Abstract
The presence of dyes is one of the main contributors to the organic load in textile effluents. In this study a mixture of surfactants, produced from animal/vegetable fats, was used to remove the Direct Yellow 27 dye from a synthetic wastewater through an ionic flocculation process. It was evaluated the effect of contact time, temperature, and surfactant concentration on dye removal efficiency. It was also evaluated the kinetics, equilibrium, and diffusion mechanism of the process. The kinetics of the process was well described by both Pseudo-second order and Elovich models. The transport of dye molecules to the surfactant flocs is controlled by the external layer. Equilibrium data showed a good fit to the Langmuir model. A removal rate of 93% was achieved in a single stage, after 5 h of contact time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water pollution is a consequence of several human activities, causing serious health problems to the population. Textile wastewater is of great concern due to its toxicity levels. The dye bath generates large volumes of effluents containing heavy metals, aromatic compounds, halogenated hydrocarbons, surfactants, antifoaming agents, sodium salts, and dyes unfixed to the textile material [1]. Dyes are toxic substances with mutagenic and carcinogenic properties that can also cause allergic skin reactions [2]. Azo dyes are molecules that are not decomposed under sunlight or radiant energy due to their high photolytic stability [3]. The azo bond (–N=N–) is responsible for the resistance against aerobic bacterial attack [4]. Therefore, it is important to use the appropriate wastewater treatment to obtain effective pollutant removal. With this goal, many researchers are being challenged to develop novel wastewater treatment processes.
Among the techniques used for wastewater treatment, one can highlight the coagulation-flocculation method [5], advanced oxidation processes [6], nanofiltration [7], and the adsorption process. In the latter, different types of adsorbents are used, such as silica gel [8], alumina [9], and zeolites [10]. Processes involving the azo dye degradation can generate aromatic amines, which are considered carcinogenic and can accumulate in the food chain, harming human health and endangering ecosystems [3].
Surfactants have been studied as separating agents in several processes, such as cloud point extraction [11], microemulsion [12], and micellar enhanced ultrafiltration [13]. Another promising process is the ionic flocculation, which uses low cost carboxylate surfactants. Carboxylate surfactants are molecules obtained easily through the saponification reaction of vegetable oils and animal fats using sodium hydroxide. The chemical structure of a biosourced carboxylate surfactant consists of a nonpolar tail group (linear hydrocarbon chain) bound to a polar head (carboxylate group). In aqueous media, these surfactants react with calcium ions forming low solubility salts, producing scummy deposits [14]. According to Zapf et al. [15], in most systems with divalent counterions, the Krafft temperature is much higher than the one of single-charged systems. For carboxylate surfactants, this temperature is higher than 100 °C, making them unattractive for applications in aqueous media. On the other hand, this insolubility in water and the presence of a nonpolar tail make these surfactants attractive for ionic flocculation, because the insoluble surfactant, under stirring, aggregates to form flocs capable of adsorbing organic compounds in aqueous media.
In this work, ionic flocculation was used to remove Direct Yellow 27 dye from a synthetic textile wastewater. A new separating agent, consisting of insoluble flocs of calcium surfactant, was obtained by adding a calcium chloride solution to the surfactant solution. The experiment evaluated the effects of contact time, surfactant concentration, and temperature on process efficiency. The authors also investigated the equilibrium and diffusion mechanism of the dye molecules to the flocs.
Materials and Methods
Materials
The base soap was the surfactant used in this study, which was produced from a mixture of coconut oil (5%) and beef tallow (95%) [16]. The Direct Yellow 27 (DY27) dye (molar mass = 662.62 g/mol, λ max = 393 nm, C25H20N4Na2O9S3) was obtained from Ciba-Geigy. Calcium chloride (CRQ) was used for surfactant flocculation. Tests were carried out in a thermostatic bath provided with a stirring system (Water Separability Tester, Koehler Instrument Company, Inc., USA). Dye concentrations before and after experiments were measured by UV–Vis spectrometry (Varian Analytical Instruments, Cary 50 Conc, USA). All chemicals were used without previous purification, and distilled water was employed to obtain solutions and synthetic wastewater containing dye.
