Abstract
In this work, three types of nonionic surfactant as corrosion inhibitors were synthesized. The chemical structure of the prepared inhibitors was confirmed using FT-IR and 1H-NMR spectroscopy. The surface tension and thermodynamic properties of these inhibitors were investigated. The corrosion inhibition efficiency of these surfactants was investigated on a carbon steel surface in 1 M HCl solution by weight loss and electrochemical measurements. Untreated and treated steel surfaces were characterized by scanning electron microscopy. Results show that the inhibition efficiency of the prepared inhibitors increases with increasing the ethylene oxide units. Also, the potentiodynamic polarization curves indicated that the investigated inhibitors behave as a mixed type inhibitor. Adsorption of these surfactants on the carbon steel surface was found to obey Langmuir’s adsorption isotherm. The computed quantum chemical properties viz., electron affinity (EA), highest occupied molecular orbital (E HOMO), the lowest unoccupied molecular orbital (E LUMO), energy gap ΔE = E HOMO − E LUMO, dipole moment (μ), polarizability and total energy (E T) show good correlation with experimental inhibition efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The petroleum industry is affected by strong economic losses derived from the serious damages caused by the metallic corrosion on piping and plant systems [1]. Both general and localized corrosion types are encountered in this industry [2]. The corrosion rate depends on the fluid composition, for example, oil type, oil/water ratio, water salinity, and the acidity of water caused by CO2 and H2S [3]. Inhibitors are generally used in these processes to control metal dissolution as well as consumption of acid [4]. Organic compounds containing nitrogen, sulfur, oxygen, and phosphorus are usually used as inhibitors against the corrosion of steel in acidic media [4]. The corrosion inhibition efficiency of organic compounds is related to their adsorption properties. Adsorption depends on the nature and the state of the metal surface, on the type of corrosive medium and on the chemical structure of the inhibitor [5]. Recently, the application of surfactants as effective corrosion inhibitors has attracted the attention of many researchers. Surfactant as corrosion inhibitors have many advantages such as high inhibition efficiency, low price, low toxicity, and easy production [5]. Generally, in aqueous solutions the inhibitory action of surfactant molecules may due to the physical (electrostatic) adsorption or to the chemisorption onto the metallic surface, depending on the charge of the solid surface and the free energy change of transferring a hydrocarbon chain from water to the solid surface. The adsorption of a surfactant markedly changes the corrosion resisting property of a metal. For these reasons, studying the relation between adsorption and corrosion inhibition are significantly important. The ability of nonionic surfactants to protect carbon steel pipelines in acid media using polarization, open circuit potential, weight loss, and surface tension measurements have been studied [3], as well as quantum chemical investigation [6]. The effect of the molecular structure on the chemical reactivity has been object of great interest in several disciplines of chemistry and quantum-chemistry calculations have been widely used to study the reaction mechanisms and to interpret the experimental results as well as to solve chemical ambiguities. This is a useful approach to investigate the mechanisms of reaction in the molecule and its electronic structure level and the structure and electronic parameters can be obtained by means of theoretical calculations using the computational methodologies of quantum chemistry [7]. The geometry of the inhibitor in its ground state, as well as the nature of their molecular orbitals, E HOMO (highest occupied molecular orbital) and E LUMO (lowest unoccupied molecular orbital) are involved in the properties of activity of inhibitors. The objective of this work is to study the inhibition performance of new synthesized nonionic surfactants on carbon steel in 1 M HCl solution using weight loss, electrochemical techniques and we attempt to find good theoretical parameters to characterize the inhibition property of the these inhibitors. Also, the correlations between the inhibition efficiency of the prepared inhibitors for the corrosion of carbon steel in 1 M HCl and some quantum parameters have been discussed.
Experimental
Materials
Two technical grades steric acid, ethylene diamine and xylene were obtained from Sigma-Aldrich Co., Germany, as well as analytical grade HCl (concentration of 37 %), isopropanol and NaCl were obtained from Fluka, Germany. Also a technical grade of ethylene oxide gas was acquired from Sidi Kerier Petrochemicals Co. (SIDPEC), Alexandria, Egypt. The water used in all experiments was double distilled, deionized and filtered prior to use.
