Abstract
A novel cationic biodegradable dimeric (gemini) surfactant, ethane-1,2-diyl bis(N,N-dimethyl-N-hexadecylammoniumacetoxy) dichloride (16-E2-16), containing an ester-linked spacer was synthesized. Its pure and mixed micellization properties with monomeric surfactants cetyl trimethyl ammonium chloride, cetyl pyridinium chloride, sodium dodecyl sulfate, sodium dodecyl benzene sulfonate, cetyl alcohol ethoxylate (20EO) and tert-octylphenol ethoxylate (9.5EO) were investigated by surface tension measurements at 30 °C. The critical micelle concentration (CMC) of 16-E2-16 is well below that of cetyl trimethyl ammonium chloride containing the same number of carbon atoms in the hydrophobic tail per polar head. At different mole fractions of the gemini surfactant, the CMCs of the gemini-conventional binary mixtures were determined and were found to be less than the ideal CMC values in all the cases indicating synergistic interactions. Aggregation number and Stern–Volmer constant, obtained by the fluorescence quenching technique, also support the synergistic behavior of the surfactant systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactants are amphiphilic molecules consisting of a hydrophilic polar head group and a hydrophobic hydrocarbon tail which decrease the surface as well as interfacial tension at the liquid–air interface and form aggregates called micelles at a concentration known as the critical micelle concentration (CMC) [1]. Gemini surfactants are made of two hydrophobic chains, two polar head groups covalently linked through a spacer, which significantly influences their properties. Having this unique chemical structure, geminis possess properties superior to those of conventional surfactants (such as low CMC, high surface activity, better solubilization, better wetting properties, specific rheological properties, and unusual aggregation behavior [2, 3]). The environment is affected considerably by the toxicity of surfactants during their use in commercial and industrial applications, which can be avoided by the use of cleavable surfactants [4–10]. The ester bond in the spacer makes the geminis more cleavable/biodegradable with lower aquatic toxicity compared with other cationic surfactants (conventional as well as gemini) [11–13]. The polar bond which contributes to the water solubility makes them degradable. For aquatic organisms including microbes, the biodegradable gemini is less toxic than the other surfactants which cause cellular breakdown, membrane disruption, protein unfolding and alter other enzymatic activities [14].
The properties of mixed surfactant solutions are more interesting because not only are the properties of individual components combined, but also synergism is observed in properties of interest. Recently, several research papers have appeared in the literature regarding the mixing behavior of geminis with conventional surfactants where combinations of different types of surfactants have been studied [12, 15–17]. However, there are scanty reports on the study of systems containing a biodegradable/cleavable gemini surfactant as one of the components. We have examined the micelles formed from the dimeric surfactant, ethane-1,2-diyl bis(N,N-dimethyl-N-hexadecylammoniumacetoxy) dichloride, abbreviated 16-E2-16 in the following, where E2 represents two ester groups in the spacer. The hydrophilic head groups of the gemini surfactant are connected by a covalent linkage through the ester bonds. Based on the behavior of other cationic gemini surfactants having ester groups, this compound is possibly more biodegradable and less toxic than common cationic gemini surfactants. The investigations were made by using surface tension and fluorescence quenching techniques.
Materials and Methods
Materials
The cationic surfactants cetyl pyridinium chloride CPC (Merck) and cetyl trimethyl ammonium chloride CTAC (99 %, Acros), anionic surfactants sodium dodecyl benzene sulfonate SDBS (TCI) and sodium dodecyl sulfate SDS (99 %, Sigma), nonionic surfactants cetyl alcohol ethoxylate (20EO) C16EO20 (Brij 58 from Merck) and tert-octylphenol ethoxylate (9.5EO) TOPEO9.5 (Triton TX-100 from Fluka) were used as received. The anionic surfactant SDS was recrystallized twice before use. N, N-dimethylhexadecylamine (≥95 %, Aldrich), ethylene glycol (99 %, Sigma Aldrich), chloroacetyl chloride (98 %, Loba chemie) and pyrene (99 %, Fluka) were also used without further purification.
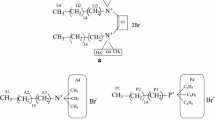
The chemical structures of the surfactants used in this study are shown in Scheme 1. For SDBS, only one of the main chemical species is represented.
Structure of surfactant molecules used in this study: ethane-1,2-diyl bis(N,N-dimethyl-N-hexadecylammoniumacetoxy) dichloride (16-E2-16), sodium dodecyl sulfate (SDS), sodium dodecyl benzene sulfonate (SDBS), cetyltrimethylammonium chloride (CTAC), cetylpyridinium chloride (CPC), polyoxyethylene (20) cetyl ether (C16EO20), tert-octylphenoxy polyethoxyethanol (TOPEO9.5)
Synthesis of the Gemini Surfactant
The cationic ester-bonded gemini surfactant 16-E2-16 was synthesized in two steps (Scheme 2) [7].
