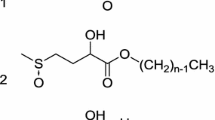
Abstract
The oligomers (m = 1,2,3,4 and 5) in the profile of compounds obtained in the ethoxylation of saturated cardanol with ethylene oxide have been synthesised. The first member which occurs only in traces in the reaction has now been synthesised by an improved method. This monoethoxylate of 3-pentadecylphenol and a diethoxylate of 5-pentadecylresorcinol (2-hydroxyethyl derivatives) have been synthesised by the base-catalysed reaction with ethylene carbonate. Cardanol and cardol separated from technical cashew nut-shell liquid (CNSL) have been similarly reacted as have saturated cardanol and saturated cardol obtained by reduction of the side-chain. Reaction of the anhydrous potassium salt of 3-pentadecylphenol in dichloromethane with ethylene sulfate has afforded a derivatives having a sulfate group in place of the terminal hydroxyl group and avoiding the use of sulfuric acid. Similarly CNSL and saturated CNSL reacted with ethylene sulfate gave sulfo derivatives which possess excellent surfactancy. By contrast, with sulfuric acid, 3-pentadecyl phenol monoethoxylate gave a trisulfo product comprising a terminal side-chain sulfate and a ring disulfonic acid. 3-Pentadecylphenol reacted with sulfuric acid in carbon tetrachloride to give 4-hydroxy-6-pentadecylphenol-1,3-disulfonic acid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenolic lipids, notably technical cashew nut-shell liquid (CNSL), have been examined previously [1] as a source of various classes of surfactants. Thus, cationic compounds [2, 3], non-ionic [4, 5] and anionic compounds (4 and Tyman and Durrani, Unpublished data) have been synthesised, although members of the latter have not been well characterised [6]. Technical CNSL which is obtained by the industrial process of thermal decarboxylation [1] of the natural cashew nut, contains the component C15 phenols cardanol (1, 65–70%), cardol (3, 15–20%), 2-methylcardol (5%) and polymeric material. Each of the component phenols contains saturated and unsaturated constituents, the saturated (n = 0), the 8Z-monoene (n = 2, the 8Z,11Z-diene (n = 4) and the 8Z,11Z,14-triene. (n = 6) In the present study, 3-pentadecylphenol (saturated cardanol), (1, n = 0) and 5-pentadecylresorcinol (saturated cardol), (3, n = 0) were used for simpler analytical characterisation and obtained by separation of CNSL and catalytic hydrogenation [1, 2, 7, 8]. They have been converted to their ethoxylates (2, n = 0, m = 1) and (4, n = 0, m = 1) by base-catalysed reaction with ethylene carbonate, and then used for the synthesis of novel anionic compounds. Thus, sulfation/sulfonation of saturated cardanol monoethoxylate with sulfuric acid afforded a monosulfate, disulfonic acid (9, n = 0) by reaction at the OH group and substitution in the aromatic ring.
By contrast, reaction of phenoxides from saturated and unsaturated cardanol, and more practically of saturated and technical CNSL with ethylene sulfate avoids the use of sulfuric acid and can give novel anionic derivatives.
Although it is known [6] that saturated cardanol is readily sulfonated with excess sulfuric acid in halogenated solvents at ambient temperature, the use of ethylene sulfate with the phenoxide is an improvement which avoids both a reaction with sulfuric acid and the need for an initial hydroxyethylation step.
Results and Discussion
In previous work [3] on the reaction of ethylene oxide with saturated cardanol (3-pentadecylphenol) and with cardol a range of oligomeric ethoxylates (2, m = 1-40) (Scheme 1), was obtained but the first member of the series (2, m = 1, n = 0) was formed in very small proportion. It was more easily derived by the reaction of saturated cardanol with chloroacetonitrile, hydrolysis and hydride reduction or from saturated cardanol and ethyl chloroacetate followed by reduction [9].
