Abstract
Upon dilution by the petroleum reservoir connate water, the anionic commercial surfactant blend often used in enhanced oil recovery by low tension, becomes more lipophilic at the interface because of so-called selective partitioning. Hence, the optimum formulation is not maintained when the injected slug moves through the reservoir. An opposite variation is found for ethoxylated nonionic surfactant systems. As a consequence of these antagonistic influences, the optimum formulation shift produced by dilution may be eliminated by using an appropriate mixture of anionic and nonionic commercial surfactants, so that the two effects exactly cancel out.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Enhanced oil recovery again appears to be attractive now and for the years to come, since it seems to be the best way to ensure the energy supply for liquid fuels in the short term, provided that the crude oil price stabilizes in the 60–80 $/Bbl range or above it, a very probable eventuality.
In the mid 1970s, a huge research and development effort made by major oil companies and western governments showed the feasibility of the so-called surfactant flooding techniques to attain an ultimate recovery in the swept zone higher than 50–60% of the original oil in place, i.e., about twice the current ultimate recovery by conventional methods [1]. The principle of the method is to achieve a so-called optimum formulation in which the interfacial tension becomes quite low, i.e., less than 0.01 mN/m, so that capillary forces which trap the oil globules essentially vanish [2, 3].
Such an occurrence was shown to be associated with a three-phase behavior in which a bicontinuous microemulsion is in equilibrium with excess oil and water phases [4].
Half a century ago, Winsor established that this situation takes place when the interaction of the surfactant adsorbed at interface with the water phase, exactly balances its interaction with the oil phase. He suggested using the ratio R of both interactions as a yardstick to find the actual formulation conditions to attain such a so-called optimum formulation; he listed the variables likely to alter the R ratio and discussed how to handle them; however, the R theory, though very pedagogical and enlightening, could be used only in a qualitative way [5].
In the mid 1970s, extensive experimental studies showed that this optimum condition could be numerically expressed as an empirical relationship between formulation variables [6, 7], which was shown later to express a zero surfactant affinity difference (SAD), i.e. the equality of the standard chemical potential of a surfactant molecule in both water and oil phases [8–10].
The zero SAD condition has been further studied for other applications and is now written as a dimensionless expression of the hydrophilic-lipophilic deviation (HLD) that could be expressed as follows in simple systems [11].
For anionic surfactants such as alkylaryl sulfonates, alkyl sulfates or soaps
and for polyethoxylated nonionic surfactants
where S is the salinity of the aqueous phase in wt% NaCl; ACN is the alkane carbon number, a characteristics of the oil phase when it is an n-alkane, which is substituted by the equivalent ACN or EACN when it is not; σ is a characteristic parameter of the surfactant and determines its relative affinity of the oil and water phase, but with a much better accuracy than the HLB number; σ increases with the alkyl tail length, i.e. when the surfactant becomes more lipophilic, which results in an increase in HLD [12]. β is the equivalent characteristic parameter for an ethoxylated alcohol or phenol surfactant which may be written as α—EON, in which the effects of the head group (EON is the ethylene oxide number, i.e. the average number of ethylene oxide groups per molecule) and tail (α also increases linearly with the tail length) are separated. The f(A) and ϕ(A) are similar functions that depend on the alcohol co-surfactant type and concentration. For a lipophilic alcohol their value is negative and increases almost linearly with the alcohol concentration.
For a given surfactant (σ or β), oil (ACN or EACN) and temperature, there is a value of salinity S for which HLD = 0, which is referred to as optimum salinity for minimum tension or three-phase behavior [13].
T is the temperature and Tref a reference value usually 25 °C. k, b, aT and cT are constants that depend on the system nature. It is worth noting that in Eqs. (1) and (2), the sign before the temperature effect is different for the two types of surfactants, hence indicating antagonistic effects of the temperature for a surfactant blend, which may be combined to cancel out each other. This feature has allowed to design ionic-nonionic mixed systems whose formulation is insensitive to temperature [14]. Such a principle of combining two antagonisms will be used to attain insensitivity to dilution in what follows.