Synthesis of New Nonionic Surfactants
Three types of new nonionic surfactants used in this study were synthesized as shown in Fig. 1. This process was carried out in two steps as follows.
Synthesis of Steric Acid Monoamide
A solution of steric acid (6.88 g, 0.02 mol) in xylene (50 ml) was added dropwise by syringe to a solution of ethylene diamine (EDA) (2.06 g, 0.02 mol) in xylene (25 ml) under reflux. After addition was completed (1 h), the reaction mixture was refluxed for 4 h. After removing the solvent, the residue was recrystallized from ethyl acetate to give N-[2-(2-hydroxyethylamino)ethyl]octadecanamide (SD) [8].
Ethoxylation of Steric Acid Monoamide
SD was charged into a high pressure stainless steel autoclave with sodium metal (0.3 wt %) as a catalyst at 150 °C with continuous stirring, while passing a stream of nitrogen gas through the system for 10 min to flush out the air. The nitrogen stream was then replaced by introducing three different molar units (5, 20 and 40) of ethylene oxide gas (e.o.). The reaction completion was established when the pressure reached its minimum value. At this stage, heating was stopped and the contents were cooled. After cooling, the product obtained was neutralized with HCl (concentration of 37 %) [9]. Then dissolved in isopropanol and salted out with supersaturated NaCl solution. The ethoxylated products (called SD1, SD2 and SD4) which are obtained appeared as brown viscous liquids.
Surface Tension (γ) Measurements
Surface tension measurements were obtained using a De-Noüy Tensiometer (Krüss-K6 type) and applying the platinum ring technique. Different concentrations of the synthesized nonionic surfactants in bidistilled water were prepared at 303 K. The apparent surface tension was measured five times for each sample within a 2 minute interval between each reading [10]. The CMC was determined from the abrupt change in the slope of the surface tension (γ CMC) versus logarithm of the concentration curve (lnC). From the slope of the linear decrease of γ, the maximum surface excess concentration (Γ), the minimum surface area per surfactant (A min), the Gibbs free energy of adsorption (∆ G ads) and the Gibbs free energy of micellization, (∆ G mic) were calculated by use of the Gibbs adsorption equations [10].
Evaluation of Weight Loss
Fresh 1 M HCl solutions were made from HCl (concentration of 37 %). The concentrations of the inhibitors employed were varied from 0.1 to 0.5 mmol dm−3 (7–12 g dm−3) in 1 M HCl. All the test solutions were prepared from analytical grade chemical reagents in distilled water without further purification. The carbon steel sheets of (7.0 × 2.0 × 0.3 cm) dimensions were immersed in a closed beaker in 1 M HCl with and without the addition of different concentrations of inhibitors for 4 h at 303 K. Experiments were carried out in triplicate. The average weight loss of the three parallel carbon steel sheets was calculated as follows [11]:
where; W 1 and W 2 are the average weight of specimens before and after exposure, respectively.
Electrochemical Measurements
A carbon steel working electrode of the following chemical composition (wt%) was used in the experiments: 0.07 % C, 0.24 % Si, 1.35 % Mn, 0.017 % P, 0.005 % S, 0.16 % Cr, 0.18 % Ni, 0.12 % Mo, 0.01 % Cu and the remainder Fe. A pre-treatment was carried out prior to each experiment, in which specimen surface was mechanically ground with 400, 600, 800 and 1,000 emery paper, washed in acetone and bidistilled water then dried and put into the cell [12]. The electrochemical measurements were carried out using a Volta lab 40 (Tacussel-Radiometer PGZ301) potentiostate and controlled by Tacussel corrosion analysis software model (Voltamaster 4) under static conditions. The corrosion cell used had three electrodes. The reference electrode was a saturated calomel electrode (SCE). A platinum electrode was used as auxiliary electrode. All potentials given in this study referred to this reference electrode. The working electrode was immersed in test solution for 30 min to a establish steady state open circuit potential (E ocp). After measuring the E ocp, the electrochemical measurements were performed. The polarization curves were obtained in the potential range from −850 to −350 m V(SCE) with a 1 mV s−1 scan rate.