-
1.
Firstly, ethane-1,2-diyl bis(chloroacetate) was prepared by heating a mixture of chloroacetyl chloride (0.22 mol) and ethylene glycol (0.1 mol) at 50 °C for 8 h in nitrogen atmosphere. The product thus obtained was dissolved in ether, dried over magnesium sulfate and the solvent distilled off under reduced pressure. Low melting colorless needle-shaped crystals of ethane-1,2-diyl bis(chloroacetate), 15.15 g (65.36 %) were obtained.
-
2.
In the second step, the target compound was obtained by heating ethane-1,2-diyl bis(chloroacetate) with N, N-dimethylhexadecylamine (molar ratio = 1:2.1) in ethyl acetate for 10 h. When the solvent was removed under reduced pressure, white crystalline solid of 16-E2-16 was obtained which was further purified by repeated crystallization in ethyl acetate-ethanol mixtures (5:1), m. p.: 180–184 °C, 36.81 g (78.7 %). The structure was confirmed by FT-IR, 1H-NMR and mass spectroscopy. The 1H-NMR and electrospray ionization (ESI, +) mass spectra of 16-E2-16 are shown in Figs. 1S and 2S (Supporting material).
FT-IR (KBr, ν cm−1): 2,922.77, 2,855.44 (C–H); 1,749.36 (C = O); 1,473 (C–O); 1,184.71 (C–N); 719.47.
1H-NMR (300 MHz, CDCl3, δ scale): 0.86–0.90 (t, 6H, −2 × CH 3 , alkyl chain); 1.25–1.34 (m, 52H, −2 × (CH 2 )13, alkyl chain); 1.76 (m, 4H, −2 × N+CH2CH 2 ); 3.53 (s, 12H, −2 × N+(CH 3 )2); 3.79 (s, 4H, −2 × CH 2 O); 4.49 (t, 4H, −2 × N+CH 2 ); 5.36 (s, 4H, −2 × N+CH 2 COO).
MS–ESI + (m/z): 718 (M–Cl−), 667 (M–Cl−–CH3Cl−), 457 (M + H+–C14H29N+(CH3)2CH2OOCH = CH2), 130 (CH3)2N+CH2OOCH = CH2).
Anal. Calc. for C42H86O4N2Cl2: C 66, H 11.4, N 3.7 (Found: C 63.76, H 10.39, N 3.63).
The above spectral data correspond to the structure of 16-E2-16.
Methods
Determination of the Critical Micellar Concentrations
A mixed micellization study at different mole fractions of 16-E2-16 (0.2, 0.4, 0.6, 0.8) was done with six different monomeric surfactants. The CMCs of the pure/mixed surfactant systems were determined by the surface tension method. For each surfactant, surface tension (γ) measurements of the pure as well as four binary mixtures prepared in double distilled water were made with a Krüss 11 Tensiometer (K11MK3, Germany) by the platinum ring detachment method at 30 °C. The temperature was maintained by circulating water from an Orbit RS 10S thermostat to the sample holder. Each experiment was repeated to achieve good reproducibility. The accuracy of the surface tension measurements was within ±0.1 mNm−1.
Determination of Aggregation Numbers
The aggregation numbers (N agg) of pure/mixed surfactant micelles were determined by steady-state fluorescence measurement of pyrene (recorded by a Hitachi F-4500 Fluorimeter, λ ex = 334 nm, excitation slit width = 5.0 nm, emission slit width = 2.5 nm). The requisite volume of ethanolic 3.0 mM pyrene solution was transferred to a standard volumetric flask and the solvent was evaporated. The surfactant solution was added to it to keep the pyrene concentration constant at 3 × 10−3 mM. The quencher (CPC) concentration was varied from 0 to 1.4 × 10−3 mM. It is considered that the fluorescence lifetime of pyrene was longer than the residence time of quencher in the micelle, ensuring Poisson distribution. Total surfactant concentration of the pure/mixed systems was taken as 0.2 mM. The fluorescence intensities I 1 (λ em = 373 nm) and I 3 (λ em = 384 nm) correspond to the first and third vibronic peaks, respectively which decrease with the increase in quencher concentration.
Biochemical Oxygen Demand (BOD) Test
The test for BOD was done to find out the tendency of the gemini surfactant to biodegrade. The inherent biodegradability of 16-E2-16 was evaluated by the BOD test by the oxygen consumption method which took 5 days to complete [18]. A 100-mg sample was added to 100 ml of the basic culture solution. The change in the BOD (mg) of the system was monitored for 5 days. The biodegradability was calculated as
(TOD refers to the theoretical oxygen demand when the test compound is completely oxidized).
The gemini surfactant 16-E2-16 showed good biodegradability. Its biodegradability was found to be 23 % after 5 days.
