Abstract
Both genetic and environmental factors (e.g. nutrition, life style) contribute to the development of the plurimetabolic syndrome, which has a high prevalence in the world population. Dietary n-3 PUFAs specially those from marine oil (EPA and DHA) appear to play an important role against the adverse effects of this syndrome. The present work examined the effectiveness of fish oil (FO) in reversing or improving the dyslipidemia, insulin resistance and adiposity induced in rats by long-term feeding a sucrose-rich diet (SRD). We studied several metabolic and molecular mechanisms involved in both lipid and glucose metabolisms in different tissues (liver, skeletal muscle, fat pad) as well as insulin secretion patterns from perifused islets under the stimulation of different secretagogues. Dietary FO reverses dyslipidemia and improves insulin action and adiposity in the SRD fed rats. FO reduces adipocytes cell size and thus, the smaller adipocytes are more insulin sensitive and the release of fatty acids decreases. In muscle, FO normalizes both the oxidative and non-oxidative glucose pathways. Moreover, FO modifies the fatty acid composition of membrane phospholipids. In isolated β cells, lipid contents and glucose oxidation return to normal. All these effects could contribute to the normalization of glucose-stimulated insulin secretion and muscle insulin insensitivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Both genetic and environmental factors (e.g. nutrition, life style) contribute to the development of the plurimetabolic syndrome. This syndrome, which has a high prevalence in the world population, refers to a cluster of metabolic abnormalities including among others: insulin resistance, dyslipidemia [increased triglyceride and decreased high density lipoprotein cholesterol (HDLC) levels], hypertension, central obesity, glucose intolerance and type 2 diabetes. All of them are well-documented risk factors for cardiovascular disease [1].
The composition of the macronutrients in the diet plays an important role in modifying several key factors of this syndrome. In this regard, numerous clinical and animal studies on n-3 polyunsaturated fatty acids (PUFAs), especially those from marine oil—eicosapentaenoic acid (20:5n-3 EPA) and docosahexaenoic acid (22:6n-3, DHA)—have shown their beneficial effects impacting on normal health and chronic diseases (e.g. the plurimetabolic syndrome). In addition to their use as fuels and structural components of the cell, the dietary intake of marine n-3 PUFAs (fish oil) has proven to be effective in lowering both triglyceride (Tg) and VLDL-Tg concentration in experimental animals and normal and hypertriglyceridemic men (see review) [2, 3]. It has been shown that fish oil (FO) in rats decreases the mRNA encoding of several enzymes proteins involved in “the novo” hepatic lipogenesis suppressing the nuclear abundance and expression of sterol regulatory element binding protein 1 (SREBP-1), and enhances fatty acid oxidation throughout a peroxisome proliferator-activated receptor (PPARα)—stimulated process [4, 5]. Moreover, the effects of n-3 PUFAs includes the alteration of the fatty acid composition of membrane phospholipids that modify membrane-mediated processes such as insulin transduction signals, activities of lipases and biosynthesis of eicosanoids [6].
Numerous studies from our group (see review [2]) and others [7–10] have shown that normal rats fed a sucrose-rich diet for a short period (3–5 weeks) develop hypertriglyceridemia, increase plasma free fatty acid (FFA) levels and enhanced triglyceride accumulation in liver and heart muscle. This is accompanied by normoglycemia with hyperinsulinemia, insulin resistance, increase in the first peak of glucose stimulated insulin secretion in perifused islets and hypertension. A different picture emerges after a long-term feeding (15–40 weeks) of a sucrose-rich diet. In addition to the altered lipid metabolism and ectopic fat deposition in several non adipose tissues, rats develop visceral adiposity with a moderate increase in body weight, hyperglycemia with normoinsulinemia and a lack of the first peak with an increase in the second phase of glucose stimulated insulin secretion from perifused islets [2, 11–14]. Interestingly, this animal model exhibits many of the hallmarks present in the plurimetabolic syndrome in humans. Moreover, the temporal metabolic changes described may reflect the early start of type 2 diabetes mellitus, since many patients have chronically elevated plasma FFA and Tg levels, altered peripheral insulin sensitivity and loss of the first peak of insulin in response to glucose.
Most experimental studies examining the effect of dietary nutrients (e.g. n-3 fatty acids from marine source) on dyslipidemia and insulin resistance have focused on the development of the impairment. In this vein, it has been shown that FO prevents the onset of insulin resistance and dyslipidemia when fed to rats with high fat [2, 15–17] or high sucrose diets [2, 18, 19]. However, relatively few studies have examined the effectiveness of FO in reversing diet-induced insulin resistance [20–23]. Therefore, the rats fed a long-term sucrose-rich diet seem to be an appropriate experimental model to investigate the effect of dietary n-3 PUFAs (fish oil) to improve or reverse these metabolic abnormalities.
The present study was conducted on rats fed a SRD during 8 months, in which a stable dyslipidemia and insulin resistance had been present before the source of fat in the diet (corn oil) was replaced by an isocaloric amount of FO for the last 2 months. We analyzed several aspects of lipid and glucose metabolism in the liver, skeletal muscle and adipose tissue of these rats. In addition, we studied the insulin secretion patterns from “in vitro” perifused isolated islets under the stimulation of different secretagogues (glucose, palmitate) and peripheral insulin sensitivity (euglycemic–hyperinsulinemic clamp).
