Abstract
The rheological properties of aqueous systems composed of each of the four homologous cationic surfactants (3-alkoxy-2-hydroxypropyl trimethyl ammonium bromides, C n HTAB, n = 12, 14, 16 and 18) in the presence of an anionic surfactant, sodium octanoate (SO), have been studied by using steady state and frequency sweep rheological measurements. The effects of surfactant concentration, hydrophobic chain length and temperature were investigated. In C14HTAB solution, the viscosity shows shear thinning in the concentration range of C C14HTAB >320 mmol/kg. Addition of SO promotes the micellar growth and results in the generation of wormlike micelles. Zero-shear viscosity (η 0) of the binary surfactant system exhibits a maximum point in the investigated concentration range, suggesting the interaction between C14HTAB and SO molecules is strongest at the optimal ratio of C14HTAB with SO. The decrease in viscosity was attributed to be the transition from entangled wormlike micelles to branching micelles after the maximum point, cryo-TEM images revealed the changes in the structure of the wormlike micelles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is known that the mixtures of oppositely charged surfactants can self-assemble into such microstructures as micelles, vesicles, lamellae, columnar and the cubic mesophases, depending on the different groups and the shape of the surfactant molecules. Among these microstructures, viscoelastic wormlike micelles formed in surfactants and its mixed systems have many potential applications, thus, there has been much interest in studying the properties of the wormlike micelles, in particular those stimuli-responsive surfactants systems in recent years [1–6].
The addition of one oppositely charged surfactant, even a small amount, to another surfactant solution is expected to enhance the formation of wormlike micelles because counterions reduce the micellar surface potential via charge neutralization and also increases the ionic strength by virtue of the released counterions [7, 8]. Thus, the rheological property of the binary solution of cationic and anionic surfactants is better than that of either parent surfactant. However, so far studies on the rheological behavior of the aqueous mixed system of counterionic surfactants are rather scarce [9–13], and this is in contradiction to the increasing development of mixed wormlike micellar solutions both in fundamental and in practical aspects. Therefore, we focus our attention in this work on the interactions of such a class of cationic surfactants, 3-alkoxy-2-hydroxypropyl trimethyl ammonium bromides (referred to as C n HTAB, n = 12, 14, 16 and 18) and a counterionic surfactant. One of the readily available anionic surfactant, sodium octanoate (SO), is selected to form a mixed wormlike micelles solution with C14HTAB. The influences of the hydrophobic effect, electrostatic interaction and hydrogen bonding on the viscoelastic behaviors of charged wormlike micelles have been investigated. The obtained experimental data should be helpful not only for a solid understanding of cationic/anionic surfactant mixed systems, but also for further broadening of the potential applications of these mixed systems in the fields of household chemicals and functional materials.
Experimental
Materials
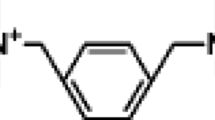
3-Alkoxy-2-hydroxypropyl trimethyl ammonium bromides (C n HTAB, n = 12, 14, 16 and 18) were synthesized in our laboratory according to the methods reported in the literature [14]. The products were purified by recrystallization from acetone five times, and then dried under a vacuum for 2 days. The purity of these products was confirmed by the 1H-NMR spectrum and elemental microanalysis. The detailed descriptions are available in the Electronic Supplementary Information 1, 2 (ESI 1, 2). Sodium octanoate (SO, Aldrich Chemical Reage Co.) was a special grade reagent and was used as received. The molecular structures are shown in Scheme 1.
Methods
Rheological Measurements
Samples were prepared by mixing C n HTAB, SO and water at a given molality and were homogenized by a magnetic stirrer at 50 °C, and then they were stored in a constant temperature water bath at 25 °C to equilibrate. A stress controlled rheometer (AR2000ex, TA instruments, USA) with cone-plate geometry of 2° cone angle and 20 mm diameter was used for rheological measurements. The gap between the center of the cone and plate was 50 µm. The measuring unit was equipped with a temperature unit (Peltier plate) providing rapid change of the temperature and giving accurate temperature control (uncertainty: ±0.05 °C) over an extended time. Frequency sweep measurements were performed in the linear viscoelastic regime of the samples, as determined previously by dynamic stress sweep measurements for all samples, and the frequency (ω) varying from 0.01 to 100 rad/s. For the steady-shear experiments, an equilibration time of 90 s was given at each data point. Each measurement was repeated three times to ensure good reproducibility (the deviation was less than 1 %).