It is worth remarking that the HLD is a generalized formulation concept which unifies and fully quantifies the early yardsticks proposed to estimate the interaction of the surfactant with oil and water phases, such as the HLB, PIT and Winsor Ratio, whose historical background may be found elsewhere [15]. As discussed in a recent paper [16], the HLD is related to physical concepts such as the surfactant packing parameter and the spontaneous curvature of the interface. At HLD = 0 the spontaneous curvature is zero and the affinity of the surfactant for the aqueous phase exactly equilibrates its affinity for the oil phase. When HLD <0 (respectively HLD >0) the dominant surfactant affinity is for the aqueous (respectively oil) phase. From the physical chemistry point of view, HLD represents the standard free energy of transfer of a surfactant molecule from aqueous phase to oil [11].
Experimental Section
PS MW425 is a petroleum sulfonate sodium salt sold commercially by Witco Chemicals as TRS 10–80, with an average molecular weight of 425, and an activity of 60%. PSHL is another petroleum sulfonate sodium salt blend sold commercially by Witco Chemicals as Petronate HL with a molecular weight range 440–470, 65% active. C12OXS is a dodecylorthoxylene sulfonate sodium salt from Exxon Chemicals; NP6EO is a commercial ethoxylated nonyl phenol, 100% active sold by Stepan as Makon 6. Alkanes and sodium chloride are reagent grade products. Isoparaffin is an Isopar M brand sold by Exxon.
All systems were equilibrated at 30 °C for 48 h in a capped vial, before the samples of oil and water were withdrawn to measure the tension. The interfacial tension is measured at 30° ± 2 °C with a spinning drop tensiometer, model TGG-M3 made by CITEC-ULA.
Results and Discussion
Formulation Scan
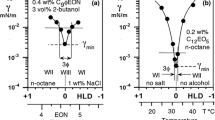
A formulation scan is a series of surfactant-oil–water systems with all formulation variables constant but one, the so-called one-dimensional formulation variable. The corresponding experimental technique has been used for the last 30 years and is well documented in the literature [2, 4, 6–10]. Figure 1 indicates various formulation scans, exhibiting the variation of interfacial tension with different scanned variables, e.g. the alkane carbon number (ACN), the salinity of the aqueous phase (S), the hydrophilicity of the surfactant as its average ethylene oxide number (EON) and the isopentanol concentration which produces a similar effect through the f(A) function. The abscissa scale indicates both the actual scanned variable value, and the HLD calculated according to Eqs. (1–2). It is worth noting that HLD increases from right to left or conversely, depending on the sign preceding the actual formulation variable in Eqs. (1–2).
The plots in Fig. 1 indicate that when an HLD scale is used, a very general variation pattern is attained, that allows one to compare cases, particularly by the value of the minimum tension and its variation close to optimum.
It is seen that in all cases a very slight departure from HLD = 0 results in a considerable increase in the tension. In practice, it implies that any slight formulation departure from the optimum would result in a severe degradation of the oil recovery performance, and this is a main issue as far as enhanced oil recovery processes are concerned.
It is difficult in practice to attain an optimum formulation in the reservoir, and even more challenging to maintain it when the injected fluid (generally a water solution) flows through the reservoir, because of several phenomena, which are not easy to avoid or prevent [17].
The first problem is that the salinity of the injected fluid varies when it flows through the reservoir. This is due to the mixing with the reservoir fluids, or the desorption of ions from rocks and clays. These phenomena result in a change in electrolyte content that produces a formulation shift away from the optimum. This may be avoided somewhat by injecting a pre-flush slug, which stabilizes the salinity before the surfactant slug arrives.
The second problem has to do with the adsorption of the surfactant on the rock surface, which results not only in a loss, but also in a change in the surfactant mixture composition because some species are more likely to adsorb than others. This alters the surfactant parameter value in Eqs. (1–2). This may be somewhat reduced by a pre-flush containing a cheap sacrificial agent, e.g., a lignin derivative, that saturates the adsorption sites, or by adding some chemical which inhibits the surfactant adsorption by electrostatic repulsion, e.g., some alkaline substance.
The problem to which which no solution has yet been found, is the fractionation between phases of surfactant mixtures, which results in a variation of the interfacial formulation as the slug advances. For compelling cost issues the surfactant has to be inexpensive, hence it is not pure, but rather a blend of very different species, e.g., a petroleum sulfonate. When such a mixture equilibrates in a surfactant-oil–water system, the different species adsorb at the interface and migrate into the oil and water according to the partition coefficient that is characteristic of each species [18–23].