Materials and Methods
Animal Model and Diets
Two months old male Wistar rats, initially weighting 170–185 g were maintained under controlled temperature (22 ± 1 °C), humidity and air flow conditions, with a fixed 12 h light:dark cycle (light 07:00–19:00). After a 1-week acclimation period, the rats were randomly divided into two groups: experimental and control. The first group received a purified sucrose-rich diet (SRD) containing by weight 62.5% sucrose, 17% of vitamin free casein, 8% corn oil, 7.5% cellulose, 3.5% salt mixture (AIN-93-MX), 1% vitamin mixture (AIN-93M-VX), 0.2% choline chloride, and 0.3% methionine. The control group received the same semisynthetic diet but with sucrose replaced by starch (CD). The experimental group received the SRD for 6 months after which the rats were randomly divided into two subgroups. The rats of the first subgroup continued on the SRD up to 8 months. The second subgroup, SRD + fish oil, (SRD + FO) received the SRD in which the source of fat (corn oil 8/100 g) had been replaced by cod liver oil (7/100 g) plus (1/100 g) of corn oil during the last 2 months on the diet. The control group received the CD throughout the experimental 8-month period. The SRD without the addition of FO used from months 6 to 8 and the CD were balanced for cholesterol and vitamins D and A, present in the FO. Diets were isoenergetics, providing approximately 16.3 kJ/g of food and were available ad libitum. Diets were prepared every day by adding the oils and base mixture containing the other nutrients. The oils and base mixture were separately stored at 4 °C until preparation of the diet. Fish oil was kept under a nitrogen atmosphere during storage. The weight of each rat was assessed twice each week during the experimental period. At the end of the 8 months dietary period, except as otherwise indicated, the food was removed and experiments were performed between 09:00 and 12:00 h. The Human and Animal Research Committee of the School of Biochemistry, University of Litoral, Santa Fe, Argentina, approved the experimental protocol.
Analytical Methods
Rats were anesthetized with intraperitoneal pentobarbital sodium (60 mg/kg body weight). Blood samples were obtained from the jugular vein, rapidly centrifuged, and plasma was either immediately assayed or stored at −20 °C. Plasma triglyceride, FFA, glucose, leptin and adiponectine as well as immunoreactive insulin levels were determined as previously described [23]. Liver, pancreas, gastrocnemius muscle and white adipose tissue (epididymal and retroperitoneal) were rapidly removed from the anesthetized rats, and except otherwise indicated they were frozen, clamped in liquid nitrogen and stored at −80 °C. The weight of the epididymal and retroperitoneal adipose tissue was recorded.
Liver Tissue Assays
Homogenates of frozen liver were used for the determination of triglyceride content [23]. A cytosolic fraction of liver homogenates samples was obtained by centrifugation at 100,000×g, and the fatty acid synthase (FAS) activity was assayed immediately as described by Halestrap [24]. Carnitine palmitoyltransferase I (CPT-I) activity was determined spectrophotometrically following the release of CoA-SH from palmitoyl-CoA in liver homogenates in the presence and absence of l-carnitine [25]. The fatty acid oxidase activity (FAO) was measured by a modification of the procedure reported by Vamecq [26] and the H2O2 release was determined spectrophotometrically from a coupled peroxidative reaction [27]. The malic enzyme (ME) activity was assayed by the method proposed by Ochoa with a minor modification according to Hsu et al. [28]. Pyruvate dehydrogenase complex (PDHc), and glucose-6-P-dehydrogenase (G-6-PDH) activities were analyzed as previously described [21]. The triglyceride secretion rate (VLDL-Tg secretion) was evaluated in 12-h fasting rats following the procedure described by Lombardo et al. [20].
Gastrocnemius Muscle Assays
Triglyceride, long-chain acyl-CoA (LC ACoA), diacylglycerol (DAG), malonyl-CoA, glycogen, and glucose-6-phosphate content as well as the activities of glycogen synthase (GSa), PDHc and PDH kinase were analyzed in muscle homogenate as previously described [29]. Gastrocnemius lipids were extracted, total phospholipids separated by TLC and their fatty acid analyzed by a procedure described in detail by Brenner et al. [14]. The protein mass expression of nPKCθ in the cytosol and membrane fraction of the gastrocnemius muscle were measured as described by D’Alessandro et al. [29].
Euglycemic Hyperinsulinemic Clamp Studies
Whole body peripheral insulin sensitivity was measured using the euglycemic hyperinsulinemic clamp technique as described in detail elsewhere [12]. At the end of the second hour of the clamp studies, gactrocnemius muscles were quickly removed, frozen and stored at −80 °C for the assay of both glycogen and glucose-6-phosphate concentration and GSa activity [12].