Cryogenic Transmission Electron Microscopy (Cryo-TEM)
Aggregate structures were determined by cryo-TEM. Samples for cryo-TEM measurement were prepared in a controlled environment vitrification system (CEVS, Cryoplunge TM3, Gatan, USA) at 25 °C at 95 % relative humidity. A drop of solution (1–5 µl) was placed on a TEM grid covered with a perforated carbon film and blotted with a filter paper to form a thin solution film on the grid. Samples were quenched rapidly in liquid ethane to form a vitrified sample and then transferred to liquid nitrogen until examination and were examined on a JEOL JEM 1400 TEM operating at 120 kV. The cryo transfer holder temperature was maintained below −166 °C during imaging. Images were recorded on a high-resolution cooled CCD camera.
1H-NMR Measurements
1H-NMR spectra of C n HTAB were measured using an MP-400 nuclear magnetic resonance spectrometer (Varian Company, US) at the proton resonance frequency of 400.15 MHz. All spectra were determined in deuterium oxide containing TMSP as an internal reference.
Results and Discussion
Effect of Surfactants Concentration
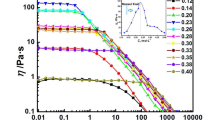
Transparent viscoelastic solutions were formed in all determined samples and a systematic study was carried out using steady state and frequency sweep rheological measurements. The steady shear viscosities of pure C14HTAB aqueous solutions at different concentrations are shown in Fig. 1 at 25.0 ± 0.2 °C. At low concentrations, these solutions form spherical micelles with very low viscosity and show Newtonian fluid behavior. However, when the surfactant concentration were higher than 320 mmol/kg, the systems formed long micelles, which can be proved from shear thinning behavior in steady shear viscosity [15, 16]. When SO was added to C14HTAB aqueous solution at 100 mmol/kg, the area per head group of each monomers markedly decreased due to the ion pairing formation which facilitates long micelles formation. The long micelle solution had higher viscosity than that of the system in the absence of SO and the steady shear viscosity exhibited shear thinning behavior (steady shear viscosity of C14HTAB/SO is shown in ESI 3). By extrapolating the plateau value, the zero-shear viscosity (η 0) at different SO concentrations were obtained and are shown in Fig. 2. It was observed that with increasing concentration of SO, the η 0 increases quickly and reaches a maximum at 60 mmol/kg SO and then decreases at high SO concentration. The maximum η 0 value (214.3 Pa·s) is 5 orders of magnitude larger than its initial viscosity without SO (0.002 Pa·s). The relationship between η 0 and SO concentration obeys the power law, \( \eta_{0} \propto C^{3.49} \) before the maximum, which was close to those wormlike micelles formed by nonionic surfactants investigated by Cates and co-workers (about 3.5) [17, 18]. The similar curve of a single pronounced peak was also observed in other cationic surfactant systems [7, 11–13, 19] and some conventional ionic surfactant/salt aqueous systems [20, 21]. For the viscosity going through a maximum as a function of surfactant ratio or salt content, there are two hypotheses in the literature. The first is that the micelles grow up to the peak and shrink beyond the peak [22–24]. The second hypothesis is that the micelles grow while staying linear up to the peak, remain linear micelles transform into a branched networks structure [8, 11, 15, 25–27] as observed from the presence of 3-way connections on the Cryo-TEM images [19, 27, 28]. Theoretical studies have predicted that branching of wormlike micelles should lower the viscosity [29]. For our system, the addition of the SO screens the interaction between the head groups and promotes an increase in micellar size, which further gives rise to an entangled network of wormlike micelles, but decreases η 0 surpassing the maximum. For the decision on which model described above is right, Raghavan pointed out that the most reliable way to confirm branches in wormlike micelles is the technique of cryo-transmission electron microscopy (cryo-TEM). In order to further confirm the structural changes in the micelle, we completed the cryo-TEM experiments. Photographs for several typical samples of the 100 mmol/kg C14HTAB solutions containing different relative amount of SO, 0, 40, 60 and 70 mmol/kg, respectively, are shown in Fig. 3.
