Abstract
Two novel environmentally friendly fluorinated surfactants, disodium monofluoroalkyl phosphates were synthesized using phosphorus pentoxide, i.e. H(CF2)6CH2OPO(ONa)2 and H(CF2)6CH2OCH2CH2OPO(ONa)2. The novel synthesized fluorinated surfactants have a high thermal stability on the basis of Thermogravimetric Analysis. Their surface properties were also examined with the aim to have similar surface tensions of dilute aqueous solutions when compared with the conventional fluorocarbon surfactants, ammonium perfluorooctanoate. The two synthesized monofluoroalkyl phosphates can respectively reduce the surface tension of water to 23.73 mN/m at 78.3 mmol/L and 21.38 mN/m at 20.93 mmol/L. In addition, the Krafft points are both below 0 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluorinated surfactants with a fluorocarbon chain as a hydrophobic group reduce the surface tension of water and form micelles at concentrations lower than those for the corresponding hydrocarbon surfactants [1]. Their outstanding chemical and thermal stability expands their applications to extreme conditions which are too severe for hydrocarbon surfactants [2–4]. Yet, they still have shortcomings that need to be overcome, such as their high price and potential environmental hazard. It has been demonstrated recently that perfluorooctanoate of ammonium (PFOA) displayed ubiquity, animal-based toxicity, and some associations with human health [5–7]. With the rising of public awareness for environment protection and extensive attention internationally to biodegradability of the fluorinated surfactants, the use of long-chain (≥C8) perfluorinated surfactants will be evidently forbidden in future [8].
Because fluorinated surfactants play a very important role in preparation of high performance fluorinated material, exploring novel fluorocarbon surfactants with a good environmental protection function is an important thesis for research workers. There are many research protocols proposed in the literatures, and one of which is that long carbon–fluorine chains of traditional fluorinated surfactants are “diluted” with hydrocarbon chains [9]. While the hydrocarbon content rises in these hybrid surfactants, they still keep the high surface tension lowering ability, characteristic of fluorinated surfactants.
Based on the above points, we expected to synthesize novel fluoroalkyl phosphate surfactants which contain short carbon–fluorine chains. Because phosphate surfactants can show outstanding properties, such as excellent wetting, thermal stability, alkali resistance and antistatic property [10]. What is more, the phosphate surfactants have reduced environmental presence of the surfactants themselves because of expected facile biodegradation [11].
This article reports the synthesis of two novel fluoroalkyl phosphate surfactants, disodium mono (dodecafluoroheptyl) phosphate and disodium mono (dodecafluoroheptyl oxyethyl) phosphate, in which single dodecafluoroheptyl acted as a fluorophilic segment, with the aim of obtaining such fluorinated surfactants that are more environmentally friendly than the conventional long-chain perfluorinated surfactants. Comparisons are also made in the article of the surface chemical properties of the newly synthesized surfactants with ammonium perfluorooctanoate, PFOA. Moreover, the effect of the introduction of oxyethylene into the formula was also obtained.
Experimental
Materials
1H,1H,7H-dodecafluoroheptanol was purchased from the China Fluoro Technology Co., Ltd., Shandong China; phosphorus pentoxide was obtained from the Tianjin Chemical Reagent Factory Tianjin China; benzene and toluene were provided by the Tianjin Guangcheng Chemical Reagent Co., Ltd., Tianjin China; ethyl ether was purchased from the Tianjin Kermel Chemical Reagent Co., Ltd., Tianjin China, as well as ethanol and sodium hydroxide. All of those chemicals were analytical reagents (AR) and were used without further purification. Ethylene glycol mono(1H,1H,7H-dodecafluoroheptyl) ether was made by Shandong Provincial Key Laboratory of Fluorine Chemistry and Chemical Materials, whose purity is up to 96% above. Ammonium perfluorooctanoate, PFOA, was also prepared by Shandong Provincial Key Laboratory of Fluorine Chemistry and Chemical Materials.
Synthesis of Disodium Mono(dodecafluoroheptyl) Phosphate
The disodium mono(dodecafluoroheptyl) phosphate was prepared from phosphorus pentoxide and alcohols in accordance with Eqs. 1 and 2.