Adipose Tissue Assays
Preparation of Isolated Adipocytes
The adipocytes were isolated from the epididymal fat pad according to the method described by Robdell [30]. Fat cell size, number and triglyceride content were determined in the isolated adipocytes [31]. Aliquots of isolated epididymal fat cells were incubated in a shaking Dubnoff water bath at 37 °C for 1 h; glycerol release was measured under these experimental conditions as an index of basal lipolysis. More details of the methodology employed have been previously described [31]. Lipoprotein lipase (LPL) and G-6-PDH activities in epididymal fat pads were also measured as previously described [31].
Total RNA Preparation and Relative Quantitative RT-PCR Analysis
Adipose tissue total RNA was prepared from fat pad tissue (epididymal and retroperitoneal) of each dietary group. Total RNA samples were stored at −80 °C until quantification of the target mRNAs (ob mRNA and adiponectin mRNA). A real time, two-step RT-PCR assay was developed for mRNA relative quantification as previously described [23]. The relative-quantitative data were expressed as the ratio of the level of adiponectin or leptin mRNA to that of 18S rRNA in arbitrary units.
Pancreas Tissue Assays
Perifusion of Isolated Islets
Islets were isolated by collagenase digestion and collected under a stereoscopic microscope. After a 30 min prewash period, a group of 30–40 islets were perifused with a Krebs Ringer buffer pH 7.4 at 37 °C (O2 95:CO2 5) containing high glucose concentration (16.5 mM) or glucose 16.5 mM plus palmitate 0.5 mM until the end of the perifusion (40 min). In all the experiments, aliquots from the effluent for insulin assays were collected after different periods of time and stored at −20 °C until insulin analysis. More details of the methodology employed have been previously described [22]. Islet triglyceride content and PDHc activity were assayed in isolated islets as previously described [22].
Statistical Analysis
Results were expressed as mean ± SEM. The statistical significant between groups was determined by one way analysis of variance followed by the inspection of all differences between pairs of means by the Newman Keul’s test. P values lower than 0.05 were considered to be statistically significant.
Results
Blood Variables
At the end of the dark period (7 am), plasma triglyceride, FFA and glucose concentration were higher (p < 0.05) in rats fed a SRD compared with age-matched controls fed a CD (Table 1). A complete normalization of all of these variables occurred in rats fed a SRD + FO during the last 2 months of the experimental period. Moreover plasma insulin levels did not differ between the groups (data not shown).
Liver Tissue
Main Effects of FO (n-3 PUFAs) on the Hepatic Liver Metabolism of SRD-fed Rats
Both triglyceride content and VLDL-triglyceride secretion were significantly higher in the liver of rats fed a SRD compared to those fed a CD (Table 2). The activities of the enzymes related to the “novo lipogenesis” were significantly increased in the SRD-fed rats, whereas the fatty acid oxidase (FAO) activity was similar and CPT-1 activity was significantly decrease compared to that in the control group fed a CD. The presence of FO in the SRD collectively acted to normalize or improve liver triglyceride contents and the VLDL-Tg secretion rate as well as the lipogenic enzyme activities, while increasing the mitochondrial and peroxisomal fatty acid oxidation (CPT-1 and FAO activities).
Skeletal Muscle
Effects of FO on Metabolite Concentrations, Enzyme Activities and PKCθ Mass Expression in Gastrocnemius Muscle of SRD-fed Rats
Compared to CD-fed rats, the gastrocnemius muscle of dyslipemic insulin-resistant, SRD-fed rats at the basal state (ex vivo) shows a significant increase (p < 0.05) of triglyceride, LC ACoA and DAG contents without changing malonyl-CoA levels (Table 3). Qualitative and quantitative analyses of Western blots showed that the relative abundance of nPKCθ isozyme was also significantly increased in the membrane fraction of the gastrocnemius muscle of the SRD, whereas the cytosol fraction was slightly but not significantly decreased. Moreover, both a reduced active form of PDHc and an increase in PDH kinase activities were observed in SRD-fed animals, suggesting an impaired glucose oxidation. Table 3 also shows that FO reduced the increased triglyceride contents to basal levels within the skeletal muscle cells, and restored the PDHc activity. Besides, FO improved long-chain acyl CoA and DAG concentration and the nPKCθ mass expression in the membrane fraction of the skeletal muscle of SRD-fed rats. Values were still higher than those observed in the CD-fed rats.
On the other hand, dietary FO reversed the impaired insulin-stimulated glucose-6-phosphate concentration, glycogen storage and glycogen synthase activity during the euglycemic hyperinsulinemic clamp (Fig. 1). Moreover, the glucose infusion rate (GIR), which measures whole body peripheral insulin action “in vivo”, was lower (p < 0.01) in the SRD-fed group [24.17 ± 4.34 μmol/(kg min), n = 6] compared to CD-fed rats [64.45 ± 5.00 μmol/(kg min), n = 6]. However, in the SRD + FO group, the GIR did not differ from the controls [68.10 ± 6.30 μmol/(kg min), n = 6].