Figure 3a shows that some spherical micelles exist in the system, their diameter is smaller than 10 nm. When SO is added to the system (Fig. 3b, 40 mmol/kg SO), the micrograph shows a number of cylindrical micelles of different length. In Fig. 3c, very long wormlike micelles are observed in the system, some wormlike micelles overlap and become entangled. As a consequence, it is impossible to identify where they begin and end. After the maximum (Fig. 3d), a micellar network formed by branched micelles was found and the micrograph shows a number of three-dimensional connections (red arrows). So the results from the cryo-TEM observation support the second hypothesis for our system in which the decrease in viscosity was attributed to be a shift from entangled wormlike micelles to branched micelles, which was in good agreement with the results from the steady shear rheology.
Significantly, the maximum viscosity occurs at a mole ratio of C SO/C C14HTAB about 0.6 where one SO molecule roughly connects with two C14HTAB molecules in the micelle (illustrated in Fig. 2). The peak shows the optimal composition of C14HTAB and SO for the formation of the longest micelle or the strongest network structure. Obviously, it does not reflect an optimal extent of charge neutralization in the micelle where the counterions of C14HTAB micelle are substituted completely by SO anions. A possible mechanism for the micellar branching may come from the transition of curvature of the micellar interface. The curvature of the aggregate is strongly influenced by the packing parameter p:
where v is the hydrophobic volume, l is the hydrophobic chain length of the surfactant molecule, and a the head group area of that molecule. When p is between 1/3 and 1/2, the surfactant molecules will packed into cylindrical micelles. When p is between 1/2 and 1, flexible bilayers or vesicles form [30]. Wormlike micelles correspond to a locally cylindrical geometry with two end caps that are generally thought to be of hemispherical geometry having higher curvature. At low SO concentrations, the electrostatic repulsion between headgroup of C14HTAB molecules are partially screened and short rodlike micelles are formed which have a weak rheological response (low viscosity). As the SO content is increased, because of the strong interaction between C14HTAB and SO, a is reduced and thus the packing parameter is increased, micelles grow rapidly into wormlike ones until the maximum. After that, continuing to increase SO to the solution, the electrostatic interactions are more effectively enhanced, the head group area could be further reduced and the tail volume increased. This provides favorable conditions for cross-link structure forming, rather than highly curved end-caps to promote branched micelle formation, leading to the decrease in zero shear viscosity. In addition, increasing the anionic surfactant concentration increases the concentration of counterions released into the solution. The effect is thus similar to salt addition and the maximum is initiated at a lower SO content for a higher C14HTAB concentration. Such a mechanism could explain the observed results.
When C SO > 90 mmol/kg, viscosity of the solution drops off slowly and then without obvious change, suggesting that the long micelle is damaged gradually and the shape and structure of C14HTAB/SO micelles change no further upon addition of SO. In an equimolar ratio, there are no precipitate or packed vesicles formation like those of catanionic systems [8, 31, 32], indicating that a dense packing is sterically favorable for long micelles instead of precipitates or vesicles [10, 33, 34].
The frequency sweep is a popular method for studying the formation of long micelles or network structure. Figure 4 shows the dynamic rheological spectrum of 100 mmol/kg C14HTAB/60 mmol/kg SO aqueous solution where the zero-shear viscosity shows a maximum (the dynamic rheological spectra of the 100 mmol/kg C14HTAB and 30–150 mmol/kg SO are shown in ESI 4). Oscillatory shear measurements of viscoelastic micelles generally have single stress relaxation time (\( \tau_{\text{R}} \)) at low shear frequency which is consistent with Maxwell fluids. The storage modulus (\( G^{\prime} \)), and the loss modulus (\( G^{\prime \prime } \)), are given by the following relations [35–38].
\( \tau_{\text{R}} \) is obtained from \( \omega_{C}^{ - 1} \), and \( G^{\prime} \) is equal to \( G^{\prime\prime} \) in the frequency \( \omega_{C} \) At high \( \omega \), \( G^{\prime} \) becomes a constant plateau module. Figure 3a represents a good fitting result for the typical case of 100 mmol/kg C14HTAB/60 mmol/kg SO aqueous solution in the range of low and medium frequencies (solid lines). Viscous modulus (G″) appears as a maximum in \( \omega_{C} \), then decreases, and then passes through a minimum with increasing frequency. After this minimum, \( G^{\prime\prime} \) increases again indicating at least a second relaxation time at a frequency beyond the limit of the rheometer (100 rad s−1), but there is not enough high-frequency data to accurately determine the second maximum in the process of our measurements. The semicircular curve of G″–G′ is another way of verifying viscoelastic wormlike micelles behavior [38]. It can be expressed as Eq. (4).
