Abstract
A new oligomeric surfactant: N,N,N′,N″,N″- pentamethyl diethyleneamine—N,N″-di-[tetradecylammonium bromide] referred to as 14-2-N(CH3)-2-14 was synthesized, purified and characterized by Elemental Analysis, 1H and 13C NMR and Electrospray. The micellar properties of this compound were determined by electrical conductivity and surface tension methods. Optical microscopy was also employed to study the behavior of anhydrous surfactant and the binary water/surfactant system as a function of temperature. The critical micellar concentration (cmc), degree of counterion binding and thermodynamic parameters of micellization (standard molar Gibbs energy, enthalpy and entropy of micellization) were determined by electrical conductivity measurements in the temperature range [24–54 °C]. Surface tension measurements also provide information about the dependence of the surface tension at the cmc (γcmc), pC20 (negative logarithm of the surfactant’s molar concentration C20, required to reduce the surface tension by 20 mN/m, the surface excess (Γmax) at air/solution interface, the minimum area per surfactant molecule at the air/solution interface (Amin), surface pressure at the cmc (Пcmc), critical packing parameter(CPP) and the standard free energies of micellization (\(\Updelta G_{m}^{0}\)) and of adsorption (\( \Updelta G_{\text{ads}}^{0} \)).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Numerous amphiphilic structures with multiple headgroups and hydrophobic chains in different distributions have been reported. They are used as models to study the aggregation of membranes and their disruption caused by the hydrophobic effect, and they are also employed for a wide range of applications [1–4]. Good examples of such compounds are bolaamphiphiles [5, 6] and Gemini surfactants [7–13]. Owing to their extraordinary activity, the Gemini surfactants have excellent properties of solubilization, soil cleanup and oil recovery. The knowledge of the thermodynamic properties of surfactants in solution is necessary to understand their behavior and optimize their use in formulated products as well as in processes.
This paper examines the synthesis of the Gemini surfactant: N,N,N′,N″,N″-pentamethyl diethyleneamine—N, N″-di-[tetradecylammonium bromide] referred as 14-2-14(CH3)-2-14. Two techniques have been proposed for the determination of the critical micellar concentration of Gemini surfactants: conductometry and surface tension.
Thermodynamic parameters concerning association behavior were determined by electrical conductivity and surface tension measurements. Polarization microscopy was also employed to study the behavior of the anhydrous surfactant and for the binary water/surfactant system as a function of temperature.
Experimental
Synthesis
The Gemini N,N,N′,N″,N″-pentamethyl diethyleneamine—N,N″-di-[tetradecylammonium bromide] referred to as 14-2-14(CH3)-2-14 was obtained by the following reaction (Scheme 1).
It is pertinent to mention that the ratio of compound A and B is closely related to the conditions of the reaction (solvent, order of addition of reagent, duration and the intensity of heating…). For instance, an excess of tetradecyl bromide and a solvent with a high boiling point like butanol or propan-2-ol can lead to the synthesis of the trimeric surfactant (compound B). In order to favor the preparation of compound (A), we followed the procedure below.
To 20 mmol (3,466 g) of N,N,N′,N″,N″-pentamethyldiethylenetriamine from Fluka (reference 26828) dissolved in dry acetone (35 mL) was added 50 mmol (13,865 g) of tetradecyl bromide from Fluka (reference 18390) dropwise with continuous stirring under reflux for 48 h. The reaction was carried out at 56 °C. The reaction was followed by thin layer chromatography (Silica gel plate 60 F254) eluted with a mixture of butanol/pyridine/glacial acetic acid/water (60:20:6:24) in volume or acetone/methanol (90/10); the layer chromatography plates were sprayed with the Dragendorff or Ninhydrine reagents. After completion of the reaction, the solvent was removed by rotary evaporation at 30 °C under reduced pressure, leaving a waxy product. The resulting product was dissolved in the minimum volume of absolute ethanol and successive extractions were carried out with ether and hexane in the order to eliminate the excess of alkyl bromide. The obtained product was treated with a large excess of diethyl ether until precipitation took place. A white product was finally obtained by filtration under vacuum. The product was dried and stored in a desiccator. In general, the yield of reaction was less than 30%. It must be noted that it is likely that the reaction above can give rise, in addition to 1,3-dialkyled ammonium dibromide product, to 1,2 and 2,3-dialkyled ammonium dibromides which are in fact indistinguishable from each other. Although the analytical conditions in thin layer chromatography have been well examined, we have always observed a single spot. Also the result of NMR spectroscopy is close to 1,3-dialkylammonium bromide therefore we retained the product A: (N,N,N′,N″,N″-pentamethyl diethyleneamine—N,N″-di-[tetradecylammonium bromide] referred as 14-2-14(CH3)-2-14) to conduct this study.