Hemolytic Activity Test
The toxicity of 16-E2-16 was determined by the hemolytic activity test (by using a BMG FLUOstar Galaxy 384 microplate reader) following a reported procedure [4]. Surfactant solutions of different volumes (ranging from 12.5 to 300 μg/ml) were taken for the study. From the hemolysis results, the dose–response curve was obtained. The concentration that induces the hemolysis of 50 % of the cells (HC50) in the erythrocyte suspension was subsequently calculated. The HC50 value of 16-E2-16 was found to be 228.88 μg/ml whereas for CTAC HC50 value was 0.00312 μg/ml. It is evident from Fig. 1 that the toxicity of 16-E2-16 is extremely low compared to the conventional surfactant CTAC [19].
Results and Discussion
Surface tension (γ) has long been established as one of the important physical parameters for determining the CMC of surfactants. When surfactant molecules are added to water, the excess surface concentration remains constant while γ decreases linearly. After saturation, the added surfactants assemble to form aggregates called micelles and γ remains constant. The CMC is obtained from the break point in the γ versus log[surfactant] profile. Constant value of γ at CMC is a measure of the effectiveness of the surfactant.
The situation in a number of cases is not as simple as described above. Instead of two (usual), three main regions are often encountered, respectively, at very low, intermediate, and high surfactant concentrations. The reason advanced for observing the first low concentration region is given due to surface active trace impurities [20–22]. Significant lowering of tension in the pre-CMC region has been documented [22–24] by the impurities present even at ppm levels.
As all the plots show clear and sharp breaks between straight lines drawn through intermediate and high surfactant concentration regions, the CMC values were obtained from such profiles (Fig. 2; Table 1). Compared with the corresponding conventional monomeric surfactant CTAC, 16-E2-16 is more efficient at lowering the surface tension of water and has a much lower CMC due to greater hydrophobicity of the dimer owing to the double-tailed structure. The CMC values of the pure surfactants decrease in the order: SDS > SDBS > CTAC > CPC > TOPEO9.5 > C16EO20 > 16-E2-16. The nonionics have naturally lower CMC values than the cationic/anionic surfactants. The surface tension curves for all the six mixtures at different molar ratios are provided in Fig. 3S (Supporting material).
Synergistic Interactions Between the Surfactants in Mixed Micelles
Ideal CMC (CMCideal) values for various mixed surfactant systems were calculated using the Clint Eq. 1 [25]
where α 1, α 2 are the stoichiometric mole fractions, CMC1 and CMC2 are the CMC values of the gemini and conventional surfactants, respectively. Lower values of CMC12 (i.e., the experimental CMC) than CMCideal indicate their nonideal behavior, which is a required condition for synergism between the constituents. The nature and strength of interactions between the surfactant molecules in the mixed micelles have been interpreted in terms of interaction parameter (\( \beta^{m} \) = \( \left[ {W_{12} - (\frac{1}{2}W_{11} + \frac{1}{2}W_{22} )} \right]/RT \), W being the molar interaction energy between the indicated constituents). According to Rubingh [26], if two surfactants form mixed micelles, then \( X_{1}^{m} \) (micellar mole fraction of gemini) is related to α 1, CMC1, CMC2, and CMC12 as
The value of \( X_{1}^{m} \) was obtained by solving Eq. 2 iteratively, which was then used to calculate \( \beta^{m} \)
\( \beta^{m} \) indicates the magnitude of interaction between the two surfactants in the mixed micelles. Higher negative value of \( \beta^{m} \) implies a reduction in the free energy of micellization, which makes the system thermodynamically more stable. When there is repulsion between the components, \( \beta^{m} \) is positive and the interaction is antagonistic in nature. But for all the mixed systems studied, we have obtained negative \( \beta^{m} \), indicating the presence of synergistic interactions between the surfactants.
The activity coefficient (\( f_{i}^{m} \)) of individual components within the mixed micelles can be obtained by using Eqs. 4 and 5
The \( f_{i}^{m} \)< 1 and \( f_{1}^{m} \) > \( f_{2}^{m} \) results imply the formation of mixed micelles with a higher participation of gemini than conventional surfactants.
The excess free energy of mixing (\( \Updelta G_{ex}^{m} \)) can be calculated using the activity coefficients by Eq. 6
Negative \( \Updelta G_{ex}^{m} \) values (Table 1S, Supporting material) indicate that the stability of mixed micelles is higher than single surfactant micelles.