Figure 4b shows the Cole–Cole plot from the data presented in Fig. 4a, and the solid lines correspond to Maxwellian evolution. Compared with the standard model, the experimental curve of G′–G″ behaves as a Maxwell fluid, having stress relaxation behavior characterized by a single terminal relaxation time except for \( G^{\prime\prime} \) at high angular frequencies. We can see a departure in the measured evolution corresponding to the contribution of the breathing and Rouse modes. This accord with the fact that wormlike micelles are in a dynamic equilibrium and in the rapid breaking and recombination processes [35, 39, 40].
In this mixed system the directive driving force of long micelle-formation comes mainly from the strong interaction between the two surfactants with opposite charges, including the hydrophobic effect and electrostatic attraction. Besides, the role of hydrogen bonding appears to merit attention. In our recent publications, the amphiphilic molecule with substituted hydroxyl group was used in studies to analyze the effect of intermolecular hydrogen bonding on the wormlike formation. It was found that the intermolecular hydrogen bonding interaction increased the cohesive strength between the molecules and sped up the micellar growth [41]. In this work, C14HTAB molecule and SO molecule contain respectively the hydroxypropyl group and carboxy group, so hydrogen-bonding interactions among the C14HTAB, SO and water molecules possibly play an important role for the wormlike micelle formation.
When the concentration of SO is fixed at 60 mmol/kg (corresponding to the peak in Fig. 2), the solution of 60 mmol/kg SO is Newtonian fluid, the zero-shear viscosities and the plateau modulus of C14HTAB/SO mixed solutions monotonically increase with increasing C14HTAB concentration and show the power law dependence with the exponents of 1.3 and 0.96 (shown in Fig. 5), respectively. While the relaxation time \( \tau_{\text{R}} \) as a function of C14HTAB concentration exhibits a downtrend in a power law −2.59 (inserted figure). Although the rheological properties are monotonic functions of C14HTAB concentration, the behavior still does not follow that predicted for ionic micelles. All exponents are very low compared with the theoretical value which predicted by Turner in some reversible scission wormlike micelles [36], namely, \( \eta_{0} \propto C^{3.7} \), \( G_{0} \propto C^{2.3} \) and \( \tau_{\text{R}} \propto C^{1.3} \). This is a behavior of typical polyelectrolyte solutions that the increasing in \( \eta_{0} \) and decreasing in \( \tau_{\text{R}} \) with increasing surfactant concentration [21]. These results are similar to that from mixed solution of CTAT/SDBS and a fixed sodium dodecylbenzenesulfonate concentration [39]. The increase of viscosity can be taken as evidence of micellar growth. The plateau modulus \( G^{\prime}_{\infty } \) usually depends on the number density of the aggregates ρ e by the equation \( G^{\prime}_{0} = \rho_{e} K_{\text{B}} T \) [39]. The linearly increase of plateau modulus \( G^{\prime}_{\infty } \) with the increasing C14HTAB corresponds to the increase of connection degree or branching and therefore reflects the decreasing mesh size \( \xi_{\text{M}} \) (shown in ESI 5) of the network. The increase of \( G^{\prime}_{\infty } \) suggests that increasing C14HTAB concentration at the fixed C14HTAB/SO ratio does not radically alter the wormlike micellar structure in the examined concentration range, only decreasing the crimp degree of wormlike micelles.
Kaler had mentioned that the total persistence length of ionic micelles, \( l_{\text{p}} \) can be calculated from the intrinsic persistence length, \( l_{\text{p}}^{0} \), and electrostatic contribution, \( l_{\text{p}}^{\text{e}} \), by the formula \( l_{\text{p}} = l_{\text{p}}^{0} + l_{\text{p}}^{\text{e}} \) [8]. The electrostatic persistence length, \( l_{\text{p}}^{\text{e}} \), depended on micellar charge and Debye length, \( k^{ - 1} \). The mean distance between charged groups \( L_{0} \) and \( k^{ - 1} \) determine \( l_{\text{p}}^{\text{e}} \) according to the Odijk–Skolnick–Fixman (OSF) theory, as \( l_{\text{p}}^{\text{e}} = ( {{\raise0.7ex\hbox{${l_{\text{B}} }$} \!\mathord{/ {\vphantom {{l_{\text{B}} } 4}}\kern-0pt} \!\lower0.7ex\hbox{$4$}}} )( {{\raise0.7ex\hbox{${k^{ - 1} }$} \!\mathord{/ {\vphantom {{k^{ - 1} } {L_{0} }}}\kern-0pt} \!\lower0.7ex\hbox{${L_{0} }$}}} )^{2} \), here \( l_{\text{B}} \) is the Bjerrum length. Thus in this mixed system increasing C14HTAB concentration decreases the \( k^{ - 1} \), results in a decrease of \( l_{\text{p}} \) and reduces the longest relaxation time, \( \tau_{\text{R}} \).