Glucose-6-phosphate and glycogen concentration and glycogen synthase (GSa) activity in gastrocnemius muscle at the start (open bars 0 min) and at the end (closed bars 120 min) of the clamp studies in rats fed control (CD), sucrose-rich (SRD) or SRD + fish oil (SRD + FO). Values are expressed as mean ± SEM, n = 6. *p < 0.05 120 versus 0 min in CD, SRD or SRD + FO; (open circles) p < 0.05 SRD 120 versus CD and SRD + FO 120 min
The normalization of peripheral insulin sensitivity in the SRD + FO fed rats was accompanied by a significant increase in the n-3 PUFAs, specially EPA and DHA, in the phospholipids of the gastrocnemius muscle. The n-3/n-6 ratio was significantly higher (p < 0.05). Values were as follows: % fatty acids; n-3PUFAs (mean ± SEM n = 6): 13.61 ± 0.44 in CD, 5.58 ± 0.58 in SRD and 22.23 ± 0.61 in SRD + FO. The ratio n-3/n-6 was 0.35 in CD, 0.14 in SRD and 0.81 in SRD + FO, respectively.
Adipose Tissue
Effects of Dietary FO on Body Weight, Fat Pad Morphology, Triglyceride Content and Enzyme Activities
Rats fed a SRD developed visceral adiposity (increase in epididymal and retroperitoneal fat tissues) and a moderate increase in body weight (Table 4). Isolated adipocyte from epididymal fat tissue showed a significant increase in triglyceride contents, and cell volume without changes in total cell number compared to CD-fed rats. Lipoprotein lipase and glucose-6-phosphate dehydrogenase activities were significantly increased (p < 0.05) in the SRD. Dietary FO markedly reduced the fat pad mass and the hypertrophy of fat cells (decreased cell volume and triglyceride contents). However, only a slight but not significant decrease of total body weight was observed. In addition, we previously reported [31] that the histograms of adipose size distribution that showed a significant increase (37%) of the mean cell diameters in the SRD-fed rats compared to CD-fed animals were significantly improved in the group of rats fed the SRD + FO. The presence of FO in the SRD reduced the high levels of lipoprotein lipase and glucose-6-phosphate dehydrogenase activities. Basal lipolysis, which was significantly increased in isolated adipocytes of SRD-fed rats, was improved when FO replaced corn oil as a source of fat in the diet. Values were as follows (mean ± SEM n = 6) μmol glycerol/(106 cells × h); 0.7 ± 0.1 in CD; 3.4 ± 0.3 in SRD and 1.3 ± 0.2 in SRD + FO, p < 0.05 SRD versus CD and SRD + FO; p < 0.05 SRD + FO versus CD.
Plasma Leptin and Adiponectin Levels and Gene Expression in White Adipose Tissue
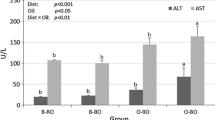
Both plasma leptin and adiponectin levels were significantly decreased in the SRD-fed rats. By shifting the source of fat in the diet to FO, the plasma levels of both adipokines reached values similar to those found in the rats fed a CD (Fig. 2a). Figure 2b shows obmRNA in adipose tissue (epididymal and retroperitoneal) of various dietary groups. Ob mRNA in both adipose tissues did not change between the different groups. Adiponectin mRNA showed a similar profile in both white adipose tissues. The presence of FO after the installation of insulin resistance induced by the SRD (Fig. 2b) had not additional effects. Therefore, Fig. 2b represents the obmRNA and adiponectin mRNA levels of both adipose tissues.
Plasma leptin and adiponectin levels (a) and white adipose tissue (WAT) (retroperitoneal and epididymal) ob and adiponectin mRNAs expression (b) of rats fed a control (CD), sucrose-rich (SRD) or SRD + fish oil (SRD + FO). Levels of mRNA were determined by real-time quantitative RT-PCR. The values are expressed as mean ± SEM, n = 6. *p < 0.05 SRD versus CD and SRD + FO
Pancreas
Insulin Secretion from Perifused Isolated Islets. Effect of Dietary Fish Oil
Perifused islets from SRD-fed rats showed an alteration in the biphasic patterns of glucose stimulated insulin secretion with an absence of the first peak and an increase in the second phase of hormone secretion compared to CD-fed rats. Dietary FO completely normalized glucose induced insulin secretion in the SRD-fed rats (Fig. 3a). Furthermore, as expected, a short-term exposure of β cell to 0.5 mmol/L of palmitate in the perifused fluid enhanced glucose stimulated insulin secretion in rats fed a CD. In the SRD-fed group, the presence of palmitate diminished both the first peak and the second phase of insulin secretion when compared to the CD group. Dietary FO enhanced the first and second phase of hormone secretion, which was greater (p < 0.05) than those in the SRD group and comparable to the control group fed a CD (Fig. 3b).
Insulin secretion in perifused pancreatic islets from rats fed control (CD), sucrose-rich (SRD) or SRD + fish oil (SRD + FO) diets under the stimulus of glucose a or glucose + palmitate b. Values are expressed as mean ± SEM, n = 6; *p < 0.05 SRD versus CD and SRD + FO at each time point in a and b; #p < 0.05 SRD + FO versus CD at each time point in b
Triglyceride Concentration and PDHc Activity in Isolated Islets
Figure 4a shows an increase (p < 0.05) in the triglyceride content in SRD-fed rats that was accompanied by a decrease (p < 0.05) in the PDHc activity (Fig. 4b). However, in the SRD + FO fed group neither variable differed from the CD group.