The selective partitioning of the mixture different species has been reported in detail in the literature [24, 25], and is only briefly explained in what follows. The basic phenomenon is the equilibrium between the water and oil phase and the interface (or the middle phase microemulsion if any) for all surfactant species in the system, and the fact that this equilibrium is different for each species. If one of the species, e.g., a low ethoxylation nonionic, is rather lipophilic, it tends to partition into the oil phase, and thus contributes less (than a more hydrophilic species) to the interfacial surfactant mixture. This surfactant mixture at the interface is, in fact, the one that determines the actual system formulation. In the presence of different species in the system, the composition of the interfacially adsorbed mixture thus depends on these equilibria.
The point is that partitioning between the phase and the interface depends on the concentration of each species and on the water/oil ratio, because the total number of molecules of each species and the phase volumes enter the definition of concentrations that appear in the partition coefficient [24]. This variation of the partitioning with the surfactant concentration is easily corroborated by the fact that the slope of the tie lines in the two-phase region of a surfactant-oil–water ternary diagram is not constant [26, 27]. When the partitioning of a species changes, its interfacial adsorption changes, hence resulting in a variation of the surfactant mixture at the interface, i.e. a shift in their σ or β characteristic parameter in Eqs. (1) and (2). Consequently, as the injected fluid moves through the porous media and contacts the connate water, it is diluted, and when it contacts oil, the water-to-oil ratio is altered, with a resulting change in partitioning and in interfacial formulation [28].
In practice, it means that when the surfactant solution is diluted, there is a shift of the formulation at which the minimum interfacial tension takes places. It has been actually reported that the lower the surfactant concentration, the stronger the effect [29, 30]. This is unfortunate since the current trend is to look for surfactants that produce a low tension at very low concentration.
The present paper reports a potential way to counterbalance this effect by mixing two surfactant systems whose shifts are antagonistic, and thus might cancel each other out.
Optimum Formulation Shift with Anionic Surfactant Mixture—Petroleum Sulfonate Case
Commercial petroleum sulfonates contains alkylaryl sulfonates with a wide molecular weight range, e.g. 400–500 Da, a variety of structures, particularly as far as the alkyl group(s) number, length and branching are concerned, with sometimes disulfonate species. These surfactants, as most ionic surfactants, have a high critical micelle concentration (CMC) in the water phase and a low one in the oil phase [31]. As a consequence, in the optimum formulation three-phase behavior case, the concentration of the surfactant is very limited (i.e. negligible) in the oil phase, thus most of the surfactant is located in the water phase and at the interface, or in the middle phase when there is enough surfactant. As the total concentration is decreased, the selective partitioning increases, a higher proportion of the species migrate to the water phase, these are the most hydrophilic ones, thus leaving a less hydrophilic mixture at the interface or in the middle phase.
The optimum formulation is generally detected at high surfactant formulation by the occurrence of three-phase behavior, whereas it is pinpointed by the minimum interfacial tension in low concentration systems, i.e., those which are below the so-called critical microemulsion concentration, which is typically a few times larger than the CMC [32].
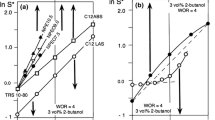
Figure 2 indicates the aspect of the interfacial tension curve versus formulation (as the brine salinity) for a commercial petroleum sulfonate surfactant (PSHL) which is actually a blend of high and low molecular weight products, with species spread over a wide range, mainly in the 440–470 interval. In this and the following figures only the data point corresponding to the minimum tension and its two-side data point are indicated to avoid the overlapping of the experimental results. In any case the position of the minimum tension, is extremely well defined. As reported a long time ago [1–8], the tension exhibits a very deep minimum at the optimum formulation, which is essentially the same when the surfactant concentration is above the CMC (about 0.02 wt % at this salinity). When the concentration is about or lower than the CMC, there is still an interfacial tension minimum, but it is displaced to the left (lower optimum salinity) as the concentration decreases. Moreover, the tension minimum tends to increase, as expected when the concentration becomes lower than the CMC.
This shift has been found to be characteristic of petroleum sulfonate complex blends, and sometimes even for ordinary laundry grade dodecylbenzene sulfonate, whose molecular weight dispersity is much less. On the other hand, it was found that there is no shift in optimum formulation versus concentration for isomerically pure sulfonate surfactants, for which the minimum tension increases as concentration decreases while remaining at an unchanged formulation [6, 8]. The meaning of the shift with concentration may be easily interpreted by using the HLD equation at the reference temperature, and in absence of alcohol, i.e.