Regular solution theory (RST) is mostly used rather than other models due to its simplified approach. However, in some cases, it is considered irrelevant when the hydrocarbon chain length and the ionic strength variations are taken into account for the determination of interaction coefficients. Another suitable model, i.e., Motomura’s approach [27], is then used to evaluate the micellar composition and physicochemical parameters. It is applicable to any kind of surfactant mixture and is independent of counterions of the amphiphiles. Accordingly, mixed micelles are considered as a macroscopic bulk phase and the related energetic parameters of such systems can be evaluated in terms of excess thermodynamic quantities. The micellar composition was determined by the relationship
In the above equation, \( X_{1}^{M} \) = the micellar mole fraction of 16-E2-16, \( \bar{\alpha }_{i} \) = bulk mole fraction, ν i = number of ions dissociated by the ith component and δ = Kronecker delta. δ = 1 for identical counterions and δ = 0 for different counterions. In the present case, Eq. 7 is modified as:
-
1. For gemini-anionic mixture: \( \nu_{1} \) = \( \nu_{1a} \) + \( \nu_{1c} \) = (2 + 1) = 3, \( \nu_{2} \) = \( \nu_{2b} \) +\( \nu_{2d} \) = (1 + 1) = 2
-
$$ X_{1}^{M} = \left( {\frac{{3\alpha_{1} }}{{\alpha_{1} + 2}}} \right) - \frac{1}{{(\alpha_{1} + 2)CMC}}\left( {\frac{{3\alpha_{1} }}{{\alpha_{1} + 2}}} \right)\left( {\frac{{2 - 2\alpha_{1} }}{{\alpha_{1} + 2}}} \right)\left( {\frac{{\partial \overline{CMC} }}{{\partial \bar{\alpha }_{1} }}} \right) $$(10)
-
2. For gemini-cationic mixture (with the same counterion):
-
\( \nu_{1} \) = \( \nu_{1a} \) + \( \nu_{1c} \) = (2 + 1) = 3, \( \nu_{2} \) = \( \nu_{2b} \) + \( \nu_{2d} \) = (1 + 1) = 2
-
$$ X_{1}^{M} = \left( {\frac{{3\alpha_{1} }}{{\alpha_{1} + 2}}} \right) - \frac{{\frac{1}{{(\alpha_{1} + 2)CMC}}\left( {\frac{{3\alpha_{1} }}{{\alpha_{1} + 2}}} \right)\left( {\frac{{2 - 2\alpha_{1} }}{{\alpha_{1} + 2}}} \right)\left( {\frac{{\partial \overline{CMC} }}{{\partial \bar{\alpha }_{1} }}} \right)}}{{1 - \frac{1}{{2\bar{\alpha }_{1} + 3\bar{\alpha }_{2} }}}} $$(11)
-
3. For gemini-nonionic mixture: \( \nu_{1} \) = \( \nu_{1a} \) + \( \nu_{1c} \) = (2 + 1) = 3, \( \nu_{2} \) = \( \nu_{2b} \) + \( \nu_{2d} \) = (1 + 0) = 1
-
$$ X_{1}^{M} = \left( {\frac{{3\alpha_{1} }}{{3\alpha_{1} + \alpha_{2} }}} \right) - \frac{1}{{(3\alpha_{1} + \alpha_{2} )CMC}}\left( {\frac{{3\alpha_{1} }}{{3\alpha_{1} + \alpha_{2} }}} \right)\left( {\frac{{\alpha_{2} }}{{(3\alpha_{1} + \alpha_{2} }}} \right)\left( {\frac{{\partial \overline{CMC} }}{{\partial \alpha_{1} }}} \right) $$(12)
The mole fraction of surfactants in ideal state was calculated using Eq. 13
We see that \( X_{1}^{M} \) (Table 1) as well as the \( X_{1}^{m} \) and \( X_{1}^{\text{ideal}} \) values (Fig. 3) for mixed systems increase with increase of α 1. Also, for all the systems, \( X_{1}^{m} \) is greater than α 1. Even at lower α 1, contribution of 16-E2-16 in mixed micellization is higher than that of single chain surfactants. This is because of the two hydrophobic chains trying to be accommodated in the mixed micelles at the same time.
Cationic Dimeric + Cationic Monomeric Surfactants
As the gemini 16-E2-16 is a dimer of CTAC, the physicochemical behavior of the binary gemini-CTAC and, for comparision, gemini-CPC systems were studied. We see (Table 1) that the CMC12 values decrease slowly with the increase of α 1 which suggests that CTAC/CPC can easily partition into the micelles formed by 16-E2-16. Higher \( X_{1}^{m} \) values in case of gemini-CPC indicate a more favorable condition for the formation of mixed micelles where contribution of 16-E2-16 is higher than that of the conventional surfactant [17].
Cationic Dimeric + Anionic Monomeric Surfactants
Formation of mixed micelles between the dicationic gemini and anionic surfactants SDS/SDBS is affected by the electrostatic interaction between them. For the gemini-SDS system, higher \( \beta^{m} \) for the higher α 1 is due to strong coulombic attraction between the oppositely charged head groups of the surfactants which facilitates micellization. The 16-E2-16 forms more stable mixed micelles with SDS than the other monomeric surfactants as can be seen from the \( \Updelta G_{ex}^{m} \) values. The negative \( \beta^{m} \) values are due to the attractive interaction between the surfactants, i.e., nonideality of the mixed surfactant systems. All the mixed systems have lower CMCs than the individual surfactants; among them gemini-SDS provides better mixed micelles than the other systems, in conformity to our earlier results [17]. SDBS behaves like SDS.
