Abstract
In the development of new detergent products, it is important to test the foaming behavior of different types of surfactants. Different types and concentrations of surfactant solutions prepared with three types of water are expected to present differences in their foamability. In this study, foam volumes produced by cetyl trimethyl ammonium bromide (CTAB; C19H42BrN), Tween 80® (T80; C64H124O26) and sodium dodecylsulfate (SDS; C12H25NaO4S) aqueous solutions (0.5, 1.0 and 1.5%, w/v) were compared using a stirring system, rotating at 8,000, 9,500 and 13,500 rpm. The foamability produced by CTAB, SDS and T80 solutions, in a concentration range between 0.2 and 1.0% (w/v), prepared using deionized, hard and hypersaline water were also compared. Foam volumes were higher at a stirring speed of 9,500 rpm than at 8,000 rpm. However, the results obtained at 9,500 and 13,500 rpm were not significantly different. In general, SDS solutions produced higher foam volumes than CTAB and T80 solutions. Water characteristics did not seem to influence significantly the foamability of the three types of surfactants in the studied concentrations. These studies related with foaming behavior appear to be an important step in the pre-formulation of detergent products, particularly in cosmetics and pharmaceutics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A pure liquid does not produce foam, but the decrease in surface tension by the addition of surfactants allows foam production. Foam, meaning “bubbly liquid”, is obtained when a non-equilibrium dispersion of gas bubbles in a relatively small volume of liquid containing surfactants exists [1]. These surface active macromolecules adsorb at the gas/liquid interfaces and are responsible for both the tendency of a liquid to foam and the stability of the resulting dispersion [2, 3].
The two most important foaming properties of a liquid are how easily foam is formed (its foamability) and how easily the foam collapses (its stability) [4]. Foam stability refers to the intrinsic resistance of the lamella to a decrease in interfacial area and does not imply its stability in a thermodynamic sense [5]. It has been shown that the amount of foam formed depends on the machinery responsible for its production as well as on the properties of the liquid composition; whereas the stability of the foam produced, within specified mechanical limitations, is primarily a function of the liquid composition. It has been pointed out that ease of foam formation, volume, and stability are properties that do not necessarily have any direct relation [6]. Many physical factors are involved in the control of foamability and foam stability. While foamability depends on surface tension and critical micelle concentration (CMC), foam stability can be influenced by two independent factors: molecular packing in adsorbed surfactant film and hence surface viscosity of the film at the air/water interface, and layering or structuring of micelles within the bulk water in foam lamellae [7].
The mechanisms leading to film rupture and thus to foam collapse are not well known yet. However, previous investigations have shown that foam undergoes a self-destruction process mainly due to liquid drainage, bubble disproportion and coalescence [8]. To keep foam from collapsing, it is necessary to oppose the drainage by surface tension gradients induced by surfactants. It is well known that some surfactants which greatly reduce surface tension are not very effective in producing and stabilizing foam whereas others have indeed good foamability and foam stability [9].
Several industries benefit from the development of surfactant technology, namely in the formulation of food, plastics, paints, coatings and inks, agrochemicals, metalworking fluids and lubricants, pulp and paper, explosives, textile processing products and many others. Surfactants are classified as cationic, nonionic, anionic and amphoteric or zwitterionic by the presence of formally charged groups in their head. When dissolved in water, surfactants concentrate at water/air or water/oil interfaces. They give rise to a wide range of surface chemistry functions in detergency, pharmaceutical and cosmetic areas like wetting, emulsifying, solubilizing, foaming/defoaming, rheology modifying, antistatic, glossing, lubricity and surface conditioning.