As surfactant concentration decreases and selective partitioning increases, the tension minimum moves to the left, i.e. the optimum S decreases to compensate for the increase in the interfacial surfactant mixture parameter σ in order to keep HLD = 0 at an optimum formulation. In an ACN scan an increase in the preferred ACN has been reported, which, according to Eq. (3) is another way to compensate for an increase in σ in order to keep HLD = 0 [6]. A consequence of this effect is that a variation of the total concentration of such a surfactant blend is able to alter formulation, and if the proper range is selected, a formulation scan can result from a change in concentration, as it was reported 30 years ago [2].
Optimum Formulation Shift with Nonionic Surfactant Mixture—Polyethoxylated Nonylphenol Case
Commercial polyethoxylated nonionic surfactants are produced by the random polycondensation of ethylene oxide on an alcohol or a phenol, and the result is often a Poisson type distribution of the number of ethylene oxide groups (EON), which is wider or narrower depending on the used catalyst [33, 34].
In practice, for a balanced nonylphenol with an average ethylene oxide number (EON) of 5–6, the actual mixture of oligomer species ranges from EON = 0 to 10. There is typically a large proportion (e.g. 30%) of species (EON ≤3), which are essentially insoluble in water, and strongly tend to migrate in oil. On the other hand nonionic surfactants are known to exhibit a very low CMC in water, which means that the amount of surfactant in the excess water in a three-phase optimum system is essentially negligible. Consequently, the surfactant species partition between the oil phase (where the most lipophilic low EON oligomers migrate) and the interface or microemulsion (where the remaining oligomers are found) [35].).
As surfactant concentration decreases and selective partitioning increases, a higher proportion of hydrophobic oligomers migrate in the oil phase, and thus the remaining mixture at interface becomes more hydrophilic [24, 25].
This is also easily interpreted with the HLD expression for nonionic surfactants:
As the surfactant concentration decreases and partitioning increases, the tension minimum moves to the right as shown in Fig. 3, i.e., the optimum salinity increases to compensate for the decrease in β in order to keep HLD = 0 at the optimum formulation. Since β is equal to α—EON and because α is essentially the same for all oligomers, this β reduction is due to the increase in interfacial average EON. It is worth noting than in the present case the shift does not take place just at very low concentration (as in the previous case) but also at relatively higher ones, much higher than the CMC. Such a shift in interfacial EON has been reported for this type of surfactant even at high surfactant concentration, e.g., up to 3 wt % [7].
Eliminating the Optimum Formulation Shift by Blending Two Mixtures with Antagonistic Shifts, e.g. a Petroleum Sulfonate and a Polyethoxylated Alkylphenol
It has been known for a long time that surfactant mixing provides a property averaging effect, particularly intermediate formulation [36]. However, mixtures do not always follow a linear rule and the departure from it could become considerable when very different substances are mixed [36, 37]. On the other hand, mixtures may be used to eliminate an effect. This is for instance the case for the antagonistic influences of temperature on ionic and polyethoxylated nonionic surfactants, which may cancel out each other when the proper anionic-nonionic blend is found [36, 38].
The same is intended in what follows with the effect of partitioning, by blending different proportions of the two previous mixtures. When the anionic (respectively nonionic) surfactant effect dominates the optimum salinity shift with concentration reduction is to the left (respectively right) as seen in Figs. 2 and 3. The basic premise on what follows is that for some appropriate anionic-nonionic mixture, the two influences are likely to cancel out. The experiments shown in Figs. 2 and 3 are thus repeated for mixtures containing different (wt%) proportions of anionic and nonionic blends. The dilution effect is studied over a total concentration range from 0.05 to 0.005 wt%, in which the right shift exhibited by the nonionic product (see Fig. 3) is higher than the left shift shown by the anionic blend (see Fig. 2). Consequently, it is expected that in this dilution range, a mixture containing more anionic product than nonionic would be required to attain the exact trade-off of opposite contributions.