Cationic Dimeric + Nonionic Monomeric Surfactants
Except for lower α 1, we observed a decrease in CMC12 with the increase of α 1 for the gemini- C16EO20/TOPEO9.5 systems. The negative \( \beta^{m} \) values show the existence of synergistic interactions between the surfactants in mixed micelles. Interactions between the components are usually considered to be the result of two contributions: one is due to the interactions between the hydrophobic parts of the surfactants in the micellar core, the other is because of the electrostatic interactions between the hydrophilic head groups of surfactants at the interface. For binary systems containing TOPEO9.5 as one of the components, Wang et al. [5] explained the non-ideality by considering that the intercalation of TOPEO9.5 molecules among the dimeric surfactant molecules within the micelle results in a decrease in electrostatic repulsions at the interface, this promoting micellization. Lower values of \( \beta^{m} \) and \( X_{1}^{m} \) for gemini-C16EO20 than gemini-TOPEO9.5 imply higher synergism in the latter system.
Surfactant–Surfactant Interaction in Mixed Monolayer Systems
Before the formation of mixed micelles, a mixed monolayer is formed at the air/water interface by adsorption of surfactants onto it. Rosen’s Eq. 14 [28] (analogous to Rubingh’s equation) is used to explain the formation of a mixed monolayer of surfactant molecules
\( X_{ 1}^{\sigma } \) indicates the mole fraction of 16-E2-16 in the mixed monolayer. C 1, C 2 and C 12 are the concentrations of gemini, conventional and mixed monolayers, respectively. At the air/water interface, interaction between the surfactants can be explained by Eq. 15
where \( \beta^{\sigma } \) is the interaction parameter of the surfactant in the mixed monolayer. As can be seen from Table 2, negative \( \beta^{\sigma } \) values (similar to \( \beta^{m} \)) show attractive interaction between the surfactant molecules at the interface. For gemini-SDS/SDBS, \( X_{ 1}^{\sigma } \) values are lower than the \( X_{1}^{m} \) (Fig. 3) suggesting that the mixed monolayer possesses less gemini surfactant molecules than the mixed micelles. For gemini-CPC/CTAC, \( X_{ 1}^{\sigma } \) > \( X_{1}^{m} \) indicating higher contribution of gemini surfactant molecules on mixed monolayer, which is supported by the \( \beta^{\sigma } \) values. For gemini-TOPEO9.5, contribution of \( X_{ 1}^{\sigma } \) towards mixed monolayer is not observed due to the presence of bulky polyoxyethylene group, whereas, with C16EO20, it is again lower than \( X_{1}^{m} \).
For all the systems (other than gemini-CTAC), \( \beta_{ave}^{\sigma } \) > \( \beta_{ave}^{m} \)—this shows higher synergism between the surfactants in the monolayer than in the mixed micelles. For the gemini-TOPEO9.5 mixture, antagonism was observed in the monolayer due to the presence of phenyl group that may hinder the packing of the hydrophilic groups at the interface, whereas in the mixed micelles the interaction is synergistic. Higher \( \beta_{ave}^{\sigma } \) values of gemini-SDS/SDBS than the other mixed systems show higher synergism and more nonideality at the air/water interface as there is electrostatic interaction of oppositely charged head groups in the mixed monolayer [1, 29].
The activity coefficients (\( f_{i}^{\sigma } \)) at mixed monolayer could be correlated to \( \beta^{\sigma } \) and \( X_{ 1}^{\sigma } \) as
\( f_{1}^{\sigma } \) and \( f_{2}^{\sigma } \) values are less than unity showing the nonideal behavior on mixed monolayer systems except for gemini-TOPEO9.5 (Table 2).
Surface and Interfacial Properties
The maximum surface excess or surface saturation (Γmax ) and minimum area per surfactant head group adsorbed at the interface (A min) are effective measures of the extent of adsorption of various components at the interface. In the submicellar region, Γmax and A min can be calculated from the surface tension data by fitting the Gibbs adsorption isotherm [1]
and
where n represents the number of ionic species whose concentrations at the interface vary with [surfactant] in solution, C the concentration of the surfactant, dγ/d logC the slope of the γ versus log C plot, N A Avogadro’s number, and R and T have their usual significance. For pure conventional nonionic, cationic/anionic and gemini n values are 1, 2 and 3, respectively. For gemini-nonionic and gemini-cationic/anionic mixed micelles the n values are 4 and 5, respectively.