Methods
Preparation of Samples
Samples were prepared by dissolving the surfactant in distilled water into 100-mL test tubes to obtain solutions with surfactant concentration ranging from 290 to 650 ppm. The systems were vigorously stirred (680 rpm, 30 min) until all the surfactant was solubilized. After this step, the calcium chloride stock solution (3600 ppm) was added and homogenized, under stirring (100 rpm, 5 min), to obtain samples with calcium concentration/surfactant concentration ratio equal to 1:2. This proportion was fixed to ensure the formation of calcium surfactant flocs. After floc formation, the required volume of the dye stock solution (2000 ppm) was added, under stirring (100 rpm), to obtain 100 ppm initial dye concentration. All procedures were carried out in a thermostatic bath (30, 40, and 50 °C).
Kinetic Study
For the kinetic study, 4-mL sample aliquots were taken at regular time intervals (0.5, 5, 15, 30, 45, 60, 90, 120, 150, 180, 240, and 300 min). The kinetic models of Pseudo-second order [17] and Elovich [18] were used to describe the experimental data. The linearized equations for the Pseudo-second order and Elovich models are given by Eqs. 1 and 2, respectively:
where q t and q e are the flocs uptake capacities at any time and at equilibrium, respectively, k 2 is the rate constant of the Pseudo-second order model, a and b are the constants of the Elovich model.
The validity of each model was evaluated considering the error function, based on the normalized standard deviation, given by Eq. 3:
where, q exp and q mod are the uptake capacities obtained experimentally and using the models, respectively, and N is the number of experimental points.
Diffusion Mechanism
Although the kinetic models are useful to describe the uptake capacity at different contact times, they are not able to determine which stage controls the diffusion in a process. For this purpose, two models were tested: the intra-particle diffusion model and the Boyd diffusion model.
The determination of diffusivity is not a simple task and, in diffusion studies, the diffusion rate can be written in terms of the square root of time [19]. The uptake capacity using the intra-particle model can be obtained by using Eq. 4:
where, k ip (mg/g min0.5) is the intra-particle diffusion rate constant and C is a constant that is proportional to the thickness of the external layer [20], i.e., the higher the value of C is, the thicker the layer will be [21].
The second model used to describe the diffusion mechanism was the Boyd model. This model is given by Eq. 5 [20]:
where, F = q t/q e is the fractional attainment of equilibrium at time t and B t is a function of F. The value of B t can be obtained by using Eqs. 6 and 7:
The analysis was performed by plotting the calculated B t values against time t. If the plot is linear and passes through the origin, then pore diffusion controls the rate of mass transfer. If the plot is nonlinear or linear without passing through the origin, then the diffusion in the film or chemical reaction controls the rate of mass transfer [21–23].
Equilibrium Study
The Langmuir model was used to evaluate equilibrium data (Eq. 8):
where, q m is the maximum uptake capacity, n is the Langmuir model constant, and C e is the concentration at equilibrium in the aqueous phase. The equilibrium isotherms were obtained by plotting C e/q against C e.
Results and Discussion
Kinetic Evaluation
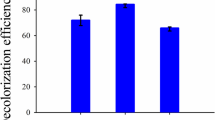
Figure 1 shows the plot of dye removal efficiency versus contact time, at 30, 40, and 50 °C. The surfactant concentration was fixed at 650 ppm based on a previous study, in which this concentration presented the best results for dye removal [16].
It can be observed in Fig. 1 that dye removal efficiency increases with contact time, achieving up to 93% dye removal. It is also observed that equilibrium is attained at 180 min contact time. Equations 1–3 were applied to determine which kinetic model appropriately describes the kinetic data. Table 1 shows the constants of the Pseudo-second order and Elovich models. Analyzing the data of R 2 and the error function one can conclude that the applied models are adequate to describe the kinetic data.
According to Wu et al. [18], the Elovich model is used when chemisorption occurs and the mass transfer rate decreases with contact time due to saturation of available sites in the surface. Dotto and Pinto [24] state that the Pseudo-second order kinetic model assumes that uptake process is a pseudo-chemical reaction. Therefore, the adsorption of DY27 in the surfactant floc is considered as a chemical process.