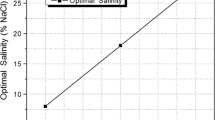
An approximate calculation may be carried out to estimate the proper anionic-nonionic proportion if the two opposite variations of HLD are expressed exactly in the same units. In Eqs. (3) and (4) the only term that has exactly the same meaning is ACN. Hence, the comparison should be made in ACN units, which means that the expressions must be divided by k, a constant that depends on the surfactant type. In the present case the values of k are essentially the same, i.e. 0.16 for petroleum sulfonates and 0.15 for ethoxylated nonylphenols. When the surfactant concentration decreases from 0.01 to 0.001 wt %, the corresponding variation of optimum salinity from surfactant are from 4.2 wt% NaCl to 3% for the petroleum sulfonate and from 3.8 to 8% for ethoxylated nonylphenol. Since the constant b is 0.13 for NaCl, these variations of HLD in equivalent ACN units are calculated to be 0.15 ln (3/4) = −0.04 and 0.16 × 0.13 (3.5–8.5) = 0.11, respectively. Hence, the effect of the nonionic blend is approximately three times larger. This means that the balanced antagonism is expected to be attained for three times more anionic than nonionic species in the mixture if the mixing rule is linear.
The best compensation of the opposite effects is actually found to occur for a mixture containing 80% of petroleum sulfonate and 20% of polyethoxyated nonionic, which is consistent with the previous guess. Figure 4 data show that for this mixture there is essentially no variation of the optimum (salinity) formulation with a variation of the total surfactant concentration from 0.05 to 0.005 wt%.
It is worth noting that although this evidence confirms the premise of the method, there are still several factors to control in practice, which would require further systematic studies to be quantified. It is known that the selective partitioning, hence the formulation shift with concentration, increases with the spread of the distribution of the species that make up each mixture [25, 28]. Moreover, it can be seen in Figs. 2 and 3 that the shift also depends on the concentration range, being less important as the total concentration increases. This is related with the fact that the selective partitioning and resulting interfacial formulation change tend to increase as the concentration decreases, as explained elsewhere [28]. Finally, and as seen in Figs. 2, 3, and 4, the value of the minimum interfacial tension, which is determinant for the performance of the process, always tends to increase as the concentration decreases, but in a different way for the base surfactants and their mixtures.
On the other hand, Fig. 4 shows that the optimum salinity at which the tension minimum occurs is the same at 0.5 wt% NaCl for all tested concentrations, but that it is much lower than the optimum salinity corresponding to the previously shown anionic and nonionic systems (S = 3–4 wt% NaCl) at a similar surfactant concentration (about 0.01 wt%).
This decrease in optimum salinity indicates that the anionic-nonionic mixture is considerably less hydrophilic than each of its components alone. This is likely to be due to the shielding effect the polyethylene oxide chain exerts on the sulfonate group (see inset drawing in Fig. 4), which results in a reduced interaction with water, hence a lower optimum salinity, and other property changes. This means that, in practice, the use of an anionic-nonionic blend would require a more hydrophilic formulation to compensate for this decrease in hydrophilic interaction. It also means that this spontaneous change in salinity may be taken advantage of in order to apply the so-called salinity gradient process [39].
Insensitivity to Dilution over a Concentration Range
The presented experimental evidence sustains the premise that a proper mixture of two surfactant blends exhibiting opposite interfacial formulation shifts and could achieve optimum formulation insensitivity over the total surfactant concentration range.
This is a first step toward resolving the challenge of maintaining the optimum formulation in an enhanced oil recovery process when the surfactant slug is diluted as it moves through the reservoir.
Nevertheless, the scarcity of data available in the literature and our preliminary evidence indicate that the HLD change with dilution is not necessarily linear nor exhibits the same relative variation with dilution over a wide range. As expected from the partitioning phenomenology, it seems that it depends on each mixture type and on the range of concentration. Consequently, a fine-tuning adjustment of each of the mixed blends might be necessary to improve the antagonism and maximize the range over which the exact compensation applies. Other effects such as the water/oil ratio influence on partitioning will also have to be scrutinized.