It was of interest to synthesise this material by a more direct specific method and to examine its utility as an intermediate for anionic surfactants. Hydroxyethylation of phenols by reaction with ethylene carbonate although not of long chain members has been described under basic conditions with triphenylphosphine [10] or imidazole [11]. In our present work, (Scheme 1), reaction of saturated cardanol (3-pentadecylphenol) (1, n = 0) with ethylene carbonate and triphenylphosphine afforded 2-hydroxyethoxy-3-pentadecylbenzene (2, m = 1, n = 0), as shown in Scheme 2, by way of an intermediate carbonic acid and then loss of carbon dioxide. It seems that the reaction is an S n 2 type by attack on the methylene rather than at the carbonyl group of ethylene carbonate. Elimination of CO2 was very slow and the presence from mass spectrometric evidence, in the molecular ion of isolated material, of a mass 44 units higher than that of (2, m = 1, n = 0) indicated the likely presence of the carbonic acid rather than at first considered due to an oligomer (2, m = 2, n = 0) in which the additional CH2CH2O would also possess a mass 44 mass units higher than (2, m = 1, n = 0). However, the OH group is a weak nucleophile compared with the phenoxide ion and oligomer formation is in fact negligible.
In a similar way, saturated cardol (5-pentadecylresorcinol) (3, n = 0) reacted with ethylene carbonate to give the diethoxylate (4, m = 1, n = 0), probably by way of the monosubstituted compound (7, m = 1, n = 0), but only traces of this could be located by TLC in the synthesis of the diethoxylate.
Nevertheless, this monosubstituted compound was synthesised by reaction of cardol with ethyl bromoacetate in the presence of potassium carbonate to afford (5, n = 0) hydrolysis of which gave the acid (6, n = 0). Reduction preferably of the trimethylsilyl derivative with lithium aluminium hydride then gave (7, m = 1, n = 0).
The preceding ethoxylates were next examined as intermediates for obtaining anionic compounds.
An attempt to sulfate (2, n = 0) by reaction with conc. sulfuric acid afforded the monosulfate disulfonic acid (9, n = 0) by way of the sulfate (8, n = 0) which, however, could not be isolated. Evidently even under mild conditions (2, n = 0) is strongly activated resulting in ring substitution.
The potassium salt of sulfate (8, n = 0) was synthesised from the potassium salt of (1, n = 0) by reaction in dichloromethane with ethylene sulfate [10] as depicted in Scheme 2. Saturated cardol (3, n = 0) similarly as the di-potassium salt reacted with ethylene sulfate and afforded the potassium disulfate (10, n = 0). The water solubility of (8, n = 0) and of (10, n = 0), however, proved less than expected. By contrast, the potassium salts of unsaturated cardanol (1) reacted with ethylene sulfate and gave a more soluble sulfate (8, n = 0,2,4,6), as found generally for polyunsaturated fatty acids and their salts compared with saturated fatty acids and their salts. The Emersol process utilises the very different solubilities of saturated and their unsaturated analogues for their separation. The advantage of reaction of unsaturated systems with ethylene sulfate for introducing the sulfonic group is that polymerisation reactions which occur with conc sulfuric acid are avoided. The only other example of the incorporation of a sulfonate group avoiding the use of sulfuric acid appears to be by the prolonged reaction of 4-dodecylphenol with sodium sulfite and aqueous formaldehyde although no yields were given [13]. The product was considered to be the sodium salt of 4-hydroxy-3-sulfomethyldodecylbenzene. The reaction of a replenishable source, namely 3-pentadecylphenol, was not instanced.
Although the water solubility of (9, n = 0) was much greater than that of (8, n = 0) due to the sulfonic groups as well as the side-chain sulfate and the increased number of oxygen atoms, it seemed likely that the presence of just two sulfonic groups might provide a sufficient lipidic balance between the C15 side-chain and the anionic head group. Moreover, by sulfonation alone, the preliminary ethoxylation stage would be avoided.
Sulfonation of saturated cardanol (1, n = 0) in carbon tetrachloride with conc. sulfuric acid gave an excellent yield of the sulfonic acid (11, n = 0) which possessed good surface activity and was characterised by NMR, mass spectrometry and elemental analysis. Although saturated cardanol has been sulfonated in earlier work [2, 14], drastically in the absence of solvent, the product has never been completely characterised. Attempts to use sulfuric acid with unsaturated cardanol led to complex reactions including polymerisation and at the side-chain. Nevertheless, unsaturated cardanol in solvent with chlorosulfonic acid afforded a soluble sulfate/sulfonate derivative (Tyman and Durrani, unpublished data, 1977–1980).