Analytical Methods
1H and 13C NMR. Depth and correlation with 1H and 13C as well as Electrospray (ES+) and microanalysis were carried out in the laboratories of the Consejo Superior of Investigation of Barcelona Spain. 1H and 13C NMR analyses were realized with a Varian 300 MHz spectrometer; the chemical shifts are reported in parts per million (δ, in ppm) downfield from tetramethylsilane (TMS).
H1-NMR (300 MHz, δ, CDCl 3 /TMS): (Scheme 2)
(a) δ = 0,86 (t,3H); (b) δ = 1,26 (44H,m); (c) δ = 1,8 (4H,m), (d) δ = 2,03 (4H,t); (e) δ = 3,33 (12H,s); (f) δ = 3,99 (4H,s); (g) δ = 3,55(4H,t); (h) δ = 1,43 (3H,s)
13C NMR (300 MHz, δ, CDCl 3 /TMS):
(a) δ = 14,1; (b) δ = 22,7–29,54; (c) δ = 31,46; (d) δ = 43,04; (h) δ = 65,33
Electrospray ES(+): 729
Thailed pic P, P+2 corresponding to 646,56 and 648,56 [CH3-(CH2)-C2-N(CH3)2-CH2-CH)2, Br+
Elemental analysis
C% | H% | N% | |
|---|---|---|---|
Calculated | 61.24 | 11.17 | 5.79 |
Found | 61.14 | 11.23 | 5.84 |
Electrical Conductivity Measurements
Electrical conductivity measurements were performed for different concentrations of surfactant solutions in the temperature range 24–54 °C, using a WTWLF 90 conductimeter with a WTW KL E1 cell. The solutions were continuously stirred and thermostated at ± 1 °C. The measured conductivity was plotted as a function of the surfactant concentration, and the critical micelle concentration (cmc) was taken at the intersection of the two linear parts of the conductivity curve by the least-squares method.
Surface Tension Measurements
Surface tension measurements were carried out at 25 °C with a Lauda tensiometer. All sample solutions were aged (at least 10 min prior to measurement) before taking a measurement. Double distilled water was used for preparing the solutions. The cmc value was determined as usual from the break point of the plot of surface tension versus the logarithm of the concentration.
Qualitative Phase Behavior
Optical microscopy was employed to study the behavior of anhydrous surfactant and for binary water/surfactant systems as function of temperature. For the water mixture system, the optical observations were performed according to the “flooding” penetration method of Lawrence [14]. A Reichert Polyvar R Leica polarizing microscope equipped with a hot stage was employed. A camcorder and PC with Leica IM500 software were used to capture images. In a flooding experiment, water was allowed to diffuse in the anhydrous surfactant placed between a slide and a cover slip. After a short time, gradient in composition were produced and different separated mesophases developed around the crystalline surfactant.
Results
Conductometric Measurements
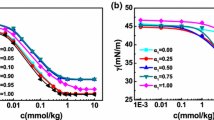
Typical plots of conductivity χ versus concentration for the Gemini surfactant (14-2-N(CH3)-2-14) at various temperatures (22–54 °C) are shown in Fig. 1; conductivity increases with increasing temperature and concentration of surfactant. Calculated values for the cmc are listed in Table 1. It may be seen from the table that the cmc increases slightly with increasing temperature.
The ionization degree α was calculated from the slopes of the two linear parts of the conductivity curves from the relation (1) [15–19]:
where S 1 and S 2 are the respective dependence χ = f(surfactant) below and above the cmc. (S 1 and S 2 are expressed in S m2 mol−1).
The values for counterion binding β were calculated according to the relation (2) [15–19]:
The calculated values for the degree of ionization α and the counterion binding of the micelle β are summarized in Table 1. With increasing temperature the degree of ionization α rises slightly, whereas the counterion binding β decreases slightly. Generally, experimental data support the suggestion that the nature of the counterion has a significant influence on the micellization of surfactants.
The cmc values determined at various temperatures were further used to calculate the thermodynamic parameters of micellization according to the Eqs. 3, 4, 5 and 6 [15–20]:
-
For the standard molar Gibbs energy of micellization \( \Updelta G_{m}^{0} \):
$$ \Updelta G_{m}^{0} = (1 + \beta ){\text{RTL}}n\left( {{\frac{\text{cmc}}{\rho }}} \right) $$(3)ρ equals to the moles of water per cubic decimeter (ρ = 55.5 mol/dm3 at 25 °C).