Effect of the Alkyl Chain Length of the Surfactant
As a comparison, we investigated the behaviors of cationic surfactants with different alkyl chain length. The aqueous solutions of 100 mmol/kg C n HTAB, n = 12, 14, 16 and 18, coexisting with 60 mmol/kg SO were selected as model systems. Figure 6 shows the steady shear viscosities of C12–18HTAB/SO aqueous solutions at 25 °C. The steady shear viscosity increase successively, on the whole, with increasing alkyl chain length. The steady shear viscosity of C12HTAB/SO, however, shows Newtonian fluid behavior with low viscosity and that of C14HTAB/SO shows shear-thinning. For C16–18HTAB/SO solutions, we note that the curves of the shear viscosity vs shear rate display a sharp shear thickening at a very low shear rate (Fig. 5). The cause may be that the temperatures of both the solutions are lower than the Kraft point of the two surfactants (26 °C for C16HTAB and 32 °C for C18HTAB). Although both the mixed systems seem to be transparent, the molecular chains of C16HTAB and C18HTAB are not fully extended, upon shear rate increasing, the interaction between C16–18HTAB and SO can be enhanced, and so peaks appear in the curves of shear viscosity vs shear rate (Fig. 6).
The dynamic shear modulus \( G^{\prime} \) and \( G^{\prime\prime} \) of 100 mmol/kg C14–18HTAB/60 mmol/kg SO as a function of angular frequency at 25 °C are shown in Fig. 7. It can be seen that no cross-over points can be detected for the samples n = 16 and 18, but the curves of \( G^{\prime} \) have plateau and \( G^{\prime} \) wholly exceed \( G^{\prime\prime} \) throughout the examined frequencies, which is in accordance with the gel-like behavior [42, 43]. For the samples of n = 14, the changing trend of storage modulus (\( G^{\prime} \)) and loss modulus (\( G^{\prime\prime} \)) fits the Maxwell model and has the characteristic of wormlike micelles, i.e., elastic modulus G′ dominating over viscous modulus G″ at high ω and viscous modulus G″ dominating over elastic modulus G′ at low ω [40]. While C12HTAB/SO aqueous solution has not showed well elasticity. In other words, the C n HTAB/SO mixed aqueous solutions change from Newtonian fluids to non-Newtonian fluids (solution of wormlike micelles), then to solutions of gel-like micelles with the increasing chain length of cationic hydrophobic group at 25 °C. These experimental results fully show that the hydrophobic interaction is strengthened with increasing alkyl chain length and long wormlike micelles and a gel is easier to form in the system. This gelation behavior may be attributed to the crystallization of wormlike micelles, which is related to the strong synergic interaction between both the surfactants under the Kraft point of C16–18HTAB [44, 45]. When the temperature is increased to above 35 °C, both the samples exhibits characteristic wormlike micelle features with strong viscoelastic behavior.
Effect of Temperature
The viscoelasticity of 100 mmol/kg C n HTAB/60 mmol/kg SO solution was studied from 15 to 35 °C. In the examined temperature range, a sample of 100 mmol/kg C12HTAB/60 mmol/kg SO did not show good rheological behavior. Figure 7 shows the variation of the dynamic rheological properties of 100 mmol/kg C14–16HTAB/60 mmol/kg SO as a function of angular frequency at different temperatures. For the sample 100 mmol/kg C14HTAB/60 mmol/kg SO (Fig. 8a), G′ dominates over G″ at high ω and G″ exceed G′ at low ω, which is a typical viscoelastic response of wormlike micelles. And the ω value of the crossover point increases from 0.1834 rad s−1 at 15 °C to 3.960 rad s−1 at 35 °C, indicating a relaxation time decrease and viscoelasticity is damaged when the temperature of the sample is enhanced. The higher angular frequency at which \( G^{\prime} \) and \( G^{\prime\prime} \) cross, the faster the relaxation process of micelles and the more difficult is the formation of a network structure [36, 46] However the sample 100 mmol/kg C16HTAB/60 mmol/kg SO (Fig. 8b) shows gel-like behavior at 15–25 °C and wormlike micelles behavior at 35 °C, indicating that the solution changes from gel-like to wormlike micelles with the increasing temperature. The dynamic rheological properties of 100 mmol/kg C18HTAB/60 mmol/kg SO (shown in ESI 6) shows the same trend with that of C16HTAB/SO system. These experimental results of the above fully consistent with the expected results that thermodynamic free energy in turn reduce in the following order, C18HTAB > C16HTAB > C14HTAB > C12HTAB, i.e., longer and more tightly packed micelles can be more easily formed at low temperature.