Surface Morphology Studies
The surface morphology of the steel specimens was examined after exposure to 1 M HCl in absence and presence of a certain concentration of the selected inhibitor (SD4). A JEOL 5410 scanning electron microscope SEM (JEOL, Japan) was used for this investigation.
Quantum Chemical Study
All required molecular parameters were calculated based on the MINDO3 semi-empirical method always used for organic inhibitor’s calculation [13] at an unrestricted Hartree–Fock (UHF) level which were implemented in Hyperchem 8.0. The molecule 2D sketch was obtained by ISIS Draw 2.1.4. The calculation of other parameters such as; log P (hydrophobic parameter), polarizability and hydration energy was carried out by the QSAR method from the optimized geometry.
Results and Discussion
Confirmation of the Chemical Structure
Industrially, fatty amides are prepared in a thermal reaction between ethylene diamine (EDA) with fatty acids to form fatty acid monoamides. Figure 2a–c show FTIR charts of synthesized monoamide (SD) and its ethoxylated (SD1 and SD4). The FTIR chart of SD Fig. 2a (Figure 2a in the supplementary file) indicating: CH3, CH– stretching at 2,924.72 and 2,853.45 cm−1, respectively; (CH2) n skeletal at 722.93 cm−1; NH2, N–H stretching at 3,300.08 cm−1; N–H deformation at 1,465.87 cm−1; stretching C–N and C=O (amide group) at 1,269.44 and 1,643.95 cm−1, respectively. On the other hand, Fig. 2b, c shows the characteristic band of SD1 and SD4 due to the ethereal band (C–O–C) appeared at 1,104.93 and 1,104.91 cm−1, respectively. The ethereal peak intensity of SD4 is larger than SD1, due to the higher (e.o.) units in SD4 than SD1.
Surface Activity and Thermodynamic Properties
The surface properties of the prepared corrosion inhibitors, including the critical micelle concentration (CMC), the values of surface tension at the CMC (γ CMC), the maximum surface pressure (π CMC), the maximum surface excess concentration at surface saturation “effectiveness”, (Γ) and the minimum surface area per surfactant molecule (A CMC) are listed in Table 1. It is obvious that, the effectiveness values (π CMC) increased with increasing e.o. chain length, while CMC and the surface tension (γ CMC) values decreased. These phenomena could be due to the hydration of surfactants in aqueous solution increase by increasing the e.o. chain length in the surfactant molecules and as increasing of e.o. units, a self orientation of the hydrophilic group (e.o. chain) toward the metal surface was be increased [14]. Also, results show that, the value of Γ decreased, while A min increased, by increasing the e.o. units. This behavior could be due to the increasing of ethylene oxide units leads to increase in molecular size of surfactant at interface, which result in a strong interaction between the hydrophilic moiety of the surfactants the and adsorbent (metal surface) [15, 16]. The orientations of the surfactant on the metal surface are schematically were illustrated in Fig. 3. From this figure, it can see that, SD1and SD2 tend to form lying completely flat and slightly flat on the surface (low Γ and high A min values), respectively, and adsorption occurs mainly by Van der Waals interaction (Fig. 3a). On the other hand, increasing of e.o. units (SD4) may lead to reorientation of the surfactant molecules (hydrophilic part) on the metal surface. This means that the surfactant becomes perpendicular to the metal surface, (high Γ and low A min values) (Fig. 3b). In perpendicular orientation, moreover, the effectiveness of adsorption may be determined by the size of the hydrophilic group when the cross-sectional area of that group is greater than that of the hydrophobic chain; the larger the hydrophilic group, the smaller the amount adsorbed at surface saturation [17]. From the thermodynamic data listed in Table 1, it can be concluded that the values of ΔG° ads are all negative and greater than those of ΔG° mic. These observations are consistent with data reported previously [18], which indicated that the surfactant molecules prefer to be adsorbed on the metal surface rather than form micelles.