Triglyceride content a and pyruvate dehydrogenase complex (PDHc) activity b in isolated islets from rats fed control (CD), sucrose-rich (SRD) or SRD + fish oil (SRD + FO) diets. Values are mean ± SEM, n = 6. *p < 0.05 SRD versus CD and SRD + FO. PDHa, the active form of PDHc, was expressed as percentage of total PDHc activity (PDHt) (PDHa: basal activity × 100/total activity). To convert triglyceride contents to pmol, multiply ng by 1.13
Discussion
This work focused on the analyses of several metabolic and molecular mechanisms concerning the effect of fish oil (FO) on the reversion or improvement of dyslipidemia and insulin resistance ensuing a long-term feeding of a sucrose-rich diet to normal rats. The study shows that in this experimental model dietary FO is associated with a number of effects that collectively act to reduce dyslipidemia and improve insulin action.
Effects on the Liver
The liver plays a central role in whole body carbohydrate and lipid metabolism and several metabolic pathways may be regulated by PUFAs through changes in the activity or abundance of different transcription factor families, among them, PPARs, SREBPs, and LXR α and β. FO decreases plasma and liver triglyceride levels, VLDL-Tg secretion, and return plasma triglyceride to basal levels in SRD fed rats. Moreover, by shifting the source of fat in the SRD from corn oil to FO, the enzymatic activities of FAS, G-6-PDH, ME and PDHc, all of them involved with “the novo” lipogenesis, decreased to values similar to those recorded in the control fed rats while FAO and CPT1 activities were enhanced. Neschen et al. [32] have recently demonstrated that dietary n-3 PUFAs administered to rats increases the fatty acid oxidation capacity of tissues through their ability to function as ligand activators of transcription factor PPARα and thereby induces the transcription of several gene-encoding proteins affiliated with fatty acid oxidation. Besides, the suppression of “the novo” lipogenesis and MUFA synthesis by n-3 PUFAs requires that the transcription factor SREBP-1c [4]. Thus, in the SRD-fed rats, the normalization of plasma and liver triglyceride levels suggests that the principal action of FO on hepatic lipid metabolism involves a shift from lipid synthesis and storage to lipid oxidation and therefore, both mechanisms, thus contributing to the hypolipemic effect of FO.
Effects on Skeletal Muscle
In the skeletal muscle, the regulation of the glucose metabolism involves a complex interplay with the other fuels, especially free fatty acids. Studies in rats and humans [33] have shown that the degree of insulin resistance is strongly correlated with the muscle accumulation of triglycerides, and especially with LC ACoA. In the SRD-fed rats, D’Alessandro et al. [29] have recently demonstrated a significant increase in both triglyceride and LC ACoA contents within the gastrocnemius muscle which was accompanied by a significant increase in DAG and nPKCθ protein mass expression in the cell membrane fraction and a decrease in PDHc activity. LC ACoA by their esterification to DAG stimulate the PKC activity. Indeed, PKC disrupts the insulin signal via serine or threonine phosphorylation of insulin receptors, insulin receptor substrate 1, and potentially, other proteins such as glycogen synthase [34]. An increase in the LC ACoA concentration could also affect translocation of the glucose transport Glut 4 by acylating proteins involved in membrane fusion processes [35]. In addition, a significant reduction of flux through PDHc may limit glucose oxidation via the glucose–fatty acid cycle [29].
Fish oil normalizes and/or improves lipid storage and glucose oxidation within the skeletal muscle as well as nPKCθ proteins mass expression in the membrane fraction. Moreover, FO reversed the impaired insulin stimulated glycogen storage, glucose-6-phosphate concentrations, GSa activity and whole body insulin insensitivity, and returned plasma glucose and FFA levels to normal without changes in insulin levels.
The hypolipidemic effect of FO decreases the availability of the lipid fuel within the skeletal muscle and could, in turn, restore glucose oxidation and help to normalize insulin resistance. A recent study in healthy humans fed a high fat diet (75% fat) for 3 days showed an increase in PDH kinase and reduced PDHc activities in the skeletal muscle. In these people, the replacement of 15% of the fat by n-3 PUFAs decreased triglycerides and improved PDH kinase without changes in PDHc activities [36]. Moreover, Aas et al. [37] showed that the chronic exposure of EPA increased the uptake and oxidation of glucose despite a marked increase in fatty acid uptake and synthesis of complex lipids in cultured human skeletal muscle.