Evidently A min decreases when Γmax increases (Table 2). For pure surfactants, the order of A min is: 16-E2-16 > SDBS > CPC > CTAC > SDS > TOPEO9.5 > C16EO20. A min of 16-E2-16 is the highest among all the studied surfactants. The hydrophilicity of the spacer may be the reason for higher value of A min for 16-E2-16 as compared to the corresponding 16-s-16 cationic gemini (i.e., alkanediyl-α,ω-bis(dimethylhexadecylammonium bromide) [30]. Lower values of A min for the nonionic surfactants C16EO20 and TOPEO9.5 are due to the negligible head–head interaction and the molecules are more tightly packed at the interface than the other surfactants. In case of the gemini-C16EO20 mixed system, Γmax increases and thus A min decreases with the increase in α 1. The A min values of gemini-SDS system are higher than the gemini-SDBS suggesting the formation of more closely packed mixed micelles with SDS (only the mixture for α 1 = 0.6 is an exception). For all the mole fractions, Γmax values of the gemini-C16EO20 systems are lower (and A min values are higher) as compared to the gemini-TOPEO9.5 system but no trend is observed for Γmax (or A min) values of gemini-CTAC/CPC.
pC 20 (i.e. − log C 20) measures the “efficiency” of a surfactant in aqueous solution. C 20 , the surfactant concentration required to reduce γ by an arbitrary 20 mNm−1, also reflects the maximum tendency of a surfactant to adsorb at the interface [1]. The values of pC 20 for all the surfactant systems are given in Table 2.
The Gibbs free energy of micellization
measures the tendency of the surfactant to form micelles (\( X_{CMC} \) indicates the CMC of the mixture in the form of the mole fraction). The values are found to be negative for all the systems (Table 1S). Among pure systems, the absolute value of ΔG o m is the highest for gemini and the lowest for SDS. Order of average ΔG o m for the mixed systems is gemini-C16EO20 > gemini-CTAC > gemini- TOPEO9.5 > gemini-SDBS > gemini-SDS > gemini-CPC. Higher ΔG o m for gemini-C16EO20 is due to more favorable micellization. For all mole fractions of 16-E2-16, the values of ΔG o m (Table 1S) are negative which again supports the occurrence of the mixed micellization process.
The standard free energy of adsorption at the interface (\( \Updelta G_{ads}^{\text{o}} \)) can be correlated to ΔG o m by Eq. 21 [31]
where π CMC = γ w − γ CMC is the surface pressure at the CMC, γ w and γ CMC are the surface tensions of pure water and the surfactant solution at the CMC [17]. Higher values of \( \Updelta G_{ads}^{\text{o}} \) than ΔG o m (Table 1S) indicate that the 16-E2-16 molecules are adsorbed more at the interface than the mixed micelles. The order of average \( \Updelta G_{ads}^{\text{o}} \) for mixed systems is gemini-CPC > gemini-C16EO20 > gemini-SDS > gemini-SDBS > gemini-CTAC > gemini-TOPEO9.5.
G min is the work required to transfer the surfactant molecules from bulk phase to interface of the surfactant solution
The lower value of G min indicates higher thermodynamically stable mixed surfaces.
The order of G min for the mixed surface is gemini-CPC > gemini-C16EO20 > gemini-TOPEO9.5 > gemini-SDS > gemini-SDBS > gemini-CTAC (Table 1S).
Spectroscopic Studies
The aggregation number of the pure/mixed micelles can be determined by the steady-state fluorescence experiment (if Poisson distribution is assumed to be valid for the equilibrium of the solubilizate between the aqueous and micellar phases). Addition of a fluorescent probe (pyrene) to the micellar system will enable it to partition among micelles with a quencher and with empty micelles. When pyrene occupies an empty micelle, the ratio of fluorescence intensities in absence (I o) and presence (I) of quencher is [32]
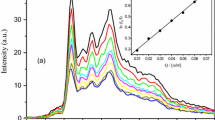
On the basis of pyrene spectra recorded at different quencher concentrations (Fig. 4), linear plots as per Eq. 23 made possible the evaluation of N agg values (Table 3). The values were higher for the mixed systems, indicating mixed micellization. The lower value of N agg for the gemini than for the conventional surfactants may be due to the hydrophilic nature of the spacer containing ester groups. For micellar aggregation, many factors are taken into account such as electrostatic interaction between head groups of the ionic surfactants and steric interaction between head groups of the surfactant components. For gemini-SDS/SDBS, N agg increases with α1 as electrostatic attraction between the head groups is higher which results in more compact micelles. For gemini-CTAC, the aggregation number decreases from α 1 = 0.2 to 0.8 forming loose micelles seemingly due to the same charge on head groups (vis-a-vis anionic ones). In the case of gemini-TOPEO9.5, as incorporation of TOPEO9.5 monomer into the micelle increases, electrostatic repulsion between the charged head groups of gemini decreases, but steric repulsion becomes preponderant due to the larger head group of TOPEO9.5 than the gemini. Steric repulsion between the head groups of TOPEO9.5 increases the area required per surfactant head group that leads to the formation of the mixed micelles to adopt a conformation with higher curvature and reduces the aggregation number of the mixed micelle from α TOPEO9.5 = 0.4 to 0.8. Thus the aggregation number is mainly controlled by TOPEO9.5.