-
For the standard molar enthalpy of micellization \( \Updelta H_{m}^{0} \)
$$ \Updelta H_{m}^{0} = - (1 + \beta )RT^{2} \left( {{\frac{{\delta {\text{Ln}}({\text{cmc}})}}{\delta T}}} \right) $$(4)Equation 4 could be also expressed in the form:
$$ \Updelta H_{m}^{0} = - (1 + \beta )RT^{2} ({\text{B}} + 2{\text{C}}T) $$where A, B and C are parameters of the second polynomial:
$$ {\text{Ln}}({\text{cmc}}) = f(T) = {\text{A}} + {\text{B}}T + {\text{C}}T^{2} $$(5)
-
For the standard molar entropy of micellization:
$$ \Updelta S_{m}^{0} = \left( {{\frac{{\Updelta H_{m}^{0} - \Updelta G_{m}^{0} }}{T}}} \right) $$(6)
The values for the thermodynamic parameters of micellization are presented in Table 1. The standard molar Gibbs energy of micellization \( \Updelta G_{m}^{0} \) was found to be negative in all cases, with only small differences over the given temperature range. The process of micellization is thus thermodynamically favored and spontaneous.
The standard molar enthalpy of micellization \( \Updelta H_{m}^{0} \) and the standard molar entropy \( \Updelta S_{m}^{0} \) increase with increasing the temperature; this indicates the formation of much ordered systems of micellization.
Surface Tension
Figure 2 shows the curve of the surface tension versus log (surfactant molar concentration) for the Gemini surfactant. The surface tension isotherm was used to determine several parameters according to the procedures described by Rosen [21]; critical micelle concentration (cmc), surface tension and the surface pressure at cmc (γcmc, Πcmc, respectively), the maximum surface excess concentration (Γmax), the minimum area per molecule (A min), the logarithm of surfactant concentration required to reduce the surface tension by 20 mN/m (pC20), the ratio cmc/C20, the standard free energy of adsorption (\( \Updelta G_{\text{ads}}^{0} \)) and the standard free energy of micellization (\( \Updelta G_{m}^{0} \)).
The surface excess concentration Гmax at the air/water interface and the minimum area per molecule, A min, were calculated according to the Eqs. 7 and 8 derived from the Gibbs adsorption isotherm [21]:
where R = 8. 31 × 107 ergs. Mol−1 K−1, γ is in dyne/cm, and Гmax is in mol/cm². The minimum area per molecule, A is in Ų, N is Avogadro’s number. The Gibbs pre-factor “n” in the equation represent the number of particles per surfactant molecule whose surface concentration changes with change in the bulk concentration of the surfactant. For monovalent ionic surfactant, n = 2. It is unclear which prefactor has to be used for a Gemini surfactant [8, 22, 23] but neutron reflectivity results from Thomas et al. [23] revealed that for a rigid xylyl spacer, a factor of 3 should be used.
However, for other Gemini surfactants with different structures, a pre-factor of 2 was found to be more appropriate.
The efficiency of adsorption pC20 is the negative logarithm of the concentration of surfactant in the bulk phase required to produce 20 mN/m (dyn/cm) reduction in the surface or interfacial tension of water [21].
The Critical Packing Parameter CPP, which can provide a good idea of the shape of aggregates forming spontaneously [21], is given by Eq. 9:
where V H(Å3) is the volume occupied by a saturated hydrocarbon chain containing n c carbon atoms and I c is the length of hydrocarbon groups in the micelle core. At saturation, a 0 can be replaced by A min and V H and I c can be calculated by the following relation [24]:
The standard free energy of micellization was calculated using the equation:
The standard free energy of adsorption was calculated according to the equation:
Values for cmc, γcmc, Гmax, A min, pC20, Cmc/C20, CPP, \( \Updelta G_{m}^{0} \) and \( \Updelta G_{\text{ads}}^{0} \) at 25 °C are presented in Table 2.
In the order to compare the performance of the studied Gemini surfactant, we have collected from the literature the surface properties data for similar Gemini species (14-4-14) as well as the corresponding monomeric compound. The only difference between the Gemini synthesized and 14-4-14 is the presence of the amine function in the spacer. The surface properties of these compounds will be discussed.