Experiments with saturated cashew nut-shell liquid which contains mainly cardanol and cardol yields (11, n = 0) and (12, n = 0) together with probably a disulfonic acid.
The surface active properties of the synthesised anionic compounds await examination.
Experimental Procedures
Spectroscopy: proton NMR spectra were carried out in the Departments of Chemistry, University of Surrey and University College, London. Accurate mass measurements and negative ion mass spectrometry (orbitrap negative analysis), the latter on ThermoFisher Scientific equipment, were carried out by the EPSRC mass spectrometry centre, University of Wales, Swansea. Infrared spectra were performed on a Perkin Elmer 502 spectrometer.
Chromatography: Analytical thin layer chromatography (TLC) was carried out on silica gel plates (0.25 mm) with fluorescent indicator UV254 and also with visualisation by iodine; and preparative TLC on 20 × 20 × 0.1 cm plates For argentation TLC analytical plates were sprayed with aqueous 10% silver nitrate solution, and dried at 100 °C. Column chromatography on columns (20 cm × 2 cm i.d.) was effected on silica gel MPD 60 Å.
Microanalyses were carried out by NRM Ltd, Bracknell. All solvents were HPLC grade. Reagents were obtained from Aldrich Chemical Co. and Technical cashew nutshell liquid (CNSL) from Cardolite, Ghent, Belgium.
Separation of Cardanol and Cardol from CNSL [2, 15]
Technical CNSL (53.1 g) in pentane-1,5-diol (50 cm3) was extracted three times with light petroleum ether (500 cm3). The combined petroleum extract was washed with water, dried and evaporated to afford cardanol, The lower layer was diluted with water (350 cm3), extracted with light petroleum, the extracts dried and evaporated to yield cardol containing some 2-methylcardol. Purified cardol was separated by flash chromatography with chloroform–ethyl acetate, (98:2) to give 3.54 g.
Reduction of Unsaturated Phenols to Saturated Cardanol (3-Pentadecylphenol) and Saturated Cardol (5-Pentadecylresorcinol) [16]
Unsaturated cardol (1.00 g) in ethanol (60 cm3) containing hydrazine hydrate (2.00 g) at 50 °C was stirred and the mixture aerated with compressed air (reaction samples were monitored by analytical argentation TLC), After 24 and 48 h, further hydrazine hydrate (2.0 g and 2.50 g), respectively, was added to complete reduction. After 72 h, the ethanol was evaporated and the residual oil dissolved in diethyl ether was washed with dilute hydrochloric acid, then with water, dried and evaporated to give the product (0.59 g), having a single band on silver nitrate TLC.
Synthesis of Intermediates and Anionic Surfactants
To identify the polyethoxy oligomers in the reaction of 3-pentadecylphenol with ethylene oxide, the first five members (2, m = 2–6) were synthesised. The synthetic compounds were chromatographically identical with those in the profile of compounds from the reaction of 0.3-pentadecylphenol with ethylene oxide.
5-(3-Pentadecylphenoxy-3-oxapentanol (2, n = 0, m = 2)
3-Pentadecylphenol (1, n = 0), (1.02 g, 3.35 mmol) and potassium metal (0.13 g, 3.41 mmol) were heated together with stirring at 90 °C under nitrogen for 10 h, after which 5-chloro-3-oxapentanol (0.42 g, 3.34 mmol) was added dropwise and the mixture heated for a further 10 h. The cooled mixture was diluted with a small volume of dry THF, filtered to remove KCl and the product separated on a Chromatotron (5% ethyl acetate in chloroform) to give the product 2 (0.45 g (45%): found, C, 76.34; H, 11.31. Reqd. for C25H44O3, C, 76.51; H, 11.22%; δH (80 MHz, CDCl3),7.26–7.14 (1H, m, HAr), 6.82–6.69 (3H, m, HAr), 4.11–3.92 (8H, m, CH2O), 2.51 (2H, t, J 7.4 Hz, CH2Ar), 1,51 (1H, s, HO, exch. D2O); 1.48–0.97 (29H, m, CH2); m/z found, 392.1. Reqd. for C25H44O4 392.620; λmax (ethanol)/nm 207 (ε 2,467), 275 (479).