Potentiodynamic Polarization Measurements
The potentiodynamic polarization curves of steel in 1 M HCl solution in the absence and presence of 0.5 mmol dm−3of the inhibitors are shown in Fig. 4. The values of related electrochemical parameters, i.e., corrosion potential (E corr), corrosion current density (i corr), anodic Tafel slope (β a), cathodic Tafel slope (β c)and inhibition efficiency η p were calculated and given in Table 2. The η p was calculated from polarization measurements according to the relation given below:
where; i corr(uninh) and i corr(inh) are uninhibited and inhibited corrosion current densities, respectively. Corrosion current densities were obtained by the extrapolation of the current-potential lines to the corresponding corrosion potentials. Herein, the corrosion rates were calculated assuming that the whole surface of steel is attacked by corrosion and no local corrosion is observed. As it can be clearly seen from Fig. 4 that, the addition of inhibitors to the corrosive solution both reduces anodic dissolution of iron and also retards cathodic hydrogen evolution reactions as would be expected. This may be ascribed to the adsorption of the inhibitor molecules over the steel surface. The presence of the prepared nonionic surfactants does not remarkably shift the corrosion potential (E corr), while the anodic and cathodic Tafel slopes change with the increase in the e.o. units in the surfactants. Therefore, the synthesized nonionic surfactants can be classified as mixed type inhibitor in HCl solution. The parallel cathodic Tafel lines suggested that the addition of inhibitors to the 1 M HCl solution do not modify the hydrogen evolution mechanism and the reduction of H+ ions at the steel surface which occurs mainly through a charge transfer mechanism. These results are in good agreement with the results obtained from weight loss measurements. According to η p values represented in Table 2, the inhibitive properties of the studied surfactant inhibitors can be given in the following order: SD4 > SD2 > SD1 with η p values of 89.18 > 81.97 > 72.96 %, respectively, i.e. the inhibition efficiency increased by increasing the e.o. units. This can be due to the increase in e.o units leading to increases in the surface coverage of the inhibitor and hence increase the adsorption onto the steel surface [19].
Weight Loss Measurements
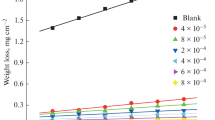
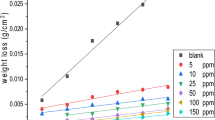
The corrosion rate (A) of steel specimens after 4 h exposure to 1 M HCl solution with and without the addition of various concentrations of the investigated inhibitors was calculated and the obtained data are listed in Table 3. The variation of A with inhibitors concentrations is shown in. The corrosion rate, A (mg cm−2 h−1), surface coverage (θ) and inhibition efficiency η w of each concentration were calculated as follows [20]:
where; ΔW is the average weight loss (mg), S is the surface area of the specimens (cm2), and t is the immersion time (h), A uninh and A inh are the corrosion rates in the absence and presence of inhibitor, respectively. Results clearly show that, η w increased with increasing inhibitor concentration, while the corrosion rate decreased. This could be due to the inhibitor molecules acting by adsorption onto the metal surface [21]. When the concentration of inhibitors is less than 0.5 mmol dm−3, the η w increased sharply with an increase in concentration, while a further increase causes no appreciable change in performance. The maximum η w values of (0.5 mmol dm−3) of inhibitors SD1, SD2 and SD4 were found to be 88.0, 89.1 and 91.5 %, respectively. It should be noted that the η w increases with increasing number of ethylene oxide (e.o.) units in the surfactant molecules. This can be attributed to the excess in e.o. units of the inhibitor which lead to more surface coverage, consequently, increase the adsorption on the steel surface. The average of the standard deviation for the three replicates of weight loss measurement was about 0.31. The obtained results from the measurements of weight loss were in a good agreement with these obtained from potentiodynamic polarization measurements.
Adsorption Isotherm and Standard Adsorption Free Energy
Corrosion inhibition by organic compounds is mainly due to their ability to adsorb onto a metal surface to form a protective film. The adsorption of organic inhibitors at the metal/solution interface takes place through the replacement of water molecules by organic inhibitor molecules according to the following process [22].