Equation 9 gives the approaching equilibrium factor (R E ), which is derived from the Elovich equation [18]. According to this factor, the kinetic curve shows four types of behavior: slow rising (R E > 0.3), mild rising (0.1 < R E < 0.3), fast rising (0.02 < R E < 0.1), and close to instantaneous equilibrium (R E < 0.02):
where, q ref is the uptake capacity at the longest operation time (300 min). The calculated R E values were 0.18, 0.17, and 0.16, for 30, 40, and 50 °C, respectively, suggesting that the kinetic curve behavior is in the mild rising zone.
In the ionic flocculation it is essential to define the operating time to reduce operational costs. The relation between uptake capacity and the operation time depends on the R E value. Equation 10, presented by Wu et al. [18], was used to determine the best operating time:
where, t x and t 0.85 are the operating times required to achieve an uptake capacity at time t and at time to obtain 85% of q e, respectively, and X is the q t/q ref ratio.
The plots of t x /t 0.85 versus X in is shown in Fig. 2. When X increases, the interaction between dye and floc increases (decreasing the surfactant consumption); however, t x /t 0.85 also increases (increasing the operation time). The optimum value for X must be based on economic criteria.
Based on Fig. 2, an operating time of 120 min was defined to reduce process costs, considering that the analysis was performed for 85% dye removal efficiency and that the floc used in this research is from natural sources and relatively inexpensive. This operating time was used in the equilibrium experiments.
Diffusion Mechanism
To understand the diffusion mechanism, the intra-particle diffusion model and the Boyd diffusion model were used to determine the rate limiting step of dye diffusion process into the surfactant floc.
The linear fit of the uptake capacity (q t) versus the square root of time (t 1/2) was evaluated to test the intra-particle diffusion model. As can be observed in Fig. 3 all plots present multilinear behavior [20, 21, 23, 25]. This multilinear behavior indicates that two or more stages are controlling the diffusion process [23, 24, 26]. Stage 1 represents the diffusion in the external layer, Stage 2 indicates the intra-particle diffusion, and Stage 3 represents the final equilibrium stage, where the intra-particle diffusion decreases due to the low concentration of dye in the solution and low availability of active sites on the floc surface [27].
It can be seen in Fig. 3 that the straight lines in the plots, for all studied temperatures, deviate from the origin, showing that the constant C of Eq. 4 is not zero, and indicating that the external layer presents a resistance for dye diffusion, i.e., in the liquid film around the floc (Stage 1). Table 2 shows the values of the intra-particle diffusion constant and the determination coefficient for the stages involved in the diffusion process.
Table 2 shows that the constant of the intra-particle diffusion rate presents its maximum value at Stage 2, for all evaluated temperatures (intra-particle diffusion). The last stage shows the lowest values for the diffusion constant, because, at this stage, the dye concentration is smaller and the diffusion process reaches its final equilibrium [26]. According to Allen et al. [19], this last stage occurs very quickly and does not represent a controlling step. Stage 1, which corresponds to diffusion in the external layer, shows a lower diffusion constant than Stage 2, suggesting that this stage controls the diffusion process.
The Boyd diffusion model was used to confirm whether the controlling step of the diffusion process occurs at Stage 1. This model allows one to identify which stage controls the diffusion process, i.e., the diffusion in the external layer or the intra-particle diffusion. Figure 4 shows the plots of B t versus time.
If the plots shown in Fig. 4 are linear and cross the origin, the pore diffusion controls the process. On the other hand, if the plots are nonlinear or deviate from the origin, the dye diffusion is controlled by the external layer [22]. In all cases evaluated in Fig. 4, the plots are nonlinear, indicating that the rate controlling step is the diffusion in the external layer, as shown by the intra-particle model.
Equilibrium Behavior
Equilibrium data were obtained for different surfactant concentrations (290–650 ppm), since in higher concentrations the improvement in dye removal efficiency is not significant to justify higher surfactant consumption. The contact time was set at 120 min based on kinetic evaluation (“Kinetic Evaluation”). The Langmuir model (Eq. 8) was applied to describe the equilibrium data of the dye adsorption on surfactant flocs. Table 3 shows the values of the Langmuir constants (q m and n), the coefficient of determination, and the values of the separation factor, R L (Eq. 11):
Table 3 shows that the experimental data are well fitted by the Langmuir model (R 2 > 0.96). According to the separation factor value, the Langmuir isotherm can be classified into four types: unfavorable (R L > 1), linear (R L = 1), favorable (0 < R L < 1), and irreversible (R L = 0). It can be observed that all separation factor values are between 0 and 1, therefore, the dye uptake in the floc can be classified as favorable. It is also observed that the values of q m increases with temperature, showing that temperature enhances the interaction of DY27 with the active sites available onto the floc surface. This might be due to the changes in the pore size of the floc as well as to an increase in the number of adsorption sites available at higher temperatures [28].