8-(3-Pentadecylphenoxy)-3,6-dioxaoctanol (2, n = 0, m = 3)
In a similar way 3-pentadecylphenol (1, n = 0) (1.16 g, 3.815 mmol) and potassium (0.304 g, 7.79 mmol) were reacted under nitrogen with 8-chloro-3,6-dioxaoctanol (0.867 g, 5.145 mmol). Chromatographic purification afforded the product 1.39 g (46%). Found, C, 74.11; H, 10.85. Reqd. for C27H48O4, 74.31, H, 11.01%; δH (80 MHz, CDCl3), 7.25–7.15 (1H, m, HAr), 6.82–6.69 (3H, m, HAr), 4.10–3.93 (12H, m, CH2O), 2.51 (2H, t, J 7.4 Hz, CH2Ar), 1.05–0.89 (29H, m, CH2); m/z found, 436.2. Reqd. for C27H48O4, 436.664; λmax (ethanol/nm), 207 (ε 2,383), 275 (490).
11-(3-Pentadecylphenoxy)-3,6,9-trioxaundecanol (2, n = 0, m = 4)
2-(3-Pentadecylphenoxy)ethanol (2, n = 0, m = 1) (1.06 g, 3.04 mmol) and potassium metal (0.11 g, 2.82 mmol) were reacted as described for the previous compound with 8-chloro-3,6-dioxaoctanol (0.46 g, 2.73 mmol). The reaction mixture was chromatographed (7% ethyl acetate in chloroform) to give the product 0.48 g (37%); found, C, 72.61; H, 10.39. Reqd. for C29H48O4 C, 72.50; H, 10.62%; δH (80 MHz, CDCl3) 7.26–7.16 (1H, m, HAr), 6.82–6.70 (3H, m HAr), 4.09–3.84 (16H, m, CH2O), 2.51 (2H, t, J 7.4 Hz, CH2Ar), 1.50–0.88 (29H, m, CH2); m/z 380.2. Reqd. for C29H52O5,380.712; λmax (ethanol/nm) (ε 2,309), 275 (507).
14-(3-Pentadecylphenoxy-3,6,9,12-tetraoxatetradecanol (2, m = 5)
In a similar way 8-(3-pentadecylphenoxy)-3,6-dioxaoctanol (2, n = 0, m = 3) (1.34 g, 3.06 mmol) and potassium metal (0.12 g, 3.08 mmol) were reacted with 5-chloro-3-oxapentanol (0.38 g 3.05 mmol) and the product separated chromatographically (10% ethyl acetate in chloroform) to afford 0.67 g (42%). Found, C, 71.01; H, 10.32. Reqd. for C31H56O5, C, 70.86; H, 10.48%; δH (80 MHz, CDCl3) 7.25–7.14 (1H, m, HAr), 6.81–6.70 (3H, m, HAr), 4.13–3.91(20H, m, CH2O), 2.51 (2H, t, J 7.4 Hz, CH2Ar), 1.55–0.88 (29H, m, CH2); m/z 524.9. Reqd. for C31H56O5 524.750; λmax (ethanol/nm), 207 (ε 2,247), 275 (534).