The acid mono(dodecafluoroheptyl) phosphate H(CF2)6CH2OPO(OH)2 was prepared according to methods described in US 2,597,702 [12]. Because of the simultaneous formation of dialkyl phosphates, the content of mono(dodecafluoroheptyl) phosphate in the mixture may be determined by titrimetric procedure [13]. Both organic phosphates and orthophosphoric acid were effectively removed by recrystallization. The finally purified acid mono(dodecafluoroheptyl) phosphate, H(CF2)6CH2OPO(OH), a white solid product, was obtained with a yield of 56.6% of the theoretical based upon the weight of alcohol consumed.
Enough sodium hydroxide/ethanol solution was added dropwise into the ethanol solution of the acid mono(dodecafluoroheptyl) phosphate with continuous stirring for about 2 h. The solid precipitate was collected by vacuum filtration, and then the product was dried in an environment of 50 °C in the vacuum drying oven. The disodium mono(dodecafluoroheptyl) phosphate, a faint yellow solid powder, was finally obtained.
Synthesis of Disodium Mono(dodecafluoroheptyl oxyethyl) Phosphate
The disodium mono(dodecafluoroheptyl oxyethyl) phosphate was synthesized according to the preparation method of disodium mono(dodecafluoroheptyl) phosphate as detailed above, which just used ethylene glycol mono(1H,1H,7H-dodecafluoroheptyl) ether as corresponding fluorine-containing alcohol.
The disodium mono(dodecafluoroheptyl oxyethyl) phosphate was obtained finally, a yellow solid or powder, with a yield 60.5% of theoretical based upon the weight of alcohol consumed.
Characterizations
Fourier transform infrared spectra (FT-IR) on the novel surfactants were performed with the Bio-Rad FTS165 at ambient temperature. 1H-, 19F- and 31P-nuclear magnetic resonance (NMR) spectra were recorded at room temperature on a Bruker Avance 400 MHz instrument with tetramethylsilane as an internal standard, using deuteroxide (D2O) as the solvent. The thermal stabilities of the synthesized fluorinated surfactants were studied by a Pryris Diamond TG/DTA (Perkin-Elmer Co., USA) in the temperature range from 37 to 400 °C with a heating rate of 10 °C/min under a dynamic nitrogen flow at 50 mL/min.
Surface tension measurements were made at 25 °C on solutions of the synthesized surfactants by the ring method using a tensiometer K 100 (Krüss, Germany) in the following way. Aqueous surfactant stock solutions at given high concentrations were prepared using accurately weighed samples. Each of the stock solutions thus prepared was diluted successively with water to give sample solutions for measurement. The critical micelle concentration (CMC) values of the surfactants were determined from the break point of the γ versus lgC plots.
Krafft point measurements were performed electroconductometrically using a DKK-TOA conductivity meter CM-60G on solutions at given high concentrations placed in a thermostat while gently heating.
For surface-active solutes the surface excess concentration, Г max can be considered to be equal to the actual surface concentration without significant error. The concentration of surfactant at the interface may therefore be calculated from surface or interfacial tension data by use of the appropriate Gibbs equation [14]. The surface concentration, Г max can be obtained from the slope of a plot of γ versus lgC at constant temperature, as Eq. 3.
where C is the concentration of surfactant, ∂γ/∂lgC is the slope in the surface tension isotherm when the concentration is near the CMC, and γ is in mN/m. R is the gas constant [8.31 J/(mol K)], Г max is in mol/1,000 m2, and T is the absolute temperature.
The area per molecule at the interface provides information on the degree of packing and the orientation of the adsorbed surfactant molecule when compared with the dimensions of the molecule as obtained by use of molecular models. From the surface excess concentration, the area per molecule at the gas–liquid interface a s m , in square angstroms is calculated from Eq. 4.
and the standard free energy of micelle formation is calculated from Eq. 5.
where N A = Avogadro’s number and Г max is in mol/cm2.