Increasing evidence suggests that the fatty acid composition of membrane phospholipids in the skeletal muscle and other target tissues is a critical factor that may induce changes in the structure and fluidity of cell membranes that could, in turn, directly affect insulin action. An increase in n-3 EPA and DHA as well as the n-3/n-6 ratio in the gactrocnemius muscle was observed after FO administration to SRD-fed rats, and this could also contribute to improve muscle insulin insensitivity. Moreover, Simoncikova et al. [38] also demonstrated that substitution of fish oil into a high fat diet in rats led to an improvement of “in vivo” insulin action. The insulin sensitive effect of FO was accompanied by a decrease of plasma FFA, triglyceride and glycerol levels and a decrease of lipid content in liver and skeletal muscle. However, another study by Podolin et al. [18] demonstrated that when the sucrose diet containing menhaden oil was given to insulin-resistant rats, insulin action on the glucose metabolism remained impaired. Differences in the amount of FO present in the diet as well as the n-3/saturated ratio between the oils employed may contribute to the effectiveness of FO in reversing insulin insensitivity.
Effects on Adipose Tissue
Dietary FO was able to reverse the preexisting metabolic and morphological changes of visceral fat pad tissue, reduce the hypertrophy of fat pad cells and improve their altered size distribution in the SRD fed rats. We have also recently observed that both the inhibitory effect of high sucrose upon the antilipolytic action of insulin and the impaired insulin stimulated glucose transport were completely normalized (unpublished results). The changes induced by FO could be possibly via mechanisms that include FO (specially EPA) binding and activation of the PPAR-γ2 isoform expression in white adipose tissue. PPAR-γ2 results in a coordinated increase in a large number of genes involved in lipid metabolism [39]. Moreover, PPAR-γ remodels the adipose tissue in adult animals, driving the formation of small insulin sensitive white adipocytes. Rossi et al. [23] have recently shown that the adiposity and insulin resistance present in the SRD-fed rats were accompanied by a decrease of plasma leptin and adiponectin levels, while no changes in the gene expression of both adipokines in visceral fats (epididymal and retroperitoneal) were observed. Leptin and adiponectin play an important role in the lipid and glucose metabolism and their regulation by FO might be implicated in the functional and morphological changes present in the adipose tissue of SRD-fed rats. Indeed, by shifting the source of fat to FO, the plasma levels of both adipokines were increased without changes in their gene expression, while insulin insensitivity and dyslipidemia were reversed and adiposity improved. Although the mechanisms by which FO increase plasma leptin and adiponectin are still unclear, these results suggest that the normal levels reached by both adipokines might play an essential role in the normalization of insulin resistance and adiposity [23]. Recently, Yamauchi et al. [40] demonstrated that insulin resistance in lipoatrophic mice was completely reversed by a combination of physiological doses of adiponectin and leptin, but only partially when either adipokine was given alone, suggesting that leptin and adiponectin may work hand in hand to sensitize peripheral tissues to insulin. Although we cannot extrapolate these results to humans, Skurnick-Minot et al. [41] demonstrated a decrease of whole body adiposity and adipocyte size in type 2 diabetic insulin resistant patients after 2 months of treatment with FO capsules (1.8 g of n-3 PUFAs). In these patients, plasma adiponectin tended to increase without any deterioration or amelioration of insulin sensitivity at this stage.
Effects on β Cell Function
A chronic exposure to hyperglycemia and high levels of plasma FFA has been shown to have a deleterious effect on both insulin secretion and action, a concept termed glucolipotoxicity. Several mechanisms have been proposed that could contribute to the dysfunction of the β cell such as changes in glucose oxidation, increase in triglyceride content within the islets, down regulation of several genes including glut 2 and glucokinase, stimulation of SREBP-1c and PPARγ among others (See [42] for a review). In long-term SRD-fed rats, we have recently demonstrated [22] highly deteriorated insulin secretion patterns in response to glucose stimulus. Furthermore, the islets acute exposure to palmitate in the perifusion medium did not improve insulin release [22]. This was accompanied by an increase in the triglyceride storage within the islets that occurred concomitantly with a reduction of the PDHc activity. A decreased flux through the PDHc is associated with lower PDHa levels, and could involve activation of the PDH kinase. The inhibition of PDHc limits the oxidative glucose metabolism, a signal for insulin secretion and synthesis. These could play a key role in the abnormal insulin secretion of rats chronically fed a SRD. Moreover, the beneficial hypolipidemic effect of dietary FO on β cell dysfunction becomes evident since after FO administration all the above alterations were completely normalized. Interestingly, an improvement by fish oil of both insulin sensitivity and i.v glucose tolerance has been shown in patients with primary hypertriglyceridemia [43].
Finally, dietary FO appears to play an important protecting role against the adverse symptoms of the plurimetabolic syndrome. The present work suggests some possible mechanisms through which FO improve or reverse dyslipidemia, β cell dysfunction and adiposity in rats fed a long-term a SRD. However, some effects of n-3 PUFAs on physiological processes such as the nature of the intracellular signal responsible for regulating the various affected transcription factors, both in human and experimental models, still remain unclear. Future research in this area will contribute to our understanding of how these singular lipids impact upon human health and disease.