Microscopical Observation
Optical microscopy was employed to study the behavior of anhydrous surfactant and for binary water/surfactant systems as the function of temperature. For the water mixture system, the optical observations were performed according to the “flooding” penetration method [14]. The main observations are summarised in Fig. 3.
Optical micrograph illustrating different phases for the anhydrous product and the phases formed by the penetration of water as function of temperature “Flooding method. a Optical micrograph for anhydrous product t = 20 °C, b Optical micrograph, flooding method, T = 45 °C, c Optical micrograph, flooding method, T = 60 °C, d Optical micrograph, flooding method, T = 70 °C, e Optical micrograph, flooding method, T = 80 °C, f Optical micrograph, flooding method, T = 85 °C
For the anhydrous product and before the melting point, a thermotropic liquid crystal phase has been observed (Fig. 3a). When small quantities of water were added (“flooding method”) and when the temperature increased and reached 45 °C, we observed the appearance of a lamellar phase together with a solid, while in the other region we observe a lamellar phase structure, Fig. 3b. This lamellar phase structure persisted up to 60 °C, where a typically lamellar phase (Fig. 3c) was observed. At 65 °C, a mesophase was observed in polarized light: a cubic phase inside and a lamellar phase outside (Fig. 3d). The phenomenon persisted with the increase of temperature and when the temperature attained 70 °C, we noted the appearance of a typical millenic phase in a certain region (Fig. 3e) and a lamellar phase elsewhere. At temperatures near the 80 °C, we only observed the millenic phase structure (Fig. 3f).
Discussion
From conductivity measurements, Fig. 1 reflects the variation of specific conductivity versus concentration of Gemini surfactant 14-2-N(CH3)-2-14 in the temperature range [24–54 °C]; these curves showed all a single break unlike Frindi et al. [27] who found two breaks for Gemini (8-3-8) and (8-6-8). The cmc is determined at the breakpoint in the specific conductivity versus concentration curve. According to Table 1, the cmc increases slightly with increasing temperature. The reports of temperature dependence of cmc are scarce. Such a report on 12-s-12 (s = 2, 3, 4) is available in the literature [28]. The cmc values increased with temperature for the three surfactants studied, which is in good agreement with our results. The effect of temperature on the micellization process is quite complex, and typically the cmc is minimal around 25 °C for ionic surfactants and at about 50 °C for nonionic surfactants [21]. The length of the alkyl chain and the nature of the counterion influence significantly the temperature dependence of cmc [29, 30]. The increase in temperature causes a decrease in hydration of polar heads that promotes micellization and also a greater disorder in the structure of water in the vicinity of the hydrophobic parts which disadvantages the micellization. As a consequence, the balance between these opposing effect determines the evolution of the cmc with temperature [16, 21].
As can be seen from the Table 1, the α values determined for the Gemini surfactant 14-2-(CH3)-2-14 are in good agreement with the results of Manet [31], the author found the α values of 0.27 at 303 K and 0.32 at 333 K for the Gemini surfactant 14-2-14, 2Br−.
It is worth noting that the α values found in the literature are quite method dependent. A recent work by Geng et al. [32] used a chemical trapping technique and to study dodecyl cationic Gemini surfactants having bromide as the counterion. They found that micelles of the Gemini surfactant with two, three and four methylene groups in the spacer had all α values of around 0.2.
We also observed that the variation of the degree of ionization α of the Gemini surfactant with temperature is insignificant; this result agrees with the finding of Tehrani et al. [33] who determined the α values at different temperatures for the monomeric surfactant and the corresponding Gemini surfactant having two and three methylene groups as the spacer and bromide as the counterion, and found that the α values were virtually independent of temperature.
All values of the free energies of micellization of the Gemini surfactants were negatives, which means that the process of micellization is spontaneous; the variation of free energy of micellization with temperature is low because the existence of an entropy-enthalpy compensation phenomenon.
The standard molar entropy of micellization \( \Updelta S_{m}^{0} \), is largely positive. The standard molar enthalpies of micellization \( \Updelta H_{m}^{0} \) can be positive or negative. The result shows that \( \left| {T\Updelta S_{m}^{0} } \right| > \left| {\Updelta H_{m}^{0} } \right| \) , indicating that the process of micellization is entropy driven. It has been shown that the weak variation in \( \Updelta G_{m}^{0} \) with increasing temperature arises from the compensation of the variation in the standard enthalpy \( \Updelta H_{m}^{0} \) and entropy of micellization \( \Updelta S_{m}^{0} \). Thermodynamics parameters for micellization of the Gemini surfactants with varying spacer length (m-s-m, m = 12) obtained by calorimetry were reported by Bai et al. [34]. They compared the results with conventional surfactants. It was found that the determined cmc values are in good agreement with those obtained by the other methods, with a maximum at s = 4–6. The enthalpies of micellization, ΔH m are all exothermic and show a marked minimum at s = 4–6. The variation of ΔH m and ΔS m shows that the balance between enthalpic and entropic contributions to the micellization process changes substantially with s.