where Org(sol) and Org(ads) are organic molecules in the solution and adsorbed onto the metal surface, respectively, x is the number of water molecules replaced by the organic molecules. It is essential to know the mode of adsorption and the adsorption isotherm that can give valuable information on the interaction of inhibitor and metal surface. Values of surface coverage (θ) corresponding to different concentrations of SD1, SD2 and SD4, which were calculated using the weight loss results at 303 K after 4 h of immersion, were used to determine which isotherm best described the adsorption process. The results obtained for the investigated inhibitors in 1 M HCl solution were tested with several adsorption isotherms. However, the best agreement was obtained using the Langmuir adsorption isothermal equation as follows [23]:
where; C i is the concentration of inhibitor and K ads the adsorptive equilibrium constant. Langmuir’s isotherm assumes that there is no interaction between the adsorbed molecules, the energy of adsorption is independent of the θ, the solid surface contains a fixed number of adsorption sites, and each site holds one adsorbed species. The linear regression parameters are listed in Table 4. Both linear correlation coefficient (r) and the slope equal to 1, indicate that the adsorption of the investigated inhibitors on steel surface obeys the Langmuir adsorption isotherm in 1 M HCl solution. The adsorptive equilibrium constant (K ads) can be calculated from reciprocal of intercept of C i/θ—C i curve. K ads is related to the standard free energy of adsorption (ΔG° ads) as shown the following equation [24]:
where; R is the universal gas constant (8.314 J mol−1 K−1), T the absolute temperature (K), and the value 55.5 is the concentration of water in solution expressed as the molar mass. The ΔG° ads values are also listed in Table 4. The high values of K ads and negative values of ΔG° ads suggested that, surfactant molecules strongly adsorb onto the steel surface. Generally, values of ΔG° ads up to −20 kJ mol−1 are consistent with the electrostatic interaction between the charged molecules and the charged metal (physical adsorption), while those more negative than −40 kJ mol−1 involve sharing or transfer of electrons from the inhibitor molecules to the metal surface to form a coordinate type of bond (chemisorption). The values of ΔG° ads for three compounds being less than (−40 kJ mol−1) indicate physical adsorption, in addition to electrostatic interaction there may be some other interactions.
Relation Between the Surface Properties and the Inhibition Efficiency
Since the corrosion inhibition mechanism of the surfactants depend on their ability to adsorb onto the corroding surface, forming a protective layer. So, the CMC is considered a key factor in determining the effectiveness of surfactants as corrosion inhibitors. This could be attributed to the fact that, above the CMC, the surface of the steel is covered with a monolayer of surfactant molecules and the additional molecules combine to form micelles in the bulk of solution. On the basis of this view, among the studied surfactants, it is SD4 which shows the lowest CMC value and hence can be considered the most effective corrosion inhibitor for steel.
Surface Examination by SEM
Figure 5a–c show the SEM images of the steel surface after polishing of the carbon steel and immersion in 1 M HCl, for a period of 4 h, in the absence and presence of 0.5 mmol dm3 of SD4, respectively. The SEM image of a polished carbon steel sample is shown in Fig. 5a as a dry sample without 1 M HCl or inhibitor). The micrograph shows a characteristic inclusion, which was properly an oxide inclusion [25]. The SEM micrographs show that the surface of steel is highly damaged in the uninhibited solution (Fig. 5b). However, a smoother surface is seen in the presence of the inhibitor SD4 (Fig. 5c), which is given a similar surface to that obtained in (Fig. 5a). These results indicate that the inhibitor molecules hinder the dissolution of steel by formation of a protective film on the steel surface [26].