References
Cheal KL, Abbasi F, Lamendola C, McLaughlin T, Reaven GM, Ford ES (2004) Relationship to insulin resistance of the adult treatment panel III diagnostic criteria for identification of the metabolic syndrome. Diabetes 53:1195–1200
Lombardo YB, Chicco A (2006) Effects of dietary polyunsaturated n-3 fatty acids on dyslipidemia and insulin resistance in rodents and humans. A Rev J Nutr Biochem 17:1–13
Connor WE (2000) Importance of n-3 fatty acids in health and disease. Am J Clin Nutr 71(suppl):171S–175S
Jump DB, Botolin D, Wang Y, Xu J,Christian B, Demeure O (2005) Fatty acid regulation of hepatic gene transcription. J Nutr 135:2503–2506
Clarke SD (2001) Polyunsaturated fatty acid regulation of gene transcription: a molecular mechanism to improve the metabolic syndrome. J Nutr 131:1129–1132
Clamp AG, Ladha S, Clark DC, Grimble RF, Lund EK (1997) The influence of dietary lipids on the composition and membrane fluidity of rat hepatocyte plasma membrane. Lipids 32:179–184
Reaven GM (1984) Diabetic hypertriglyceridemia in the rat: animal models simulating the clinical syndromes of impaired glucose tolerance, noninsulin-dependent diabetes and insulin-dependent diabetes. In: Shafrir E, Renold AS (eds) Lessons from animal diabetes. Libby, London, pp. 531–536
Pagliassotti MJ, Prach PA, Koppenhafer TA, Pan DA (1996) Changes in insulin action, triglycerides, and lipid composition during sucrose feeding in rats. Am J Physiol 271:R1319–R1326
Bezerra RMN, Ueno M, Silva MS, Tavares DQ, Carvalho CRO, Saad MJA (2000) A high fructose diet affects the early steps of insulin action in muscle and liver of rats. J Nutr 130:1531–1535
Luo J, Rizkalla SW, Lerer-Metzger M, Boillot J, Ardeleanu A, Bruzzo F, Desplanque N, Dalix AM, Durand G, Slama G (1995) A fructose-rich diet decreases insulin-stimulated glucose incorporation into lipids but not to glucose transport in adipocytes of normal and diabetic rats. J Nutr 125:164–171
Chicco A, Soria A, Fainstein-Day P, Gutman R, Lombardo YB (1994) Multiphasic metabolic changes in the heart of rats fed a sucrose-rich diet. Horm Metab Res 26:397–403
Chicco A, D’Alessandro ME, Karabatas L, Pastorale C, Basabe JC, Lombardo YB (2003) Muscle lipid metabolism and insulin secretion are altered in insulin-resistant rats fed a high sucrose diet. J Nutr 133:127–133
Lombardo YB, Drago S, Chicco A, Fainstein-Day P, Gutman R, Gagliardino JJ, Gomez Dumm CL (1996) Long-term administration of a sucrose-rich diet to normal rats: relationship between metabolic and hormonal profiles and morphological changes in the endocrine pancreas. Metabolism 45:1527–1532
Brenner RR, Rimoldi OJ, Lombardo YB, Gonzalez MS, Bernasconi AM, Chicco A, Basabe JC (2003) Desaturase activities in rat model of insulin resistance induced by a sucrose-rich diet. Lipids 38:733–742
Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW (1991) Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglycerides and w-3 fatty acids in muscle phospholipid. Diabetes 40:280–289
Taouis M, Dagou C, Ster C, Durand G, Pinault M, Delarue J (2002) n-3 Polyunsaturated fatty acids prevent the defect of insulin receptor signaling in muscle. Am J Physiol 282:E664–E671
Storlien LH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, Pascoe WS (1987) Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science 237:885–888
Podolin DA, Gayles EC, Wei Y, Thresher JS, Pagliassotti MJ (1998) Menhaden oil prevents but does not reverse sucrose-induced insulin resistance in rats. Am J Physiol 274:R840–R848
Peyron-Caso E, Fluteau-Nadler S, Kabir M, Guerre-Millo M, Quignard-Boulange A, Slama G, Rizkalla SW (2002) Regulation of glucose transport and transporter 4 (Glut-4) in muscle and adipocytes of sucrose-fed rats: effects of n-3 poly- and monounsaturated fatty acids. Horm Metab Res 34:362–366
Lombardo YB, Chicco A, D’Alessandro ME, Martinelli M, Soria A, Gutman R (1996) Dietary fish oil normalizes dyslipidemia and glucose intolerance with unchanged insulin levels in rats fed a high sucrose diet. Biochem Biophys Acta 1299:175–182
D’Alessandro ME, Chicco A, Karabatas L, Lombardo YB (2000) Role of skeletal muscle on impaired insulin sensitivity in rats fed a sucrose-rich diet: effect of moderate levels of dietary fish oil. J Nutr Biochem 11:273–280
Pighin D, Karabatas L, Rossi A, Chicco A, Basabe JC, Lombardo YB (2003) Fish oil affects pancreatic fat storage, pyruvate dehydrogenase complex activity and insulin secretion in rats fed a sucrose-rich diet. J Nutr 133:4095–4101