From surface tension measurements, the plot of surface tension versus the logarithm of the concentration of the Gemini 14-2-N(CH3)-2-14 surfactant exhibits an abrupt change in the slope at a concentration corresponding to the cmc. The cmc value and surface parameters derived from the plot are listed in Table 2, which also includes bisquaternary Gemini 14-4-14 without amine function in the spacer, and monoquaternary ammonium bromides C14 for comparison purpose.
According to Table 2, the value of cmc determined for the Gemini 14-2-NCH3)-2-14 is slightly lower than the value corresponding to the Gemini 14-4-14, and much lower than the cmc of the monomeric surfactant C14TAB. Devinsky et al. [35] reported the same result for homologous series of Gemini surfactants with flexible hydrophobic spacers (CH2)2 -Y(CH2)2, i.e., the cmc of these surfactants does not depend significantly on the chemical nature of the Y. The cmc values of Gemini surfactant [(C12H25(CH3)2N+, Br−)2 (CH2)2Y(CH2)2] were found to be 1.2, 1.1, 1.0 and 0.84 mM for Y=N(CH3), O, CH2 and S respectively.
Cmc values found for these compounds were lower than those corresponding to the monomer, meaning that the two alkyl chains of the Gemini surfactants facilitate the micellization, when compared to the hydrophobic single chain in their conventional counterparts. Aratani et al. [36] have suggested two hypotheses to explain this difference:
-
1.
The first is a repulsion between the hydrophilic cationic heads which hinders the aggregation and the formation of micelles for mono-quat, while in the bis-quat, this constraint is reduced by the covalent bonds between these two hydrophilic heads in the spacer.
-
2.
The presence of two hydrophobic chains in the structure of the bis-quat is itself a phenomenon of premicellization.
The formation of micelles in an aqueous solution of dimeric surfactant has been much investigated. In all instances, where the comparison is possible, the cmc(s) of dimeric surfactants have been found to be at least one order of magnitude smaller than the corresponding value for a monomeric surfactant. The most likely explanation for these low cmc values is that in solution of dimeric surfactants, two alkyl chains are simultaneously transferred from water to the micellar phase, resulting in a nearly double free energy change of transfer, and thus in a much lower value of the cmc [21].
The surface excess Гmax is a measurement of how much the air/solution surface has been modified by the surfactant adsorption. It depends on the molecular structure of a surfactant molecule. Adsorption behavior at the air/solution surface of the Gemini surfactant with a hydrophobic spacer is determined mainly by the hydrophobicity of the two alkyl chains and the spacer length [35, 36]. When the length of the spacer increases, it begins to exert an important influence on adsorption: Гmax decreases and consequently A min increases.
The value of Гmax determined for the Gemini 14-2-N(CH3)-2-14 was slightly lower than the one for 14-4-14 and much lower than the surfactant monomeric C14TAB value. This means that the Gemini 14-2-N(CH3)-2-14 produces a strong modification at the air/solution surface, greater than those produced by Gemini 14-4-14 and monomeric surfactant C14TAB.
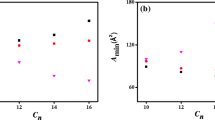
The value of A min obtained for the Gemini 14-2-N(CH3)-2-14 is slightly higher than that obtained for the Gemini 14-4-14, thus indicating a looser packing of the former at the air/solution surface.
The main factors that determine the variation of A min with spacer S were found to be the spacer conformational entropy and the attractive and repulsive interactions between surfactant molecules. Devinsky et al. [35] have studied the effect of flexible spacers type (CH2)2-Y(CH2)2 on the area per molecule A min in the case of the Gemini surfactants in C12 series. They found that A min, depends on the chemical nature of the group Y and increases in the order S (84) < N(CH3) (108) < CH2 (114) < O (128). The values of A min in parentheses are in Å2 for cationic Gemini surfactants with the following chemical structure (Scheme 3):
Y=S; N(CH3); CH2; O
A useful measure of the efficiency of a surfactant in reducing the surface tension of water is the surfactant concentration C20 required to reduce the surface tension by 20 mN/m or the logarithm of C20 (pC20). Molecules with high efficiency partition adsorb strongly at the interface even at low concentrations. Our results show that the value of pC20 obtained for the 14-2-N(CH3)-2-14 species is similar to that obtained for the Gemini 14-4-14.