In addition, the steady shear viscosity is also investigated. For example, the viscosities of C14HTAB/SO aqueous solutions at different temperatures show the typical shear thinning non-Newtonian fluid behavior. The zero-shear viscosities \( \eta_{0} \) (shown in ESI 7) decrease successively with the increasing temperature, but the maximum \( \eta_{0} \) value at C SO = 60 mmol/kg does not change with a different temperature, indicating that the similar structure of micelles is in existence in our examined temperature range. It is easy to understand that the increasing thermal motion reduces the viscosity of the solution and decreases the average micellar length [22, 47]. The activation energy, E a, can be obtained from the Arrhenius plot according to Eq. (5).
Activation energy, \( E_{\text{a}} \) is the necessary energy of an individual micelle to move in an environment of surrounding micelles. Therefore \( E_{\text{a}} \) is given by the interactions between individual aggregates [16]. Figure 9 presents the plot of ln \( \eta_{0} \) versus the reciprocal of the thermodynamic temperature for 100 mmol/kg C14HTAB/60 mmol/kg SO mixed aqueous solutions. The plot is linear and demonstrates that the main \( \eta_{0} \) follows Arrhenius-type behavior. A value of 136.35 kJ/mol for E a obtained from the slope falls into the wide range of E a values (70–300 kJ/mol) reported for surfactant micellar systems [27, 48–50].
Conclusion
In summary, the viscoelastic properties of C n HTAB aqueous systems and their mixed solutions with SO were studied using viscosity measurements. Influences of surfactant concentration, alkyl chain length of C n HTAB and temperature on the properties were investigated. In C14HTAB solution at 100 mmol/kg, only spherical micelles can be formed. The addition of SO can promote the micellar growth from spherical ones into rodlike ones and to wormlike ones by the synergistic effect of hydrophobic effect, electrostatic interaction and hydrogen bonds between the oppositely charged surfactants. Zero-shear viscosity of mixed solutions as a function of SO concentration shows a maximum peak behavior. The decrease in viscosity can be attributed to be the transition from entangled wormlike micelles to branching wormlike micelles after the maximum. Cryo-TEM observation confirmed the micellar structure changes with SO concentration increases. The viscosity and elasticity can be flexibly adjusted by selecting the suitable concentration, the ratio of the two surfactants, hydrophobic chain length of the surfactant molecule and the temperature. The steady and dynamic rheological curves show great differences with increasing the hydrophobic chain at a same ratio of C n HTAB/SO mixed solution. The activation energy, E a, obtained from the Arrhenius plot falls into the range reported for surfactant micellar systems. These results may be regarded as an important reference in theoretical research and the practical application of mixed surfactants in several new fields.