The above results can be explained further on the basis of fluorescence quenching. The strength of the hydrophobic environment can be evaluated by determining the Stern–Volmer binding constant (K sv), using the relation
A higher K sv value suggests a greater probability of finding the presence of both the fluorophore and quencher in a strong hydrophobic environment [33]. K sv is higher for the surfactant mixtures than for the pure conventional surfactants as shown in Fig. 4S and Table 3.
The ratio of fluorescence intensities (I 1 /I 3) of pyrene in micellar solutions can be directly related to the microenvironment of the solubilized pyrene. A higher value indicates a more polar microenvironment of the solubilized probe in a system. In the present case, higher I 1 /I 3 values of gemini-CTAC and gemini-C16EO20/TOPEO9.5 indicate that pyrene resides in a more polar region than the gemini or gemini-SDS/SDBS system (Table 3).
Conclusion
A cationic biodegradable gemini surfactant having ester-bonded spacer, ethane-1,2-diyl bis(N,N-dimethyl-N-hexadecylammoniumacetoxy) dichloride, (16-E2-16) has been prepared and the results of systematic investigation of mixed micellization and aggregation behavior involving 16-E2-16 with cationic, anionic and nonionic monomeric surfactants are presented. Surface tension measurements provided physicochemical properties such as the CMC, Γmax, A min, etc. Clint, Rosen, Rubingh and Motomura theories have been used to evaluate other physicochemical parameters at the air/water interface as well as within the micelles. A fluorimetric technique was used to determine the aggregation behavior of pure/mixed systems. The CMC of the gemini surfactant is significantly less than that of the monomeric surfactants. All the mixed surfactant solutions show synergism in the formation of mixed micelles which is indicated by the values of \( \beta \), \( X_{1} \), \( \Updelta G_{ex}^{m} \). Although 16-E2-16 can be regarded as the dimer of CTAC, its binary mixtures with CTAC deviate most from ideal behavior. This indicates that in the mixed micelles, the head groups of CTAC are likely to be much farther apart from each other than those in 16-E2-16. Motional freedom of the cationic head groups in a dimeric surfactant is greatly reduced compared with that of the head group in a monomeric surfactant and the micellar behavior seems to be profoundly affected by this change. With the cationic cleavable gemini used in this study, the potential risk of an adverse environmental effect of surfactants can be reduced. Here, the synergism is highest for the gemini-SDS mixed surfactant systems—the results are better than systems involving conventional–conventional mixtures.
References
Rosen MJ (2004) Surfactants and interfacial phenomena, 3rd edn. Wiley, New York
Shukla D, Tyagi VK (2006) Cationic gemini surfactants: a review. J Oleo Sci 55:381–390
Menger FM, Keiper JS (2000) Gemini surfactants. Angew Chem Int Ed 39:1906–1920
Perez L, Garcia MT, Ribosa I, Vinardell MP, Manresa A, Infante MR (2002) Biological properties of arginine-based gemini cationic surfactants. Environ Toxicol Chem 21:1279–1285
Wang X, Wang J, Wang Y, Ye J, Yan H, Thomas RK (2005) Properties of mixed micelles of cationic gemini surfactants and nonionic surfactant triton X-100: effects of the surfactant composition and the spacer length. J Colloid Interface Sci 286:739–746
Tehrani-Bagha AR, Oskarsson H, Ginkel CGV, Holmberg K (2007) Cationic ester-containing gemini surfactants: chemical hydrolysis and biodegradation. J Colloid Interface Sci 312:444–452
Gao ZN, Tai SX, Zhang Q, Zhao Y, Lü B, Ge YS, Huang L, Tang XY (2008) Synthesis and surface activity of biquaternary ammonium salt gemini surfactants with ester bond. Wuhan Univ J Natural Sci 13:227–231
Banno T, Kawada K, Matsumura S (2010) Creation of novel green and sustainable gemini-type cationics containing carbonate linkages. J Surfactants Deterg 13:387–398
Shukla D, Tyagi VK (2010) Development of cleavable surfactants. Tenside Surfactants Deterg 47:7–12
Kuo C-FJ, Lu L-H, Dong M-Y, Chang W-S, Chen K-M (2010) Preparation and properties of new ester-linked cleavable gemini surfactants. J Surfactants Deterg 14:195–201
Kralova K, Sersen F, Devinsky F, Lacko I (2010) Photosynthesis-inhibiting effects of cationic biodegradable gemini surfactants. Tenside Surfactants Deterg 47:288–293