Surfactant effectiveness is also measured by the maximum reduction of surface tension reached at the cmc (γcmc). The value of surface tension at the cmc for the Gemini studied was slightly lower than that of Gemini 14-4-14. The ratio cmc/C20 value is a measure of the surfactant preference for adsorption relative to micellization. The high value of this cmc/C20 ratio determined for the studied Gemini 14-2-N(CH3)-2-14 indicates that this surfactant shows a great preference for adsorption at the interface. This result was confirmed by the values of free energies of adsorption and micellization. Indeed, the free energy of adsorption is higher in absolute values to the free energy of micellization.
The packing parameter calculated for the Gemini 14-2-N(CH3)-2-14 is less than 1/3 indicating the formation of spherical micelles, as Gemini 14-4-14.
In order to compare the investigated compound 14-2 N(CH3)-14 with other Gemini surfactants, we have compiled in Table 3 the physicochemical parameters of some dimeric and trimeric Gemini surfactants. We first compared the cmc of the dimeric studied surfactant 14-2-N(CH3)-14 with the homologous Gemini m-4-m with m = 10.12.14; we found that the cmc of the Gemini 14-2N(CH3)-14 is smaller than the cmc(s) of the dimeric species 10-4-10, 12-4-12 and 14-4-14. The same trend is observed in the case of two series of homologous dimeric surfactants 10-2-10, 12-2-12, 14-2-14 [26, 37] and 8-3-8,10-3-10 and 12-3-12 [38, 39]. This fact is due to the well known phenomenon. i.e., the linear decrease of cmc with the length of the alkyl tail at constant spacer length [26, 37–41]. The decrease of cmc with the length of the alkyl chain is also observed for the trimeric Gemini surfactants [37]. We have also observed that the studied Gemini 14-2N (CH3)-14 has the lowest surface tension at the cmc (γcmc), and is more effective at reducing surface tension than the Gemini species10-4-10, 12-4-12 and 14-4-14.
By comparing some thermodynamic parameters listed in Table 3 for the investigated Gemini 14-2N (CH3)-14, with the trimeric 12-2-12-2-12 synthesized by Yoshimura et al. [37] and the trimeric 12-3-12-3-12 synthesized and studied by Zana et al. [39], we can see that the studied Gemini has roughly the same performance as the trimeric 12-3-12-3-12, while the trimeric 12-2-12-2-12 seems more efficient than the studied dimeric.
From polarized light microscopy, we observed the behavior of anhydrous surfactant and the binary water/surfactant system as function of temperature. Only few phase diagrams have been reported for dimeric surfactants, though the m-s-m, 2Br- surfactants have been investigated systematically. It was first shown that the pure dimeric surfactants do not give rise to thermotropic liquid crystals, a rather unexpected behavior, as the corresponding conventional monomeric surfactant show liquid crystalline thermotropic behavior. This result agrees with our finding. The absence of the thermotropic liquid crystal was tentatively attributed to the geometric constraint introduced by the spacer on the arrangement of the charged groups. For these surfactants, the concentration range of the lyotropic mesophases was found to decrease as the spacer carbon number “s” increased and completely disappeared at s = 10 and 12. These surfactants only give micellar solutions even at concentrations as high as 90%. The observed mesophases had the texture of the conventional lamellar and cylindrical phases.
Eastoe et al. [42], in their study on sugar Gemini surfactants found the following phase sequence with decreasing surfactant concentration: hydrated crystals → lamellar (Lα) → Cubic (V1) → Hexagonal (H1) → micellar (L1).