Abbreviations
- SAD:
- HLD:
-
Hydrophilic-lipophilic deviation defined in Eqs. (1) and (2)
- ACN:
-
Alkane carbon number
- PSMW425:
-
Petroleum sulfonate sodium salt with an average molecular weight of 425
- PSHL:
-
Petroleum sulfonate sodium salt with a molecular weight range of 440 to 470
- C12OXS:
-
Dodecyl orthoxylene sulfonate sodium salt
- NP6EO:
-
Commercial ethoxylated nonylphenol with an average of 6 ethylene oxide groups
- EON:
-
Average number of ethylene oxide groups per molecule
- S :
-
Salinity of aqueous phase (in wt% NaCl)
- σ :
-
Characteristic parameter of an anionic surfactant
- β :
-
Characteristic parameter of a nonionic surfactant
- f(A) and ϕ(A):
-
Functions representing the effect of the type and concentration of alcohol
- T :
-
Temperature (° C)
- k, b, aT, cT:
References
Shah DO, Schechter RS (eds) (1977) Improved oil recovery by surfactant and polymer flooding. Academic Press, New York, USA
Cayias JL, Schechter RS, Wade WH (1977) Utilization of petroleum sulfonates for producing low tension between hydrocarbons and water. J Colloid Interface Sci 59:31–38
Lake L (1989) Enhanced oil recovery. Prentice Hall, Englwood Cliffs, NJ
Bourrel M, Schechter RS (1988) Microemulsions and related systems. Marcel Dekker, New York
Winsor P (1954) Solvent properties of amphiphilic compounds. Butterworths, London, UK
Salager JL, Vasquez E, Morgan J, Schechter RS, Wade WH (1979) Optimum formulation of surfactant-water-oil systems for minimum interfacial tension and phase behavior. Soc Petrol Eng J 19:107–115
Bourrel M, Salager JL, Schechter RS, Wade WH (1980) A correlation for phase behavior of nonionic surfactants. J Colloid Interface Sci 75:451–461
Wade WH, Morgan JC, Schechter RS, Jacobson JK, Salager JL (1978) Interfacial tension and phase behavior of surfactant systems. Soc Petrol Eng 18:242–252
Salager JL (1988) Phase transformation and emulsion inversion on the basis of catastrophe theory. In: Becher P (ed) Encyclopedia of emulsion technology, vol 3. Marcel Dekker, New York, USA, pp 79–134
Salager JL (1999) Microemulsions. In: Broze G (ed) Handbook of detergents—part A: properties. Marcel Dekker, New York, pp 253–302
Salager JL, Márquez N, Graciaa A, Lachaise J (2000) Partitioning of ethoxylated octylphenol surfactants in microemulsion-oil-water systems. Influence of temperature and relation between partitioning coefficient and physicochemical formulation. Langmuir 16:5534–5539
Salager JL, Antón RE, Andérez JM, Aubry JM (2001) Formulation des micro-émulsions par la méthode HLD. In: Techniques de l’Ingénieur. Chap 157 Vol. Génie des ProcédésJ2, pp 1–20
Healy RL, Reed RN (1977) Some physicochemical aspects of microemulsion flooding: a review. In: Shah DO, Schechter RS (eds) Improved oil recovery by surfactant and polymer flooding. Academic Press, New York, pp 347–383
Kunieda H, Solans C (1997) How to prepare microemulsions: temperature insensitive microemulsions. In: Solans C, Kunieda H (eds) Industrial applications of microemulsions. Marcel Dekker, New York, pp 21–45
Queste S, Salager JL, Strey R, Aubry JM (2007) The EACN scale for oil classification revisited thanks to fish diagrams. J Colloid Interface Sci 312:98–107
Kunz W, Testard F, Zemb T (2009) Correspondence between curvature, packing parameter and hydrophilic-lipophilic deviation scales around the phase inversion temperature. Langmuir 25:112–115
Haegel FH, Lopez JC, Salager JL, Engelskirchen S (2009) Microemulsions in large scale applications. In: Stubenrauch C (ed) Microemulsions—background new concepts applications, perspectives. Blackwell Pub, Oxford UK, pp 302–344
Crook EH, Fordyce DB, Trebbi GF (1963) Molecular weight distribution of nonionic surfactants I. Surface and interfacial tension or normal distribution and homogeneous p,t-octylphenoxyethanols (OPE’S). J Phys Chem 67:1987–1994
Márquez N, Antón RE, Graciaa A, Lachaise J, Salager JL (1995) Partitioning of ethoxylated alkylphenol surfactants in microemulsion-oil-water systems. Colloids Surf A 100:225–231
Zornes DR, Willhite GP, Michnick MJ (1978) An experimental investigation into the use of high pressure liquid chromatography for the determination of petroleum sulfonates. Soc Petrol Eng J 18:207–218