Quantum Chemical Calculation
The quantum chemical method was introduced to study the relationship between the organic molecular structure and the inhibition effect [27]. The structural parameters, such as the frontier molecular orbital energy E HOMO (highest occupied molecular orbital) and E LUMO (lowest unoccupied molecular orbital) and the energy gap (ΔE), dipole moment (μ), polarizability, log P (substituent constant- measure of differential solubility of a compound in two solvents and characterizes the hydrophobicity/hydrophilicity of a molecule), and total energy (E T) were calculated and recorded in Tables 5 and 6 (Table 6 is in the supplementary file). Also, the optimized geometry structures of the compounds are illustrated in Fig. 6. The molecular orbital energies (E HOMO, E LUMO, ΔE LUMO-HOMO ) of the investigated inhibitors molecules were calculated. From Table 5 and Fig. 6, it was obvious that the higher the E HOMO energy (E H) of the inhibitor (SD4), the ease of offering electrons to the unoccupied d orbital of carbon steel increased and the higher the inhibition efficiency of the inhibitor for carbon steel in 1 M HCl solution. The lower the E LUMO energy (E L), the easier the acceptance of electrons of the d orbital of carbon steel and the higher the inhibition efficiency of the inhibitor for carbon steel. Also, the ΔE increase when increasing the inhibition efficiency indicates that the more stable the inhibitor, the stronger interaction between the inhibitor and the metal surface. Thus, the interactions are probably physical adsorption [28]. Inspection of data listed in Table 5, showed that the dipole moment (μ) of the inhibitors changed regularly, which proved that the adsorption might have arisen from the intermolecular electrostatic force, whereas, the lowest dipole moment value with maximum inhibition efficiency was observed with SD4, this may be attributed to an increase in the accumulation of inhibitor molecules on the metallic surface [28]. According to Eddy and Ebenso [29], substituent constant values (log P) account for the hydrophobicity of an actual molecule. From the results obtained in Tables 3 and 4, it can be concluded that the inhibition efficiency for all the investigated inhibitors increases with a decreasing log P value. Because the log P value depends the molecule hydrophobicity, the inhibitors containing a large number of ethylene oxide units (SD4) have the ability to form a protective film by physical adsorption between oxygen atoms in ethylene oxide units and the metal surface [29]. Another global property in our study is the electric dipole polarizability (α). The minimum polarizability principle (MPP) has been postulated, which expects that the natural direction of evolution of any system is towards a state of minimum polarizability [29]. From Table 5, it was cleared that the highest polarizability values were observed with SD4 inhibitor (maximum %η w), while the lowest polarizability was exhibited by SD1 (minimum %η w). Also, a beneficial parameter was calculated by quantum chemical methods are the total energy (E T). The total energy (E T) was obtained after geometric optimization with respect to all nuclear coordinates. According to the results of their computational study as listed in Table 5, it was found that the maximum value of E T was obtained by SD4. This indicates that the higher stability of the molecule and hence the lesser the tendency of it to the donating ability [30]. Also, hydration energy (E hyd) values for the investigated inhibitors were computed and recorded in Table 5. E hyd values computed were negative and ranged from −16.29 to −28.84 kcal mol−1 for SD1, SD2 and SD4 indicating that the dissolution of the inhibitors in water (hydration) is an exothermic process. The exothermic dissolution of the inhibitors increases and ranked as follows: SD1 > SD2 > SD4. The increase of the negativity of the E hyd might be attributed to the hydrogen bond which is formed between the inhibitor (nitrogen and oxygen of the ether linkage) and water molecules [28]. On the other hand, ionization potential (I P) of the investigated inhibitors was computed, whereas, the energy of the E HOMO is directly related to the ionization potential. From Tables 3 and 6, it can be noticed that the corrosion inhibition efficiency increases with decreasing of I P. As the ionization potential decrease, the minimum energy needed for the detachment of the orbital electrons to bind to the metal surface, protecting it from corrosive media, increases and hence the inhibition efficiency increases. The electron affinity (E A) of the prepared inhibitors is shown in Tables 3 and 6. From these tables, a direct relationship between the efficiency of the inhibitors and electron affinity was observed. As a result, high negative values of electron affinity of (SD4) suggest that surfactant molecules strongly adsorb onto the steel surface and form a more protection film on the carbon steel surface [28]. One of the important calculated quantum chemical parameters of the investigated inhibitors is electronegativity (i inhibitor) and chemical potential (μ inhibitor). Electronegativity is related to the ability of the molecule to draw electron toward itself [30]. By inspection of the obtained data listed in Table 6, it is worth noting that SD4 (4.043 eV mol−1) has the highest probability to form a coordinating bond by accepting an electron from the metal surface (7 eV mol−1), and conversely, the electronic chemical potential of the inhibitors is larger than the metallic iron (−7 eV mol−1). Another important point is that the effect of global hardness (η) and softness (σ, eV−1) on the inhibitors efficiency. Global hardness (η) is approximated as ΔE/2, and can be defined under the principle of chemical hardness and softness (HSAB). It provides information about the reactive behaviour of molecules. A high value of the absolute hardness is, thus, an indication of high stability and low reactivity. In Tables 3 and 6, it can be observed that the inhibition performance is oppositely proportional to the global hardness. This may be attributed to the chemical structure of the inhibitor, as the ethylene oxide units increase (SD4), the reactivity of it is increasing and global hardness decreasing. From Table 6, it was observed that the softness (σ) increases from 0.241 to 0.229 eV−1 for SD4 and SD1, respectively. This means that the adsorption of the tested inhibitors onto the metal surface increases with increasing the value of (σ), this may be regarded as due to the chemical structure of the investigated inhibitors, whereas, the increasing of ethylene oxide units (SD4) leads to an increase in the number of electrons transferring from the inhibitor to the metal surface, thus a strong protective film was formed on the metal surface [30].