Rossi A, Lombardo YB, Lacorte JM, Chicco A, Rouault C, Slama G, Rizkalla SW (2005) Dietary fish oil positively regulates plasma leptin and adiponectin levels in sucrose-fed, insulin-resistant rats. Am J Physiol 289:R486–R494
Halestrap AP, Denton RM (1973) Insulin and the regulation of adipose tissue acetylCoA carboxylase. Biochem J 132:509–517
Karlic H, Lohninger S, Koeck T, Lohninger A (2002) l-carnitine stimulates carnitine acyltransferases in the liver of aged rats. J Histochem Cytochem 50:205–212
Vamecq J (1990) Fluorometric assay of peroxisomal oxidase. Anal Biochem 186:340–349
Xing Xian Y, Drackley JK, Odle J (1998) Food deprivation changes peroxisomal β oxidation activity but not catalase activity during postnatal development in pig tissues. J Nutr 128:1114–1121
Hsu TH, Lardy HA (1969) Method of enzymatic analysis, Vol XIII, pp 230
D’Alessandro ME, Chicco A, Lombardo YB (2006) A long-term sucrose-rich diet increases diacylglycerol content and membrane nPKCθ expression and alters glucose metabolism in skeletal muscle of rats. Nutr Res 26:289–296
Rodbell M (1964) Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism on lipolysis. J Biol Chem 239:375–380
Soria A, Chicco A, D’Alessandro ME, Rossi A, Lombardo YB (2002) Dietary fish oil reverse epididymal tissue adiposity, cell hypertrophy and insulin resistance in dyslipemic sucrose fed rat model. J Nutr Biochem 13:209–218
Neschen S, Moore I, Regittnig W, Yu CL, Wang Y, Pypaert M, Petersen KF, Shulman GI (2002) Contrasting effects of fish oil and safflower oil on hepatic peroxisomal and tissue lipid content. Am J Physiol 282:E395–E401
Ellis BA, Poynten A, Lowy AJ, Furler SM, Chisholm DJ, Kraegen EW, Cooney GJ (2000) Long chain acyl-CoA esters as indicators of lipid metabolism and insulin sensitivity in rat and human muscle. Am J Physiol 279:E554–E560
Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron T, Kim JK, Cushman SW, Cooney GJ, Cooney BA, White MF, Kraegen EW, Shulman GI (2002) Mechanism by which fatty acid inhibits insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol-3-kinase activity in muscle. J Biol Chem 277:50230–50236
Sleeman MW, Donegan NP, Heller-Harrison R, Lane WS, Czeck MP (1998) Association of acyl-CoA synthetase-1 with Glut4-containing vesicle. J Biol Chem 273:3132–3135
Turvey EA, Heigenhauser JF, Parolin M, Peters SJ (2005) Elevated n-3 fatty acids in a high-fat diet attenuate the increase in PDH kinase activity but not PDH activity in human skeletal muscle. J Appl Physiol 98:350–355
Aas V, Rokling-Andersen H, Kase ET, Thoresen GH, Rustan AC (2006) Eicosapentaenoic acid (20:5 n-3) increases fatty acid and glucose uptake in cultured human skeletal muscle cells. J Lipid Res 47:366–374
Simoncikova P, Wein S, Gasperikova D, Ukropec J, Certik M, Klimes I, Sebokova E (2002) Comparison of the extrapancreatic action of gamma-linolenic acid and n-3 PUFAs in the high fat diet-induced insulin resistance. Endocr Regul 36:143–149
Brun RP, Spiegelman BM (1997) Obesity and the adipocyte. J Endocrinol 155:217–218
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akunuma Y, Gavrilova O, Vinson C, Reitman M, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7:887–888
Skurnick-Minot G, Laromiguiere M, Oppert JM, Quignard-Boulange A, Boillot J, Rigoir A, Slama G, Rizkalla SW (2004) Whole-body fat mass and insulin sensitivity in type 2 diabetic women: effect of n-3 polyunsaturated fatty acids. In: 64th ADA Meeting, Orlando, June 4th–8th, Diabetes 53, Suppl 2, A44 (0159)
Poitout V (2004) β-cell lipotoxicity: burning fat into heat? Endocrinology 145:3563–3565
Zak A, Zeman E, Tvrzicka E, Pisarikova A, Sindelkova E, Vrana A (1993) Glucose tolerance, insulin secretion, plasma lipid fatty acids, and the hypolipidemic effects of fish oil. In: Klimes I, Howard BU, Storlien LH, Sebokova E (eds) Dietary lipids and insulin action, vol 683. New York Academy of Sciences, New York, pp 378–379
Acknowledgments
This investigation was carried out with the financial support of the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT) and CONICET, Grants N° PICTO # 05-13260/BID 1201/OC-AR, PIP # 5619/2005. The authors thank A.M. Bernasconi, Instituto de Investigaciones Bioquímicas de La Planta (INIBIOLP) for her technical assistance in the determination of muscle phospholipids.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lombardo, Y.B., Hein, G. & Chicco, A. Metabolic Syndrome: Effects of n-3 PUFAs on a Model of Dyslipidemia, Insulin Resistance and Adiposity. Lipids 42, 427–437 (2007). https://doi.org/10.1007/s11745-007-3039-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-007-3039-3