17-(3-Pentadecylphenoxy)-3,6,9,12,15-pentaoxaheptadecanol (2, n = 0, m = 6)
Similarly, 8-(3-pentadecylphenoxy)-3,6-dioxaoctanol (2, n = 0, m = 3) (0.87 g, 2.39 mmol) and potassium metal (0.10 g, 2.56 mmol) were reacted at 90 °C for 4 h, after which 8-chloro-3,6-dioxaoctanol (0.37 g, 2.19 mmol) was added and reaction continued for a further 10 h. Chromatographic purification (15% ethyl acetate in chloroform) afforded the product 0.61 g, (46%); found, C, 69.77; H, 10.29. Reqd. for C33H58O7, C, 69.72; H, 10.38%. δH (80 MHz, CDCl3) 7.25–7.14 (1H, m, HAr), 6.81–6.70 (3H, m, HAr), 4.13–3.91 (20H, m, CH2O), 2.51 (2H, t, J 7.4 Hz, CH2Ar), 1.55–0.88 (29H, m, CH2); m/z 524.9. Reqd. for C33H58O7 524.759; λmax (ethanol/nm), 297 (ε 2,247).
3-Pentadecylphenol monoethoxylate [2-(3-pentadecyloxyphenyl)-ethanol] (2, n = 0, m = 1)
A mixture of 3-pentadecylphenol (1, n = 0) (1.52 g, 4.36 mmol) and ethylene carbonate (0.742 g, 8.43 mmol) with triphenylphosphine (0.0078 g, 30 μmol) was heated at 150–160 °C for 6 h. TLC showed two major spots and absence of 3-pentadecylphenol, an upper band of the product and a lower one of the intermediate carbonic acid due to incomplete decarboxylation. The mixture was dissolved in a small volume of hexane and kept at 0 °C. The crystalline material which separated was filtered (ethylene carbonate), washed with hexane and the filtrate kept at 0 °C to afford the product as a white crystalline solid 0.33 g, 18%; a further crop of product (0.50 g) was separated from the filtrate kept at 0 °C (total yield (46%); mp 45–46 °C; (lit.4 an oil). Found, C, 78.9; H, 11.40. Reqd. for C23H40O2, C, 79.3; H, 11.45%; R f (chloroform/ethyl acetate, 90:10) 0.63, (3-pentadecylphenol 0.76); νmax (KBr disc)/cm−1 3,370 (OH), 2,918, 2,848 (CH2), 1,750, 1,594, 1,466, 1,270, 1,090; δH (300 MHz, CDCl3) 7.1 (1H, t, J 7.6,HAr), 6.7 (3H, m, HAr), 4.05 (2H, t, J 4.4 Hz, OCH2), 3.9 (2H, t, J 4,4 Hz, CH2OH),s 2.5 (2H, t, J 7.7 Hz, CH2Ar), 1.25 (26H, m, 13CH2), 0.95 (3H, t, J 6.6, Hz CH3). m/z 348, 152, 153, 108, 107, 43. M+ 366.3368. Reqd. for (M + NH4)+. C23H40O2 366.3367.
5-Pentadecylresorcinol diethoxylate [3,5-bis(2-hydroxyethoxy-1-pentadecyl benzene] (4, n = 0, m = 1)
5-Pentadecylresorcinol (saturated cardol), (3, n = 0) (0.033 g, 0.10 mmol) and ethylene carbonate (0.032 g, 0.36 mmol) together with triphenyl phosphine (0.0053 g, 20 μmol) were heated at 160–170 °C for 3 h. TLC monitoring indicated cardol remaining and further ethylene carbonate (0.090 g, 1.02 mmol) and triphenylphosphine were added with resumption of heating. A second batch was carried out and the combined products separated by prep.TLC after removal of ethylene carbonate by washing an ethereal solution copiously with water. The recovered dried product was separated on 4 silica gel plates (20 × 20 cm) with chloroform/ethyl acetate (100:20) (a 5 × 20 cm reference plate visualised with iodine serving to locate bands). In this way a middle band on each plate containing the product was removed and eluted with ethanol. Filtration and removal of ethanol in vacuo afforded a colourless solid, mp 100–105 °C; m/z 408, 278, 77. M+ found, 408.3238. Reqd. for C25H44O4 408.3234; R f (chloroform/ethyl acetate, 30:10) 0.15, (satd. cardol 0.35, satd. cardanol 0.85); δH (300 MHz), CDCl3) 6.7 (3H, m, HAr), 4.05–3.9 (8H, 2t, 4CH2O), 2.6–2.4 (2H, t, J 7.7 Hz) CH2Ar), 1.25 (26H, m, CH2), 0.85 (3H, t, J 6.6 Hz, Me).