References
Rogers SA, Calabrese MA, Wagner NJ (2014) Rheology of branched wormlike micelles. Curr Opin Colloid Interface 19:530–535
Han YX, Feng YJ, Sun HQ, Li ZQ, Han YG, Wang HY (2011) Wormlike micelles formed by sodium erucate in the presence of a tetraalkylammonium hydrotrope. J Phys Chem B 115:6893–6902
Tian MZ, Zhu LY, Yu DF, Wang YY, Sun SF, Wang YL (2013) Aggregate transitions in mixtures of anionic sulfonate gemini surfactant with cationic ammonium single-chain surfactant. J Phys Chem B 117:433–440
Mitrinova Z, Tcholakova S, Popova Z, Denkov N, Dasgupta BR, Ananthapadmanabhan KP (2013) Efficient control of the rheological and surface properties of surfactant solutions containing C8-C18 fatty acids as cosurfactants. Langmuir 29:8255–8265
Chu ZL, Dreiss CA, Feng YJ (2013) Smart wormlike micelles. Chem Soc Rev 42:7174–7203
Yan H, Long Y, Song K, Tung CH, Zheng LQ (2014) Photo-induced transformation from wormlike to spherical micelles based on pyrrolidinium ionic liquids. Soft Matter 10:115–121
Koehler RD (2000) Microstructure and Dynamics of wormlike micellar solutions formed y mixing cationic and anionic surfactants. J Phys Chem B 104:11035–11044
Kaler EW, Herrington KL, Murthy AK, Zasadzinski JAN (1992) Phase behavior and structures of mixtures of anionic and cationic surfactants. J Phys Chem 96:6698–6707
Blanco E, Rodriguez-Abreu C, Schulz P, Ruso JM (2010) Effect of alkyl chain asymmetry on catanionic mixtures of hydrogenated and fluorinated surfactants. J Colloid Interface Sci 341:261–266
Li HG, Hao JC (2008) Phase behavior and rheological properties of a salt-free catanionic surfactant TTAOH/LA/H2O system. J Phys Chem B 112:10497–10508
Raghavan SR, Fritz G, Kaler EW (2002) Wormlike micelles formed by synergistic self-assembly in mixtures of anionic and cationic surfactants. Langmuir 18:3797–3803
Yin HQ, Lin YY, Huang JB (2009) Microstructures and rheological dynamics of viscoelastic solutions in a catanionic surfactant system. J Colloid Interface Sci 338:177–183
Fan HM, Yan Y, Li ZC, Xu Y, Jiang LX, Xu LM, Zhang B, Huang JB (2010) General rules for the scaling behavior of linear wormlike micelles formed in catanionic surfactant systems. J Colloid Interface Sci 348:491–497
Wei ZB, Wei XL, Wang XH, Wang ZN, Liu J (2011) Ionic liquid crystals of quaternary ammonium salts with a 2-hydroxypropoxy insertion group. J Mater Chem 21:6875–6882
Acharya DP, Kunieda H (2003) Formation of viscoelastic wormlike micellar solutions in mixed nonionic surfactant systems. J Phys Chem B 107:10168–10175
Shrestha RG, Shrestha LK, Aramaki K (2007) Formation of wormlike micelle in a mixed amino-acid based anionic surfactant and cationic surfactant systems. J Colloid Interface Sci 311:276–284
Cates ME, Marques CM, Bouchaud JP (1991) Dynamic relaxation of rodlike micelles. J Chem Phys 94:8529–8536
Kern F, Lemarecha P, Candau SJ, Cates ME (1992) Rheological properties of semidilute and concentrated aqueous solutions of cetyltrimethylammonium bromide in the presence of potassium bromide. Langmuir 8:437–440
Ziserman L, Abezgauz L, Ramon O, Raghavan SR, Danino D (2009) Origins of the viscosity peak in wormlike micellar solutions. 1. mixed catanionic surfactants. A cryo-transmission electron Microscopy Study. Langmuir 25:10483–10489
Cappelaere E, Cressely R (1998) Rheological behavior of an elongated micellar solution at low and high salt concentrations. Colloid Polym Sci 276:1050–1056
Pei XM, Zhao JX, Ye YZ, You Y, Wei XL (2011) Wormlike micelles and gels reinforced by hydrogen bonding in aqueous cationic gemini surfactant systems. Soft Matter 7:2953–2960
Magid LJ (1998) The surfactant-polyelectrolyte analogy. J Phys Chem B 102:4064–4074
Cappelaere E, Cressely R (2000) Influence of NaClO3 on the rheological behaviour of a micellar solution of CPCl. Rheol Acta 39:346–353
Imae T, Kohsaka TJ (1992) Size and electrophoretic mobility of C14TASal micelles in aqueous media. Phys Chem 96:10030–10035
Croce V, Cosgrove T, Dreiss CA, King S, Maitland G, Hughes T (2005) Giant micellar worms under shear: a rheological study using SANS. Langmuir 21:6762–6768
Candau SJ, Khatory A, Lequeux F, Kern F (1993) Rheological behaviour of wormlike micelles: effect of salt content. J Phys IV 3:197–209