Koukounis C (1979) Phase partitioning and fractionation of anionic and nonionic surfactant mixtures. MSc Thesis, University of Texas at Austin
Graciaa A, Lachaise J, Bourrel M, Osborne-Lee I, Schecheter RS, Wade WH (1987) The partitioning of nonionic and anionic surfactant mixtures between oil/microemulsion/water phases. SPE Reservoir Eng 2:305–314
Bourrel M, Koukounis C, Schechter RS, Wade WH (1980) Phase and interfacial tension behavior of nonionic surfactants. J Dispers Sci Technol 1:13–35
Graciaa A, Lachaise J, Sayous JG, Grenier P, Yiv S, Schechter RS, Wade WH (1983) The partitioning of complex surfactant mixtures between oil-water-microemulsion phases at high surfactant concentration. J Colloid Interface Sci 93:474–486
Graciaa A, Andérez JM, Bracho C, Lachaise J, Salager JL, Tolosa L, Ysambertt F (2006) The selective partitioning of the oligomers of polyethoxylated surfactant mixtures between interface and oil and water bulk phases, Adv Colloid Interface Sci 123–126:63–73
Kahlweit M, Lessner E, Strey R (1983) Influence of the properties of the oil and the surfactant on the phase behavior of systems of the type H2O-oil-nonionic surfactant. J Phys Chem 87:5032–5040
Kilpatrick PK, Gorman CA, Davis HT, Scriven LE, Miller WG (1986) Patterns of phase behavior in ternary ethoxylated alcohol-n-alkane-water mixture. J Phys Chem 90:5292–5299
Antón RE, Andérez JM, Bracho CL, Véjar F, Salager JL (2008) Practical surfactant mixing rules based on the attainment of microemulsion-oil-water three-phase behavior systems. Adv Polymer Sci 218:83–113
Salager JL (1977) Physicochemical properties of surfactant-oil-water mixtures: phase behavior, microemulsion formation and interfacial tension. PhD Dissertation, University of Texas at Austin
Sayous JG (1983) Etude du partage de tensioactifs nonioniques entre phases de systèmes de Winsor. PhD Dissertation (In French). Université de Pau PA, Pau, France
Rosen MJ (2004) Micelle Formation by Surfactants. In Surfactants and interfacial phenomena. Wiley-Interscience. John Wiley, Hoboken, pp 134
Acosta EJ, Harwell JH, Sabatini DA (2004) Self-assembly in linker-modified microemulsions. J Colloid Interface Sci 274:652–664
Schick M (ed) (1967) Nonionic surfactants. Marcel Dekker, New York
Goel SK (1998) Tuning the polydispersity of alcohol ethoxylates for enhanced oily soil removal. J Surfactants Deterg 1:539–545
Márquez N, Antón RE, Graciaa A, Lachaise J, Salager JL (1995) Partitioning of ethoxylated alkyl phenol surfactants in microemulsion-oil-water systems. Colloids Surf A 100:225–231
Salager JL, Bourrel M, Schechter RS, Wade WH (1979) Mixing rules for optimum phase behavior formulations of surfactant-oil-water systems. Soc Petrol Eng J 19:271–278
Salager JL, Antón RE, Forgiarini A, Márquez L (2009) Formulation of Microemulsions. In: Stubenrauch C (ed) Microemulsions—background new concepts applications, perspectives. Blackwell Pub, Oxford UK, pp 84–121
Antón RE, Salager JL, Graciaa A, Lachaise J (1992) Surfactant-oil-water systems near the affinity inversion—part VIII: optimum formulation and phase behavior of mixed anionic-nonionic systems versus temperature. J Dispers Sci Technol 13:565–579
Hirasaki G, Van Domselar HR, Nelson RC (1980) Evaluation of the salinity gradient concept in surfactant flooding. Paper SPE 8825, 1rst joint SPE/DOE symposium in enhanced oil recovery, Tulsa OK, 20–23 Apr 1980
Acknowledgments
The authors are grateful to their university research council CDCHT, for a partial financial backing. One of the authors (MAA) would like to thank FONACIT, an agency of the Venezuelan Ministry of Science and Technology for granting her a doctoral scholarship.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Arandia, M.A., Forgiarini, A.M. & Salager, JL. Resolving an Enhanced Oil Recovery Challenge: Optimum Formulation of a Surfactant-Oil–Water System Made Insensitive to Dilution. J Surfact Deterg 13, 119–126 (2010). https://doi.org/10.1007/s11743-009-1171-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-009-1171-1