(1-Hydroxy-3-carbethoxymethoxy-5-pentadecylphenol), (5, n = 0)
To 5-pentadecylresorcinol (1.00 g, 3.12 mmol) in refluxing benzene (40 cm3) containing potassium carbonate, ethyl bromoacetate (0.52 g, 3.11 mmol) in benzene (10 cm3) was added dropwise over 4 h. The mixture was refluxed for a further 20 h, then cooled and filtered and the filtrate concentrated to give an oil which was purified by column chromatography (2% ethyl acetate in chloroform) to give the product, 0.80 g, (34%); δH (500 MHz), CDCl3) 6.30 (2H, m, HAr), 6.23–6.24 (1H, d, J 2 Hz, HAr), 4.57 (2H, s, OCH2CO), 4.25–4.29 (2H, q, J 7 Hz, CH2O), 2.47–2.50 (3H, t, J 7.7 Hz, ArCH2), 1.25–1.31 (29H, m, CH2,CH3); νmax (KBr disc)/cm−1 3,450–3,370 (OH), 2,900 (CH), 1,740 (C=O), 1,600, 1,500; m/z found, 424.3417. Reqd. for C25H42O4, 424.3421.
3-Carboxymethoxy-5-pentadecylphenol (6, n = 0)
The ester 5 (0.3 g, 0.74 mmol) was refluxed with 10% aqueous potassium hydroxide (5 cm3) and methanol (15 cm3) for 1 h. The cooled mixture was acidified with HCl and the precipitate filtered and dried to give a white product in 97% yield, mp 145–147 °C; δH (500 MHz, CDCl3) 8.50 (1H, s, HAr), 6.05 (2H, m, HAr), 4,25 (2H, s, OCH2CO), 2.25 (2H, t, J 7.7 Hz), CH2Ar), 2.6 (2H, bs, OH, CO2H); νmax (KBr disc)/cm−1, 3,480 (OH), 2,920 (CH), 1,720 (C=O), 1,585; m/z found, (M++ NH4) 396.3109. Reqd. for C23H38O4,396.3108.
3-(2-Hydroxyethoxy)-5-pentadecylphenol (7, n = 0, m = 1)
3-Carboxymethoxy-5-pentadecylphenol (6, 0.010 g, 26 μmol) in dry THF at ambient temperature was treated under nitrogen with excess lithium aluminium hydride and allowed to warm to ambient temperature. After 16 h, the mixture was acidified with HCl and extracted with diethyl ether. The ethereal layer was washed with water, dried and evaporated to leave a solid. m/z, found (M+1)+, 365.3048, Reqd. for C23H40O3, 365.3050. Some residual acid was present, m/z, 396.3118.
Potassium Salt of 2-(3-Pentadecyloxy)-1-sulfooethanol (8, n = 0)
To methanolic potassium hydroxide (1.7 cm3) prepared from potassium hydroxide (1.30 g, 23.2 mmol) in methanol (10 cm3), 3-pentadecylphenol (1, n = 0) (1.0 g, 3.28 mmol) was added and the methanol evaporated to leave a dry solid. This was suspended in dichloromethane (10 cm3) and ethylene sulfate (0.50 g, 4.03 mmol) added. After an exothermic reaction the mixture was treated with more dichloromethane (5 cm3) and refluxed for 4 h. The cooled mixture containing a white solid was filtered and the solid washed with dichloromethane to give upon freeing of solvent, the product, 1.10 g, (78%); δH (300 MHz, DMSO) 7.1–7.2 (1H, t, J 8.2 Hz, HAr), 6.65–6.7 (3H, m, HAr), 3.9–4.1 (4H, 2t, CH2O), 2.45–2.50 (2H, m, ArCH2), 1.25–1.31 (29H, m, CH2, CH3); m/z found, 427.2525. Reqd. for [C23H39O5S]−, [M–K]−, 427.2524. The product contained some HO(CH2)2OSO3 K, and after correcting for this, found, S, 9.60. Reqd. 9.90%.