Fatma N, Ansari WH, Panda M, Kabir-ud-Din (2013) Mixed micellization behavior of gemini (cationic ester-bonded) surfactants with conventional (cationic, anionic and nonionic) surfactants in aqueous medium. Z Phys Chem 27:133–149
Wu Y, Iglauer S, Shuler P, Tang Y, Goddard WA (2010) Alkyl polyglycoside sorbitan ester formulations for improved oil recovery. Tenside Surfactants Deterg 47:280–287
Hamme JDV, Singh A, Ward OP (2006) Physiological aspects Part 1 in a series of papers devoted to surfactants in microbiology and biotechnology. Biotechnol Adv 24:604–620
Zhao J, Christian SD, Fung BM (1998) Mixtures of monomeric and dimeric cationic surfactants. J Phys Chem B 102:7613–7618
Azum N, Naqvi AZ, Akram M, Kabir-ud-Din (2008) Studies of mixed micelle formation between cationic gemini and cationic conventional surfactants. J Colloid Interface Sci 328:429–435
Kabir-ud-Din Sheikh MS, Mir MA, Dar AA (2010) Analysis of mixed micellar and interfacial behavior of cationic gemini hexanediyl-1,6-bis(dimethylcetylammonium bromide) with conventional ionic and nonionic surfactants in aqueous medium. J Phys Chem B 114:6023
Delzer GC, Mckenzie SW (1999) Five–day biochemical oxygen demand, U.S. Geological Survey TWRI Book, vol 9
El-Sadek BM (2011) Synthesis, micellization and hemolysis evaluation of biodegradable quaternary ammonium compounds. Adv Appl Sci Res 2:363–372
Bae S, Harke M, Goebel A, Lunkenheimer K, Motschmann H (1997) A critical comparison of adsorption models for soluble surfactants. Langmuir 13:6274–6278
Bae S, Haage K, Wantke K, Motschmann H (1999) On the factor in Gibbs equation for ionic surfactants. J Phys Chem B 103:1045–1050
Eastoe J, Sandrine N, Downer A, Paul A, Rankin A, Tribe K (2000) Adsorption of ionic surfactants at the air-solution interface. Langmuir 16:4511–4518
Li ZX, Lu JR, Thomas RK (1997) Neutron reflectivity studies of the adsorption of aerosol-OT at the air/water interface: the surface excess. Langmuir 13:3681–3685
Downer D, Eastoe J, Pitt AR, Penfold J, Heenan RK (1999) Adsorption and micellisation of partially- and fully-fluorinated surfactants. Colloids Surf A 156:33–48
Clint JH (1975) Micellization of mixed nonionic surface active agents. J Chem Soc Faraday Trans 71:1327–1334
Rubingh DN (1979) Mixed micelle solutions. In: Mittal KL (ed) Solution chemistry of surfactants. Plenum Press, New York
Motomura K, Aratono M (1993) Mixed surfactant systems. In: Ogino K, Abe M (eds) Surfactant science series. Marcel Dekker, New York
Zhou Q, Rosen MJ (2003) Molecular interactions of surfactants in mixed monolayers at the air/aqueous solution interface and in mixed micelles in aqueous media: the regular solution approach. Langmuir 19:4555–4562
Kabir-ud-Din, Fatma W, Khatoon S, Khan ZA, Naqvi AZ (2008) 1H NMR and viscoelastic studies on cationic gemini surfactants in presence of aromatic acids and salts. J Chem Eng Data 53:2291–2300
Kabir-ud-Din, Al-dahbali GA, Naqvi AZ, Akram M (2013) Adsorption and micellization behavior of cationic surfactants (gemini and conventional-amphiphilic drug systems). J Sol Chem 42:172–189
Rosen MJ, Aronson S (1981) Standard free energies of adsorption of surfactants at the aqueous at the aqueous solution/air interface from surface tension data in the vicinity of the critical micelle concentration. Colloids Surf 3:201–208
Turro NJ, Yekta A (1978) Luminescent probes for detergent solutions. A simple procedure for determination of the mean aggregation number of micelles. J Am Chem Soc 100:5951–5952
Rohatgi-Mukherjee KK (1992) Fundamentals of photochemistry. Wiley-Eastern, New Delhi
Acknowledgments
MP acknowledges financial assistance under the Department of Science and Technology, Government of India, NF is thankful to the University Grants Commission, (India) for a fellowship and to SAIF, CDRI, Lucknow, for providing the1H-NMR facility, and KU thanks the UGC for awarding a Faculty Fellowship under its BSR Program. The help rendered by the Interdisciplinary Biotechnology Unit, AMU, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Fatma, N., Ansari, W.H., Panda, M. et al. A Systematic Study of Mixed Surfactant Solutions of a Cationic Ester-Bonded Dimeric Surfactant with Cationic, Anionic and Nonionic Monomeric Surfactants in Aqueous Media. J Surfact Deterg 16, 609–620 (2013). https://doi.org/10.1007/s11743-013-1448-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-013-1448-2