Results and Discussion
Chemical Structure of Novel Monofluoroalkyl Phosphate Surfactants
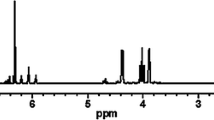
Figures 1, 2, 3 and 4 show the 1H-NMR, 19F-NMR, 31P-NMR and FT-IR spectra of the disodium mono(dodecafluoroheptyl) phosphate, respectively. 1H NMR (D2O): δ = 6.30 ppm to δ = 6.58 ppm (m, 1H, a), δ = 4.10 ppm to δ = 4.19 ppm (m, 2H, b), δ = 4.70 ppm (solvent) for Ha(CF2)6CH b2 OPO(ONa)2; 19F NMR (D2O): δ = −138.2 ppm (s, 2F, a), δ = −129.9 ppm (s, 2F, b), δ = −123.7 ppm (s, 4F, c and d), δ = −122.4 ppm (s, 2F, e), δ = −121.0 ppm (s, 2F, f) for H(CF a2 CF b2 CF c2 CF d2 CF e2 − CF f2 )CH2OPO(ONa)2; 31P NMR (D2O): δ = −1.89 ppm for the only phosphorus atoms in the targeted product; IR (KBr): 2,956 (ν C–H), 1,444 (δ C–H), 1,120 (ν C–F), 1,174 (ν P–O–C), 1,263 (ν P=O), 1,056 (ν P–ONa).
Figures 5, 6, 7 and 8 show the 1H-NMR, 19F-NMR, 31P-NMR and FT-IR spectra of the disodium mono(dodecafluoroheptyl oxyethyl) phosphate, respectively. 1H NMR (D2O): δ = 6.32 ppm to δ = 6.57 ppm (m, 1H, a), δ = 4.08 ppm to δ = 4.16 ppm (m, 2H, b), δ = 3.76 ppm (m, 4H, c and d), δ = 4.70 ppm (solvent) for Ha(CF2)6CH b2 OCH c2 - CH d2 OPO(ONa)2; 19F NMR (D2O): δ = −138.2 ppm (s, 2F, a), δ = −129.9 ppm (s, 2F, b), δ = −123.8 ppm (s, 4F, c and d), δ = −122.5 ppm (s, 2F, e), δ = −120.1 ppm (s, 2F, f) for H(CF a2 CF b2 CF c2 CF d2 CF e2 CF f2 )CH2OCH2CH2OPO(ONa)2; 31P NMR (D2O): δ = −1.83 ppm to δ = −1.90 ppm for the only phosphorus atoms in the targeted product; IR (KBr): 2,946 (ν C–H), 1,444 (δ C–H), 1301 (ν C–O–C), 1,136 (ν C–F), 1,198 (ν P–O–C), 1,263 (ν P=O), 1,041 (ν P–ONa).
Thermal Stabilities of Novel Monofluoroalkyl Phosphate Surfactants
Figure 9 shows the TGA curves of two novel synthetized surfactants. From the TGA curves, it can be seen that the disodium mono(dodecafluoroheptyl) phosphate begins to degrade at 280 °C and a rapid weight loss occurred at 300 °C attributed to the oxygenolysis of the sample. Finally, complete decomposition was at 350 °C, leaving only a mixture of sodium element oxides and phosphorus pentoxide. The disodium mono(dodecafluoroheptyl oxyethyl) phosphate begins to degrade at 230 °C and decomposes completely at about 320 °C, finally. Thermal stability characteristic of the disodium mono(dodecafluoroheptyl oxyethyl) phosphate is worse than that of the former, which may be caused by the introduction of the oxyethyl group into the molecule. In conclusion, the fluoroalkyl phosphate surfactants have good thermostability to be applied in relatively high temperature state.
Surface Properties of Novel Monofluoroalkyl Phosphate Surfactants
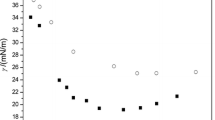
The surface tension (γ)-bulk concentration (C) dependencies for the studied compounds are presented in Fig. 10. No minimum was observed on the γ-lgC curves of the aqueous synthesized surfactants solution, indicating that there were no impurities brought into our products [15]. Table 1 lists the aqueous surface activities of novel synthesized fluorinated surfactants, PFOA, together with n-C7F15SO3Na from the published work [16]. Comparisons of the properties of disodium monofluoroalkyl phosphates with PFOA show that the γ CMC values of the two novel synthetized fluorinated surfactants are close to the that of PFOA, indicting that the two synthesized surfactants possess high surface activities. Both of the newly synthesized fluorinated surfactants have much lower γ CMC than n-C7F15SO3Na, whose γ CMC is 37.3 mN/m, although all of them possess the same length of fluorocarbon chains.
The CMC of disodium mono(dodecafluoroheptyl) phosphate is a little higher than that of PFOA, which may be attributed to the introduction of a C–H bond into the fluorophilic segment. However, the CMC of disodium mono(dodecafluoroheptyl oxyethyl) phosphate is even lower than that of PFOA, mainly because the introduction of oxyethylene make the hydrophobic group of surfactant lengthen effectively.