In our case, we observed the following sequence with increasing the temperature:
References
Pérez L, Luis Torres J, Manresa A, Solans C, Infante MR (1996) Synthesis, aggregation, and biological properties of a new class of Gemini cationic amphiphilic compounds from arginine, bis(Args). Langmuir 12:5296–5301
Vogtle F (1991) Supramolecular chemistry. Wiley, New York, pp 207–229
Graciani MM, Rodriguez A, Munoz M, Moya LM (2005) Micellar Solutions of Sulfobetaine Surfactants in Water−Ethylene Glycol Mixtures: Surface tension, fluorescence, spectroscopic, conductometric, and kinetic studies. Langmuir 21:7161–7169
Rosen MJ, Gao T, Nakatsuji Y, Masuyama A (1994) Synergism in binary mixtures of surfactants 12.Mixtures containing surfactants with two hydrophilic and two or three hydrophobic groups. Colloids Surf A 88:1–11
Wagenaa A, Engberts JBFN (2007) Synthesis of nonionic reduced-sugar based bola amphiphiles and Gemini surfactants with an α, ω-diamino-(oxa)alkyl spacer. Tetrahedron 63:10622–10629
Jarasuriya N, Bosak S, Regen SL (1990) Supramolecular surfactants: polymerized bolamphiphiles exhibiting extraordinarily high membrane-disrupting activity. J Am Chem Soc 112:5851–5854
Zana R, Benrraou M, Rueff M (1991) Alkanediyl-.alpha.,omega. bis (dimethyl alkyl ammonium bromide) surfactants. 1. Effect of the spacer chain length on the critical micelle concentration and micelle ionization degree. Langmuir 7:1072–1075
Alami E, Beinert G, Marie P, Zana R (1993) Alkanediyl-.alpha.,omega.- bis (dimethyl alkyl ammonium bromide) surfactants. 3. Behavior at the air-water interface. Langmuir 9:1465–1467
Layne KM, Debenetti PG, Prud’homme RK (1998) A theoretical study of Gemini Surfactants phase behavior. J Chem Phys 109:5651–5658
Menger FM, Littau CA (1993) Gemini surfactants: a new class of self-assembling molecules. J Am Chem Soc 115:10083–10090
Rosen MJ, Tracy DI (1998) Gemini surfactants. J Surfact Deterg 1:547–554
Zana R (2002) Dimeric (Gemini) Surfactants: Effect of the Spacer Group on the Association Behavior in Aqueous Solution. J Colloid Interface Sci 248:203–220
Menger FM, Keiper JS (2000) Gemini surfactants. Angew Chem Int Ed 39:1906–1920
Lawrence ACS (1958) Solubility in Soap Solutions. Part 10. Phase Equilibrium, and Diffusion Phenomena involving the Ternary Liquid Crystalline Phase. Discuss Faraday Soc 25:51–59
Greksakova O, Oremusova J (2000) Thermodynamics of the micellisation of cationic surfactants 1-hexadecyltrimethylammonium Bromide and 1-Hexadecylpyridinium bromide. Tenside Surfact Deterg 39:40–47
Oremusova J, Greksakovo O (2005) Effect of counterions and Temperature on the association and partition balances of Hexadecylpyridinium halides in aqueous solutions. Tenside Surfact Deterg 42:288–294
Oremusova J, Greksakovo O (2003) Determination of the critical Micelle Concentration, Hydrodynamic Micelle Radius and Experimental Partition Coefficient of N-Dodecyl-N-methylephedrinium Bromide. Tenside Surfact Deterg 40:90–95
Andriamainty F, Cizmarik J, Holikova M (2004) Study of local anesthetics. Part 166: Conductometric determination of the critical micelle concentration of local anesthetic heptacainium chloride in aqueous electrolyte solution. Scientia Pharmaceutica 72:221–225
Gonzalez-Perez A, Czapkiewicz J, Castillo JL, Rodriguez JR (2004) Temperature dependence of second critical micelle concentration dodecyl dimethylbenzyl ammonium bromide in aqueous solution. Colloid Polym Sci 281:1169–1173
El Worthy PH, Florence AT, McFarlane CB (1968) Solubilisation of Surface Active Agents. Chapman and Hall, London
Rosen MJ (2004) Surfactants and interfacial phenomena. John Wiley, New York, third edition
Li ZX, Dong CC, Thomas RK (1999) Neutron Reflectivity Studies of the Surface Excess of Gemini Surfactants at the Air−Water Interface. Langmuir 15:4392–4396
Dreja M, Pychkhout-Hintzen W, Mays H, Tieke B (1999) Cationic Gemini Surfactants with Oligo(oxyethylene) Spacer Groups and Their Use in the Polymerization of Styrene in Ternary Microemulsion. Langmuir 15:391–399