The use of dry sodium methoxide for formation of the phenoxide prior to reaction with ethylene sulfate in dichloromethane appears preferable in the cases of reactions with saturated and unsaturated CNSL.
Sulfation and Sulfonation of 3-Pentadecylphenol monoethoxylate [1-(2-sulfoxyethoxy)-4,6-disulfo-3-(3-pentadecyl) benzene (9, n = 0)
2-(3-Pentadecylphenoxy)ethanol (2, n = 0, m = 1) (0.202 g, 0.58 mmol), cooled to 0 °C and stirred was treated with conc. sulfuric acid (3 cm3) in three portions. The mixture was left for 16 h by which time it had solidified. A sample monitored by TLC showed an absence of the starting ethoxylate and neutralisation with potassium hydroxide afforded a highly foaming solution while the starting material, by contrast, did not. The mixture was neutralised with a solution of potassium hydroxide (6.3 g, 112 mmol) in water (40 cm3). The resulting solution was evaporated to dryness, the solid was pulverised and then extracted three times with ethanol. Evaporation of the extract in vacuo afforded a white and sticky residue; δH (300 MHz), D2O), 8.30–8.35 (1H, m, HAr) 6.9–7.1 (1H, m, HAr), 3.90–4.50 (4H, m, CH2O), 2.9–3.1 (2H, m, ArCH2), 1.20–1.70 (26H, m, CH2, CH3); m/z, negative ion (orbitrap negative analysis), found for trianion, 195. Reqd. for [C23H37O11S3]3−, 585/3, (195).
4-Hydroxy-6-pentadecylbenzene-1,3-disulfonic acid (11, n = 0)
To 3-pentadecylphenol (1.00 g, 3.28 mmol) in carbon tetrachloride (5 cm3) at 0 °C, conc. sulfuric acid (1.84 g, 18.8 mmol) was added dropwise. The temperature rose to 22 °C and after some time, upon cooling, a solid separated. TLC examination showed a single band and no 3-pentadecylphenol. The mixture was diluted with water, warmed to give a solution and cooled to yield a pale cream crystalline product which was filtered and washed with a small volume of water; δH (300 MHz), D2O) 7.94 (1H, s, HAr), 6.70 (1H, s, HAr), 2.65–2.70 (2H, t, J 7,7 Hz, ArCH2), 1.05–1.4 (26H, m, CH2, CH3); m/z found, 463.1837. Reqd. for C21H36O7S2 463.1830; The product proved difficult to dry; found, S,11.70. Reqd. for C21 H36O7S2.4H2O, S, 11.90%.
Saturated CNSL was reacted in chloroform solution with conc. sulfuric acid and afforded disulfonic acids of the component phenols, saturated cardanol and cardol.
Reaction of Saturated CNSL with Ethylene Carbonate
Saturated CNSL (consisting mainly of 1 and 3, n = 0), (1.60 g) was heated with ethylene carbonate (0.80 g, 9.1 mmol) and triphenylphosphine at 170–180 °C for 6 h. TLC monitoring indicated the presence of starting material and further ethylene carbonate was added and refluxing continued. With completion of reaction the reaction mixture solidified upon cooling; δH (300 MHz, CDCl3) 6.70–6.80 (3H, m, HAr), 7.15–7.20 (1H, m, HAr), 3.90–4.10 (6H, m, CH2O), 2.50–2.60 (2H, t, J 7.7 Hz ArCH2), 1.20–1.40 (26H, m, CH2, CH3), and contained primarily the ethoxylates of saturated cardanol (2, m = 1, n = 0) and of cardol (4, m = 1, n = 0).
A portion of the worked-up mixture in chloroform was treated with conc. sulfuric acid and gave a soluble sulfonate, most probably mainly a sulfate, disulfonate (9, n = 0).