Conclusions
Three nonionic surfactants SD1, SD2 and SD4 have been synthesized, and their effects on corrosion inhibition of carbon steel in 1 M HCl have been studied using the weight loss method, as well as chemical and electrochemical techniques. From the data obtained, the following points can be emphasized;
-
1.
SD1, SD2 and SD4 surfactants had good inhibition effects against corrosion of steel in 1 M HCl solution. The high inhibition efficiencies of surfactants were attributed to their adherent adsorption onto the metal surface and the formation of a protective film.
-
2.
The potentiodynamic polarization curves indicated that SD1, SD2 and SD4 behaved as mixed type inhibitors by inhibiting both anodic metal dissolution and cathodic hydrogen evolution reactions.
-
3.
The Langmuir adsorption isotherm exhibited the best fit to the experimental data with ΔG° ads of −35.71, −35.85 and −36.36 kJ mol−1 for SD1, SD2 and SD4, respectively, which indicate that the adsorption of inhibitors onto the steel surface took place through electrostatic interaction (physical adsorption), as well as electrostatic interaction there may have been some other interactions.
-
4.
A comparison of the inhibition effectiveness of the prepared inhibitors indicated that their inhibition effects were closely related to orbital energies (HOMO and LUMO), dipole moment, total energy, log P and ΔN.
References
Bentiss F, Lebrini M, Vezin H, Chai F, Traisnel M, Lagrené M (2009) Enhanced corrosion resistance of carbon steel in normal sulfuric acid medium by some macrocyclic polyether compounds containing a 1,3,4-thiadiazole moiety: AC impedance and computational studies. Corros Sci 51:2165–2175
Deng S, Yu G, Chen Z, Wu D, Xia F, Jiang N (2009) Characterization of suspended solids in produced water in Daqing oilfield. Colloids Surf A 332:63–69
Noor El-Din MR, Al-Sabagh AM, Hegazy MA (2012) Study of the inhibition efficiency for some novel surfactants on the carbon steel (Type H-11) pipelines in 0.5 M HCl solution by potentiodynamic technique. J Dispers Sci Technol 33:1–8
Behpour M, Ghoreishi SM, Khayatkashani M, Soltani N (2012) Green approach to corrosion inhibition of mild steel in two acidic solutions by the extract of Punica granatum peel and main constituents. Mater Chem Phys 131:621–633
Farag AA, Noor El-Din MR (2012) The adsorption and corrosion inhibition of some nonionic surfactants on API X65 steel surface in hydrochloric acid. Corros Sci 64:174–183
Hegazya MA, El-Tabei AS, Bedair AH, Sadeq MA (2012) An investigation of three novel nonionic surfactants as corrosion inhibitor for carbon steel in 0.5 M H2SO4. Corros Sci 54:219–230
Yinqiu W, Philip RH (1997) Thermal reactions of fatty acids with diethylene triamine. J Am Oil Chem Soc 74:1–9
Kabir-ud-Din Gaurav S, Andleeb ZN (2011) Micellization and interfacial behavior of binary surfactant mixtures based on cationic geminis and nonionic Tweens. Colloids Surf A 385:63–71
Farag AA, Noor El-Din MR (2012) The adsorption and corrosion inhibition of some nonionic surfactants on API X65 steel surface in hydrochloric acid. Corros Sci 64:174–183
SayemAlam M, Asit BM (2012) Thermodynamic studies on mixed micellization of amphiphilic drug amitriptyline hydrochloride and nonionic surfactant Triton X-100. J Mol Liquids 168:75–79
Rosen MJ (1991) Synergism in mixtures containing zwitterionic surfactants. Langmuir 7:885–888