Conclusions
This study assessed the adsorption of Direct Yellow 27 dye onto calcium surfactant flocs. The main findings are listed below:
-
The pseudo-second order and Elovich models are suitable to describe the kinetics of the process.
-
The kinetic is classified as mild rising according to the approaching equilibrium factor.
-
The transport of dye molecules to the surfactant flocs is controlled by diffusion in the external layer.
-
The dye uptake onto surfactant flocs is a favorable process, achieving up to 93% dye removal.
References
Snowden-Swan LJ. Industrial pollution prevention handbook—pollution prevention in the textile industries. New York: McGraw-Hdl Inc; 1995.
Hunger K. Industrial dyes: chemistry, properties, applications. Weinheim: WILEY-VCH Verlag GmbH & Co.; 2003.
Lau Y, Wong Y, Teng T, Morad N, Rafatullah M, Ong S. Coagulation-flocculation of azo dye Acid Orange 7 with green refined laterite soil. Chem Eng J. 2014;246:383–90.
Nawaz MS, Ahsan M. Comparison of physico-chemical, advanced oxidation and biological techniques for the textile wastewater treatment. Alex Eng J. 2014;53:717–22. doi:10.1016/j.aej.2014.06.007.
Albuquerque LF, Salgueiro AA, Melo JLS, Chiavone-Filho O. Coagulation of indigo blue present in dyeing wastewater using a residual bittern. Sep Purif Technol. 2013;104:246–9. doi:10.1016/j.seppur.2012.12.005.
Karthikeyan K, Titus A, Gnanamani A, Mandal AB, Sekaran G. Treatment of textile wastewater by homogeneous and heterogeneous Fenton oxidation processes. Desalination. 2011;281:438–45. doi:10.1016/j.desal.2011.08.019.
Gozálvez-Zafrilla JM, Sanz-Escribano D, Lora-García J, Hidalgo MCL. Nanofiltration of secondary effluent for wastewater reuse in the textile industry. Desalination. 2008;222:272–9. doi:10.1016/j.desal.2007.01.173.
Andrzejewska A, Krysztafkiewicz A, Jesionowski T. Treatment of textile dye wastewater using modified silica. Dyes Pigments. 2007;75:116–24. doi:10.1016/j.dyepig.2006.05.027.
Adak A, Bandyopadhyay M, Pal A. Removal of crystal violet dye from wastewater by surfactant-modified alumina. Sep Purif Technol. 2005;44:139–44. doi:10.1016/j.seppur.2005.01.002.
Alver E, Metin AÜ. Anionic dye removal from aqueous solutions using modified zeolite: adsorption kinetics and isotherm studies. Chem Eng J. 2012;200–202:59–67. doi:10.1016/j.cej.2012.06.038.
Melo RPF, Barros Neto EL, Moura MCPA, Castro Dantas TN, Dantas Neto AA, Oliveira HNM. Removal of Reactive Blue 19 using nonionic surfactant in cloud point extraction. Sep Purif Technol. 2014;138:71–6. doi:10.1016/j.seppur.2014.10.009.
Beltrame LTC, Dantas Neto AA, Castro Dantas TN, Barros Neto EL, Lima FFS. Influence of cosurfactant in microemulsion systems for color removal from textile wastewater. J Chem Technol Biotechnol. 2005;80:92–8. doi:10.1002/jctb.1162.
Ahmad AL, Puasa SW, Zulkali MMD. Micellar-enhanced ultrafiltration for removal of reactive dyes from an aqueous solution. Desalination. 2006;191:153–61. doi:10.1016/j.desal.2005.07.022.
Myers D. Surfactant science and technology. 3rd ed. Hoboken: Wiley; 2006.