Rosen MJ, Mathias JH, Davenport L (1999) Aberrant aggregation behavior in cationic Gemini surfactants investigated by surface tension, interfacial tension, and fluorescence methods. Langmuir 15:340–7346
El Achouri M, Gouttaya HM, Bensouda Y, Nciri B, Perez L, Infante MR (2001) Gemini surfactants of the type 1, 2-Ethanediyl bis(dimethylalkylammonium bromide), Synthesis and thermodynamic and corrosion–inhibiting properties of some Gemini surfactants in the series 1, 2-ethanediyl bis-(dimethylalkylammonium bromide). Tenside Surfact Deterg 38:208–215
Gouttaya HM (2002) Etude thermodynamiques de nouveaux tensioactifs Gemini- Application à l’inhibition de la corrosion, Thesis, Université Sidi Mohammed ben Abdellah, Faculté des Sciences, Fes, Marocco
Frindi M, Michels B, Levy H, Zana R (1994) Alkanediyl-.alpha.,omega. bis(dimethylalkylammonium bromide) Surfactants. Ultrasonic Absorption Studies of Amphiphile Exchange between Micelles and Bulk Phase in Aqueous Micellar Solution. Langmuir 10:1140–1145
Menger FM, Keiper JS, Mbadugha BN, Caran KL, Romsted LS (2000) Interfacial Composition of Gemini Surfactant Micelles Determined by Chemical Trapping. Langmuir 16:9095–9098
Rodriguez JR, Gonzalez-Perez A, Del Castillo JL, Czapkiewicz J (2002) Thermodynamics of Micellization of Alkyl dimethyl benzyl ammonium chlorides in Aqueous Solutions. J Colloid Interf Sci 250:438–443
Mukerjee P, Korematsu K, Obawauchi M, Sugihara G (1985) Effect of temperature on the electrical conductivity and the thermodynamics of micelle formation of sodium perfluorooctanoate. J Phys Chem 89:5308–5312
Manet S (2007) Effet de contre ion sur les propriétés d’amphiphiles cationiques, Thesis, Université de Bordeaux I, Ecole doctorale des sciences chimiques, Bordeaux, France
Geng Y, Romsted LS, Menger FM (2006) Specific ion pairing and interfacial hydration as controlling factors in Gemini micelle morphology. Chemical trapping studies. J Am Chem Soc 128:492–501
Tehrani-Bagha AR, Oskarsson H, Van Ginkel C, Holmberg K (2007) Cationic ester-containing Gemini surfactants: Chemical hydrolysis and biodegradation. J Colloid Interface Sci 312:444–452
Bai G, Wang J, Yan H, Thomas K (2001) Thermodynamics of Molecular Self-Assembly of Cationic Gemini and Related Double Chain Surfactants in Aqueous Solution. J Phys Chem B 105:3105–3108
Devinsky F, Lacko I, Bittererova L (1986) Relationship between structure, surface activity, and micelle formation of some new bisquaternary isoesters of 1, 5- pentanediammonium dibromides. J Colloid Interface Sci 14:314–322
Hayashi Y, Aratani K, Oida T, Shimizu T (1998) Preparation and properties of Gemini surfactants from tartric acid. Jornadas Com Esp Deterg 28:45–56
Yoshimura T, Yoshida H, Ohno A, Esumi K (2003) Physicochemical properties of quaternary ammonium bromide-type trimeric surfactants. J Colloid Interface Sci 267:167–172
Wetting SD, Verrall RE (2001) Thermodynamic studies of aqueous m-s-m Gemini surfactants. J Colloid Interface Sci 235:310–316
Zana R, Levy H, Papoutsi D, Beinert G (1995) Micellization of two triquaternary ammonium surfactants in aqueous solution. Langmuir 11:3694–3698
Zana R, Levy H (1997) Alkanediyl-α, ω-bis(dimethylalkylammonium bromide) surfactants (dimeric surfactants) Part 6. CMC of the ethanediyl-1, 2-bis(dimethylalkylammonium bromide) series. Colloids Surf A 127:229–232
Wetting SD (2000) Studies of the interaction of Gemini surfactants with polymers and triblock copolymers, Thesis, Department of Chemistry, University of Saskatchewan, Saskatoun, Saskatchewan, Canada
Eastoe J, Rogueda P, Harrison BJ, Howe AM, Pitt AR (1994) Properties of dichained “sugar surfactant”. Langmuir 10:4429–4433
Acknowledgments
We would like to thank the CNRST (Morocco) for its financial help: Project number D13/31.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Alehyen, S., Bensajjay, F., El Achouri, M. et al. Preparation of a New Oligomeric Surfactant: N,N,N′,N″,N″-Pentamethyl Diethyleneamine—N,N″-Di-[Tetradecylammonium Bromide] and the Study of its Thermodynamic Properties. J Surfact Deterg 13, 339–348 (2010). https://doi.org/10.1007/s11743-009-1162-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-009-1162-2