The two novel monofluoroalkyl phosphates have outstanding water solubility and their Krafft points are both below 0 °C owing to the two hydrophilic groups of the surfactants.
Conclusions
Two novel fluorinated surfactants, disodium monofluoroalkyl phosphate [H(CF2)6CH2OPO(ONa)2] and disodium mono(dodecafluoroheptyl oxyethyl) phosphate [H(CF2)6CH2OCH2CH2OPO(ONa)2] were successfully obtained as a pale yellow solid or a powder. Studies of the properties of the synthesized fluorinated surfactant showed that the Krafft points are both below 0 °C and the values of surface tension at CMC (γ CMC) are very close to that of PFOA. With the introduction of one mole of oxyethylene into the formula, the CMC of disodium mono(dodecafluoroheptyl oxyethyl) phosphate become lower than that of PFOA. In addition, the fluoroalkyl phosphate surfactants have good thermostability with a starting equilibrium thermal degradation temperature respectively at 250 and 300 °C to be applied under high temperature state. The synthesized fluorinated surfactants may be used as replacement of the traditional long-chain perfluorinated surfactants in order to help construction environment owing to their remarkable biodegradability.
Abbreviations
- PFOA:
-
Ammonium perfluorooctanoate
References
Kissa E (2001) Fluorinated surfactants and repellents. Marcel Dekker, New York
Griffiths PC, Cheung AYF, Jenkins RL, Howe AM, Pitt AR, Heenan RK, King SM (2004) Interaction between a partially fluorinated alkyl sulfate and gelatin in aqueous solution. Langmuir 20:1161–1167
Abe M (1999) Synthesis and applications of surfactants containing fluorine. Curr Opin Colloid Interface Sci 4:354–356
Dong SL, Li X, Xu GY, Hoffmann H (2007) A cationic fluorocarbon surfactant DEFUMACl and its mixed systems with cationic surfactants: 19F NMR and surface tension study. J Phys Chem B 111:5903–5910
Badr MZ, Birnbaum LS (2004) Enhanced potential for oxidative stress in livers of senescent rats by the peroxisome proliferator-activated receptor alpha agonist perfluorooctanoic acid. Mech Ageing Dev 125:69–75
Kawashima Y, Suzuki S, Kozuka H, Sato M, Suzuki Y (1994) Effects of prolonged administration of perfluorooctanoic acid on hepatic activities of enzymes which detoxify peroxide and xenobiotic in the rat. Toxicology 93:85–97
Kannan K, Corsolini S, Falandysz J, Oehme G, Focardi S, Giesy JP (2002) Perfluorooctanesulfonate and related fluorinated hydrocarbons in marine mammals, fishes, and birds from Coasts of the Baltic and the Mediterranean Seas. Environ Sci Technol 36:3210–3216
Yang BQ, Chen K, Xing H, Xiao JX (2009) Perfluorobutyl-based fluorinated surfactant with high surface activity. Acta Phys-Chim Sin 25:2409–2412
Ohno A, Kushiyama A, Kondo Y, Kondo Y, Teranaka T, Yoshino N (2008) Synthesis and properties of gemini-type hydrocarbon-fluorocarbon hybrid surfactants. J Fluor Chem 129:577–582
Sánchez ÓJ, Cardona CA (2008) Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresource Technol 99:5270–5295
Liu C, Zhang WF, Chen CM (1995) Application notes of surface active agent. Chemical Industry Press, Beijing
Anthony FB, Woodstown NJ (1952) Fluoroalkylphosphoric compounds. US 2,597,702
Nelson AK, Toy ADF (1963) The preparation of long-chain monoalkyl phosphates from pyrophosphoric acid and alcohols. Inorg Chem 2:775–777
Rosen MJ (2004) Surfactants and interfacial phenomena. Wiley, New York
Xiao JX, Zhao ZG (2003) Application principle of surfactants. Chemical Industry Press, Beijing
Liang ZQ, Chen P (1998) Fluorinated surfactant. China Light Industry Press, Beijing
Acknowledgments
This project was financially supported by National Natural Science Foundation of China (No. 20774037).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Wang, Q., Zhang, S., Geng, B. et al. Synthesis and Surface Activities of Novel Monofluoroalkyl Phosphate Surfactants. J Surfact Deterg 15, 83–88 (2012). https://doi.org/10.1007/s11743-011-1284-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-011-1284-1