 can be easily determined by MALDI-TOF MS without the need of major sample workup. Both markers may be of high relevance for the determination of oxidative damages induced by the storage of spermatozoa (Fig. 6).
Positive ion MALDI-TOF mass spectra of 1-palmitoyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine (PDPC) (a) and subsequent to air oxidation (b). Traces (c) and (d) correspond to pure 1-O-1′-(Z)-octadecenyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine (c) and after exposition to air (d). 2,5-dihydroxybenzoic acid (DHB) was used as matrix and all samples (1 mg/ml) were mixed 1:1 (v/v) with a 0.5 mol/l DHB solution in CH3OH. Details on methodology and phospholipid oxidation are provided in “Materials and Methods”. The given structures correspond to intact PDPC and the corresponding oxidation product. For means of clarity the corresponding Na+ adducts (but not the H+ adducts) are exclusively shown
References
Brites P, Waterham HR, Wanders RJ (2004) Functions and biosynthesis of plasmalogens in health and disease. Biochim Biophys Acta 1636:219–231. doi:10.1016/j.bbalip.2003.12.010
Nagan N, Zoeller RA (2001) Plasmalogens: biosynthesis and functions. Prog Lipid Res 40:199–229. doi:10.1016/S0163-7827(01)00003-0
Lenzi A, Picardo M, Gandini L, Dondero F (1996) Lipids of the sperm plasma membrane: from polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum Reprod Update 2:246–256. doi:10.1093/humupd/2.3.246
Thompson DH, Inerowicz HD, Grove J, Sarna T (2003) Structural characterization of plasmenylcholine photooxidation products. Photochem Photobiol 78:323–330. doi:10.1562/00318655(2003)078 < 0323:SCOPPP > 2.0.CO;2
Zemski Berry KA, Murphy RC (2005) Free radical oxidation of plasmalogen glycerophosphocholine containing esterified docosahexaenoic acid: structure determination by mass spectrometry. Antioxid Redox Signal 7:157–169. doi:10.1089/ars.2005.7.157
Khaselev N, Murphy RC (2000) Peroxidation of arachidonate containing plasmenyl glycerophosphocholine: facile oxidation of esterified arachidonate at carbon-5. Free Radic Biol Med 29:620–632. doi:10.1016/S0891-5849(00)00361-0
Schiller J, Süß R, Arnhold J, Fuchs B, Leßig J, Müller M, Petković M, Spalteholz H, Zschörnig O, Arnold K (2004) Matrix-assisted laser desorption and ionization time-of-flight (MALDI-TOF) mass spectrometry in lipid and phospholipid research. Prog Lipid Res 43:449–488. doi:10.1016/j.plipres.2004.08.001
Schiller J, Süß R, Fuchs B, Müller M, Zschörnig O, Arnold K (2007) MALDI-TOF MS in lipidomics. Front Biosci 12:2568–2579. doi:10.2741/2255
Arnhold J, Osipov AN, Spalteholz H, Panasenko OM, Schiller J. (2001) Effects of hypochlorous acid on unsaturated phosphatidylcholines. Free Radic Biol Med 31:1111–1119. doi:10.1016/S0891-5849(01)00695-5
Schiller J, Zschörnig O, Petković M, Müller M, Arnhold J, Arnold K (2001) Lipid analysis of human HDL and LDL by MALDI-TOF mass spectrometry and 31P-NMR. J Lipid Res 42:1501–1508
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 3:911–917
Watson AD, Leitinger N, Navab M, Faull KF, Hörkkö S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, Berliner JA (1997) Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem 272:13597–13607. doi:10.1074/jbc.272.21.13597
Schiller J, Arnhold J, Glander HJ, Arnold K (2000) Lipid analysis of human spermatozoa and seminal plasma by MALDI-TOF mass spectrometry and NMR spectroscopy––effects of freezing and thawing. Chem Phys Lipids 106:145–156. doi:10.1016/S0009-3084(00)00148-1
Wright LC, Nouri-Sorkhabi MH, May GL, Danckwerts LS, Kuchel PW, Sorrell TC (1997) Changes in cellular and plasma membrane phospholipid composition after lipopolysaccharide stimulation of human neutrophils, studied by 31P NMR. Eur J Biochem 243:328–335. doi:10.1111/j.1432-1033.1997.0328a.x
Schiller J, Arnhold J, Benard S, Müller M, Reichl S, Arnold K (1999) Lipid analysis by matrix-assisted laser desorption and ionization mass spectrometry: a methodological approach. Anal Biochem 267:46–56. doi:10.1006/abio.1998.3001
Schiller J, Süß R, Fuchs B, Müller M, Petković M, Zschörnig O, Waschipky H (2007) The suitability of different DHB isomers as matrices for the MALDI-TOF MS analysis of phospholipids: which isomer for what purpose? Eur Biophys J 36:517–527. doi:10.1007/s00249-006-0090-6
Petković M, Schiller J, Müller M, Benard S, Reichl S, Arnold K, Arnhold J (2001) Detection of individual phospholipids in lipid mixtures by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry: phosphatidylcholine prevents the detection of further species. Anal Biochem 289:202–216. doi:10.1006/abio.2000.4926
Schiller J, Arnold K (2000) Mass spectrometry in structural biology. In: Meyers RA (ed) Encyclopedia of analytical chemistry. Wiley, Chichester
Hillenkamp F, Peter-Katalinić J (2007) MALDI MS––a practical guide to instrumentation, methods and applications. Wiley-VCH, Weinheim
Fuchs B, Schober C, Richter G, Süß R, Schiller J (2007) MALDI-TOF MS of phosphatidylethanolamines: different adducts cause different post source decay (PSD) fragment ion spectra. J Biochem Biophys Methods 70:689–692. doi:10.1016/j.jbbm.2007.03.001
Schiller J, Müller K, Süß R, Arnhold J, Gey C, Herrmann A, Leßig J, Arnold K, Müller P (2003) Analysis of the lipid composition of bull spermatozoa by MALDI-TOF mass spectrometry––a cautionary note. Chem Phys Lipids 126:85–94. doi:10.1016/S0009-3084(03)00097-5
Leßig J, Gey C, Süß R, Schiller J, Glander HJ, Arnhold J (2004) Analysis of the lipid composition of human and boar spermatozoa by MALDI-TOF mass spectrometry, thin layer chromatography and 31P NMR spectroscopy. Comp Biochem Physiol B 137:265–277. doi:10.1016/j.cbpc.2003.12.001
Al-Saad KA, Siems WF, Hill HH, Zabrouskov V, Knowles NR (2003) Structural analysis of phosphatidylcholines by post-source decay matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Am Soc Mass Spectrom 14:373–382. doi:10.1016/S1044-0305(03)00068-0
Cerolini S, Maldjian A, Pizzi F, Gliozzi TM (2001) Changes in sperm quality and lipid composition during cryopreservation of boar semen. Reproduction 121:395–401. doi:10.1530/rep.0.1210395
Roldan ERS, Shi QX (2007) Sperm phospholipases and acrosomal exocytosis. Front Biosci 12:89–104. doi:10.2741/2050
Acknowledgments
This work was supported by the German Research Council (DFG Schi 476/5-1, Mu 1520/2-1) and the Federal Ministry of Education and Research (Grant BMBF 0313836).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Fuchs, B., Müller, K., Göritz, F. et al. Characteristic Oxidation Products of Choline Plasmalogens are Detectable in Cattle and Roe Deer Spermatozoa by MALDI-TOF Mass Spectrometry. Lipids 42, 991–998 (2007). https://doi.org/10.1007/s11745-007-3108-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-007-3108-7