Zapf A, Beck R, Platz G, Hoffmann H. Calcium surfactants: a review. Adv Colloid Interface Sci. 2003;100–102:349–80. doi:10.1016/S0001-8686(02)00065-9.
Melo RPF, Barros Neto EL, Moura MCPA, Castro Dantas TN, Dantas Neto AA, Oliveira HNM. Removal of direct Yellow 27 dye using animal fat and vegetable oil-based surfactant. J. Water Process Eng. 2015;7:196–202. doi:10.1016/j.jwpe.2015.06.009.
Ho YS, McKay G. Pseudo-second order model for sorption processes. Process Biochem. 1999;34:451–65. doi:10.1016/S0032-9592(98)00112-5.
Wu F, Tseng R, Juang R. Characteristic of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem Eng J. 2009;150:366–73. doi:10.1016/j.cej.2009.01.014.
Allen SJ, McKay G, Khader KYH. Intraparticle diffusion of a basic dye during adsorption into sphagnum peat. Environ Pollut. 1989;56:39–50. doi:10.1016/0269-7491(89)90120-6.
Zhou C, Wu Q, Lei T, Negulescu II. Adsorption kinetic and equilibrium studies for methylene blue dye by partially hydrolyzed polyacrylamide/cellulose nanocrystal nanocomposite hydrogels. Chem Eng J. 2014;251:17–24. doi:10.1016/j.cej.2014.04.034.
Hameed BH, Tan IAW, Ahmad AL. Adsorption isotherm, kinetic modeling and mechanism of 2,4,6-trichlorophenol on coconut husk-based activated carbon. Chem Eng J. 2008;144:235–44. doi:10.1016/j.cej.2008.01.028.
Loganathan S, Tikmani M, Edubilli S, Mishra A, Ghoshal AK. CO2 adsorption kinetics on mesoporous silica under wide range of pressure and temperature. Chem Eng J. 2014;256:1–8. doi:10.1016/j.cej.2014.06.091.
Tang H, Zhou W, Zhang L. Adsorption isotherms and kinetics studies of malachite green on chitin hydrogels. J Hazard Mater. 2012;209–210:218–25. doi:10.1016/j.jhazmat.2012.01.010.
Dotto GL, Pinto LAA. Adsorption of food dyes acid blue 9 and food yellow 3 onto chitosan: stirring rate effect in kinetics and mechanism. J Hazard Mater. 2011;187:164–70. doi:10.1016/j.jhazmat.2011.01.016.
Abramian L, El-Rassy H. Adsorption kinetics and thermodynamics of azo-dye Orange II onto highly porous titania aerogel. Chem Eng J. 2009;150:403–10. doi:10.1016/j.cej.2009.01.019.
Sun Q, Yang L. The adsorption of basic dyes from aqueous solution on modified peat-resin particle. Water Res. 2003;37:1535–44. doi:10.1016/S0043-1354(02)00520-1.
Duan S, Tang R, Xue Z, Zhang X, Zhao Y, Zhang W, Zhang J, Wang B, Zeng S, Sun D. Effective removal of Pb(II) using magnetic Co0.6Fe2.4O4 micro-particles as the adsorbent: synthesis and study on the kinetic and thermodynamic behaviors for its adsorption. Colloids Surf A Physicochem Eng Asp. 2015;469:211–23. doi:10.1016/j.colsurfa.2015.01.029.
Castro Dantas TN, Dantas Neto AA, Moura MCPA, Barros Neto EL, Paiva Telemaco E. Chromium adsorption by chitosan impregnated with microemulsion. Langmuir. 2001;17:4256–60. doi:10.1021/la001124s.
Acknowledgements
The authors would like to thank the Federal University of Rio Grande do Norte (UFRN), especially to the Chemical Engineering Graduate Program, the Center for Teaching and Research in Oil and Gas (NUPEG), and the Textile Engineering Laboratory for the support provided.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Melo, R.P.F., de Barros Neto, E.L., Moura, M.C.P.d. et al. Removal of Direct Yellow 27 Dye by Ionic Flocculation: The Use of an Environmentally Friendly Surfactant. J Surfact Deterg 20, 459–465 (2017). https://doi.org/10.1007/s11743-016-1913-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-016-1913-9