Croce V, Cosgrove T, Maitland G, Hughes T, Karlsson GR (2003) Rheology, Cryogenic transmission electron spectroscopy, and small-angle neutron scattering of highly viscoelastic wormlike micellar solutions. Langmuir 19:8536–8541
Lin Z (1996) Branched worm-like micelles and their networks. Langmuir 12:1729–1737
Lequeux F (1992) Reptation of connected wormlike micelles. Europhys Lett 19:675–681
Israelachvili JN, Mitchell DJ, Ninham BW (1976) Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J Chem Soc Faraday Trans II 72:1525–1568
Puvvada S, Blankschtein D (1992) Mixed surfactant systems and Modeling mixed surfactant systems. In: Holland PM, Rubingh DN (eds) Mixed surfactant systems. ACS symposium series 501, Chapters 1 and 2. American Chemical Society, Washington, DC
Herrington KL, Kaler EW, Miller DD, Zasadzinski JA, Chiruvolu S (1993) Phase behavior f aqueous mixtures of dodecyltrimethylammonium bromide and sodium dodecyl sulfate. J Phys Chem 97:13792–13802
Regev O, Khan A (1996) Alkyl chain symmetry effects in mixed cationic-anionic surfactant systems. J Colloid Interface Sci 182:95–109
Schulz PC, Rodríguez JL, Minardi RM, Sierra MB, Morini MA (2006) Are the mixtures of homologous surfactants ideal? J Colloid Interface Sci 303:264–271
Kern F, Lequeux F, Zana R, Candau SJ (1994) Dynamic properties of salt-free viscoelastic micellar solutions. Langmuir 10:1714–1723
Turner MS, Marques C, Cates ME (1993) Dynamics of wormlike micelles: the bond-interchange reaction scheme. Langmuir 9:695–701
Clausen TM, Vinson PK, Minter JR, Davis HT, Talmon Y, Miller WG (1992) Viscoelastic micellar solutions: microscopy and rheology. J Phys Chem 96:474–484
Gennee PGD (1979) Scaling concepts in polymer physics. Cornell University Press, Ithaca
Ali AA, Makhloufi R (1999) Effect of organic salts on micellar growth and structure studied by rheology. Colloid Polym Sci 277:270–275
Schubert BA, Kaler EW, Wagner NJ (2003) The microstructure and rheology of mixed cationic/anionic wormlike micelles. Langmuir 19:4079–4089
Khatory A, Lequeux F, Kern F, Candau SJ (1993) Linear and nonlinear viscoelasticity of semidilute solutions of wormlike micelles at high salt content. Langmuir 9:1456–1464
Boris DC, Colby RH (1998) Rheology of sulfonated polystyrene solutions. Macromolecules 31:5746–5755
Thurn H, Loebl M, Hoffmann H (1985) Viscoelastic detergent solutions-a quantitative comparison between theory and experiment. J Phys Chem 89:517–522
Lin YY, Qiao Y, Tang PF, Li ZB, Huang JB (2011) Controllable self-assembled laminated nanoribbons from dipeptide-amphiphile bearing azobenzene moiety. Soft Matter 7:2762–2769
Mu JH, Li GZ, Wang ZW (2002) Effect of surfactant concentration on the formation and viscoelasticity of anionic wormlike micelle by the methods of rheology and freeze-fracture TEM. Rheol Acta 41:493–499
Cates ME, Candau SJ (1990) Statics and dynamics of worm-like surfactant micelles. J Phys: Condens Matter 2:6869–6892
Kern F, Zana R, Candau J (1991) Rheological properties of semidilute and concentrated aqueous solutions of cetyltrimethylammonium chloride in the presence of sodium salicylate and sodium chloride. Langmuir 7:1344–1351
Tung SH, Huang YE, Raghavan SR (2007) Contrasting effects of temperature on the rheology of normal and reverse wormlike micelles. Langmuir 23:372–376
Yuan ZW, Lu WJ, Hao JC (2008) Gel phase originating from molecular quasi-crystallization and nanofiber growth of sodium laurate–water system. Soft Matter 4:1639–1644
Lin YY, Qiao Y, Yan Y, Huang JB (2009) Thermo-responsive viscoelastic wormlike micelle to elastic hydrogel transition in dual-component systems. Soft Matter 5:3047–3053
Acknowledgments
This work was supported by the National Natural Science Foundation of China and Shandong Province (Grant Nos. 21473084, 21073081, 21373106, ZR2012BQ013), Scientific Research, Experimental Technology (LDSY2014008) and Teaching Research Project of Liaocheng University (G20122036).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ping, A., Geng, P., Wei, X. et al. Rheological Properties of Wormlike Micelles Formed in Aqueous Systems of 3-Alkoxy-2-hydroxypropyl Trimethyl Ammonium Bromides in the Presence of Sodium Octanoate. J Surfact Deterg 18, 1117–1126 (2015). https://doi.org/10.1007/s11743-015-1724-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1724-4