Ayse OY, Ramazan S, Gülfeza K (2012) Investigation of inhibition effect of rhodanine-N-acetic acid on mild steel corrosion in HCl solution. Mater Chem Phys 131:615–620
Xianghong L, Shuduan D, Hui F (2011) Triazolyl blue tetrazolium bromide as a novel corrosion inhibitor for steel in HCl and H2SO4 solutions. Corros Sci 53:302–309
Ahmed AF, Noor El-Din MR (2012) The adsorption and corrosion inhibition of some nonionic surfactants on API X65 steel surface in hydrochloric acid. Corros Sci 64:174–183
Tadros TF (2005) Applied surfactants: principles and applications. Wiley, UK
Rosen M, Dahanayake M, Cohen A (1983) Relationship of structure to properties in surfactants. 11. surface and thermodynamic properties of N-dodecyl-pyridinium bromide and chloride. Colloids Surf A 5:159–172
Ashassi-Sorkhabi H, Shaabani B, Seifzadeh D (2005) Corrosion inhibition of mild steel by some schiff base compounds in hydrochloric acid. Appl Surf Sci 239:154–164
Rosen MJ (2004) Surfactants and interfacial phenomena. John Wiley, Hoboken
Esquena J, Solans C (2001) Influence of the HLB parameter of nonionic surfactants on normal and reversed-phase thin layer chromatography. Colloids Surf A 189:85–92
Hegazy MA, Zaky MF (2010) Inhibition effect of novel nonionic surfactants on the corrosion of carbon steel in acidic medium. Corros Sci 52:1333–1341
Dileep KY, Quraishi MA, Maiti B (2012) Inhibition effect of some benzylidenes on mild steel in 1 M HCl: an experimental and theoretical correlation. Corros Sci 55:254–266
Xianghong L, Shuduan D, Hui F, Taohong L (2009) Adsorption and inhibition effect of 6-benzylaminopurine on cold rolled steel in 1.0 M HCl. Electrochim Acta 54:4089–4098
Da-Quan Z, Qi-Rui C, Xian-Ming H, Li-Xin G, Gui SK (2009) Corrosion inhibition and adsorption behavior of methionine on copper in HCl and synergistic effect of zinc ions. Mater Chem Phys 114:612–617
Ramazan S, Ece A, Gülfeza K (2011) Adsorption and corrosion inhibition effect of 2-((5-mercapto-1,3,4-thiadiazol-2-ylimino)methyl)phenol Schiff base on mild steel. Mater Chem Phys 125:796–801
Solmaz R, Kardas G, Yazıci B, Erbil M (2008) Adsorption and corrosion inhibitive properties of 2-amino-5-mercapto-1,3,4-thiadiazole on mild steel in hydrochloric acid media, Colloids Surf A 312:7–17
Noor El-Din MR (2009) Corrosion inhibition efficiency of some new surfactants on carbon steel type H-11 in 1 M hydrochloric acid solution. J Dispers Sci Technol 30:1027–1035
Emergul KC, Hayvali M (2006) Studies on the effect of a newly synthesized Schiff base compound from phenazine and vanillin on the corrosion of steel in 2 M HCl. Corros Sci 48:797–812
Rosenfeld IL (1981) Corrosion inhibitors. McGraw-Hill, New York
Eddy NO, Ibok UJ, Ebenso EE, El Nemr A, El Ashry HE (2009) Quantum chemical study of the inhibition of the corrosion of mild steel in H2SO4 by some antibiotics. J Mol Model 15:1085–1092
Ying Y, Weihua L, Lankun C, Baorong H (2008) Electrochemical and quantum chemical study of purines as corrosion inhibitors for mild steel in 1 M HCl solution. Electrochim Acta 53:5953–5960
Acknowledgments
The authors are grateful to the Egyptian Petroleum Research Institute (EPRI) for funding and support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Noor El-Din, M.R., Khamis, E.A. Corrosion Inhibition Efficiency, Electrochemical and Quantum Chemical Studies of Some New Nonionic Surfactants for Carbon Steel in Acidic Media. J Surfact Deterg 17, 795–805 (2014). https://doi.org/10.1007/s11743-014-1565-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-014-1565-6