Reaction of Unsaturated CNSL with Ethylene Carbonate
CNSL (consisting mainly of 1 and 3, n = 0, 2, 4, 6) (9.20 g) and ethylene carbonate (5.06 g, 57.5 mmol) with triphenylphosphine (0.054 g, 0.21 mmol) were heated at 160 °C for 4 h and, after TLC monitoring which showed the intermediate carbonic ester to be present, for a further 4 h. The reaction mixture was cooled and an ethereal solution washed with water to remove ethylene carbonate, dried and evaporated to afford the hydroxyethylated product as a viscous oil; Examination by 1H NMR, showed that the unsaturation had not been affected by the reaction at 180 °C; δH (300 MHz, CDCl3) 6.65–6.80 (3H, m, HAr), 7.10–7.25 (1H, m, HAr), 5.3–5.85 (5H, m, CH=CH, CH=CH2, 3.60–4.15 (10H, m, OCH2, CH2OH), 2.5 (2H, t, J 7.7 Hz CH2Ar), 0.85–1.6 (26H, m, CH2, CH3).
The sodium salt of unsaturated CNSL can be reacted in dichloromethane with ethylene sulfate to give mainly the sodium salts of [8] and [10], a mixture which is more soluble than the potassium salt of (8, n = 0).
References
Tyman JHP (1997) Synthetic and natural phenols, Ch. 13, Elsevier, Amsterdam; E Books, Elsevier (2008)
Tyman JHP (1979) Non-isoprenoid phenolic lipids. Chem Soc Rev 8:500–538
Tyman JHP, Patel M (2007) Phenolic structure and colour in Mannich reaction products. J Chem Res, 34–37
Tyman JHP, Bruce IE (2003) Synthesis and characterisation of polyethoxylate surfactants derived from phenolic lipids. J Surfactants Detergents 6:291–297
Tyman JHP, Bruce IE (2004) Surfactant properties and biodegradation of polyethoxylates from phenolic lipids. J Surfactants Detergents 7:167–173
Sethi SC, Subba Rao BC, Kulkarni SH, Katti SS (1963) Anionic surface active agents from cardanol, tetrahydrocardanol and derivatives. Indian J Technol 1:348–355
Tyman JHP (1973) Identification of the components of a novel fraction in Cashew Nut-shell Liquid. J Chem Soc Perkin Trans 1:1639–1647
Sood SK, Tyman JHP, Durrani AA, Johnson RA (1986) Practical liquid chromatographic separations of the phenols in Technical Cashew Nut-shell Liquid. Lipids 21:241–246
Bruce IE (1991) A study of cashew nut-shell liquid purification and synthesis of non-ionic surfactants from the component phenols, PhD Thesis, Brunel University
Dressler H (1991) Hydroxylation of phenols with cyclic organic carbonates, USP, 5059723, Oct 22
Dow Chemical Co. (1982) Imidazole Catalysts for Hydroxylation of Phenols, USP 4310706, Dec 1
Tomalin DA, Falk JC (1972) The synthesis and reactions of substituted ethyl sulphates. J Heterocyclic Chem 9:891–894
Phillips Petroleum Co. (1990) o-Hydroxybenzylsulfonic acids, USP 4939293, July 3
Harvey MT (1943) Sulfonation process, USP, 2324300, July 13
Tyman JHP, Bruce IE, Payne P (1992) The phase separation of phenolic lipids from Anacardium occidentale. Natural Product Lett 1:117–120
Lam SK, Tyman JHP (1982) The conversion of Anacardic acid into Urushiol. J Chem Soc Perkin Trans 1:1852–1942
Acknowledgments
J. H. P. T thanks Prof. H. Adlercreutz, University of Helsinki, for some financial assistance, Dr. S. Grimes and Prof. J. D. Donaldson of the former Centre for Environmental Research, Brunel University and Prof. J. Sumpter of the present Institute for the Environment, for facilities. Dr. S. Berger is thanked for a sample of 5-pentadecylresorcinol. Prof. P. G. Sammes, University of surrey) is thanked for help with certain proton NMR spectra. Technicians H. Dee and P. Szadorski and post-graduate, R. Suivre are thanked for some assistance.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Bruce, I.E., Mehta, L., Porter, M.J. et al. Anionic Surfactants Synthesised from Replenishable Phenolic Lipids. J Surfact Deterg 12, 337–344 (2009). https://doi.org/10.1007/s11743-009-1116-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-009-1116-8