Cetyl trimethyl ammonium bromide (CTAB), sodium dodecyl sulfate (SDS) and Tween 80® (T80) are some of the surfactants usually employed in pharmaceutical and cosmetics industry. CTAB, cetyl trimethyl ammonium bromide, a quaternary ammonium compound, is a cationic surfactant with a CMC of 0.1 g/L, which is used in cosmetics and pharmaceutical formulations as an antimicrobial preservative against bacteria and fungi. Solutions containing 1–3% (w/v) CTAB are used in shampoos to remove scales in seborrhea. T80, a polyoxyethylene sorbitan monooleate or polysorbate 80, is a hydrophilic nonionic surfactant, with a CMC of 0.014 g/L, which is used as an emulsifying agent in the preparation of stable oil-in-water emulsions and as a wetting agent in suspensions. It is also widely used in cosmetics and food products. T80 is a synthetic surfactant with a well defined hydrophilic-lipophilic balance (HLB) value (15.0) that is used as a standard for experimental HLB determination of complex mixtures. SDS is an anionic surfactant, with a CMC of 0.23 g/L, employed in a wide range of nonparenteral pharmaceutical formulations and cosmetics. It is a detergent and wetting agent effective in both alkaline and acidic conditions [10]. Because of its foamability and degreasing properties, SDS is included in many shampoos (up to 30%) and other formulations used for short contact with skin. In research, SDS is used as standard skin irritant (1–5% aqueous solutions).
In this study, the influence of stirring speed and type of surfactant on foam volumes produced by cationic (Cetyl trimethyl ammonium bromide), anionic (Sodium dodecyl sulfate) and nonionic (Tween 80®) surfactant solutions (0.5, 1.0 and 1.5%, w/v) was assessed. The influence of the water characteristics on the foaming capacity of surfactant solutions prepared with deionized, hard and hypersaline water was also studied.
Experimental Procedures
Cetyl trimethyl ammonium bromide and sodium dodecyl sulfate were purchased from Sigma–Aldrich Chemical (St. Louis, MO, USA). Tween 80® was purchased from Merck KGaA (Darmstadt, Germany). Calcium carbonate and sodium chloride were purchased from José M. Vaz Pereira, S.A. (Lisbon, Portugal). All other substances were of analytical grade.
Initially, 50 ml of aqueous surfactant solutions at different concentrations (0.5, 1.0 and 1.5%, w/v) were measured in 100 ml graduated cylinders. Foam was produced at a temperature of 20 °C, using a Janke and Kunkel, Ultra-Turrax T25 (Staufen, Germany) agitation system, stirring at 8,000, 9,500 and 13,500 rpm, for 10 s. Foamability measurements were carried out in triplicate and recorded 3 min after stirring.
A quantitative measure of foam stability can be obtained by measuring the period between foam formation and foam breaking. Foam stability of surfactant solutions stirred at 8,000 rpm, and foam volumes remaining 60 and 120 min after stirring, were measured in triplicate.
In order to study the influence of the type of water on the foamability of surfactant solutions, a 100 ppm CaCO3 solution was used as hard water and a 35 g/L NaCl solution (NaCl concentration close to sea water) was used as hypersaline water. CTAB, T80 and SDS solutions at different concentrations (0.2, 0.4, 0.6, 0.8 and 1.0%, w/v) were prepared using deionized, hard and hypersaline water. Foam was produced following the directions specified above. The influence of the type of water and surfactant concentration on the foamability of the different solutions was compared.
Results and Discussion
Due to the influence of the type of surfactant and agitation speed, foam formation and foam stability of surfactant solutions can be hard to control.
The study of the influence of the type of surfactant showed that, in general, SDS solutions produced higher foam volumes than CTAB and T80 solutions. However, there was no significant difference in the foamability of the three surfactants over the studied concentration range, as expected since it is beyond the CMC of the three surfactants.
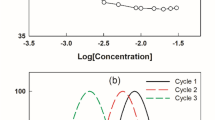
As can be seen in Fig. 1, lowest foam volumes produced by surfactant solutions at 0.5% were attained at an agitation speed of 8,000 rpm. Results obtained with samples stirred at 9,500 and 13,500 rpm were not significantly different. Similar results were obtained with surfactant solutions at concentrations of 1.0 and 1.5%.
Stability of foam over time was visually assessed by measuring the foam volume at 3, 60 and 120 min after stirring. Foam stability measurements were carried out at different surfactant concentrations and the plot of the foam volume vs time of surfactant solutions at 0.5% is shown in Fig. 2. The foam produced by a SDS solution was more stable than the foam produced by CTAB and T80 solutions; similar results were obtained with surfactant solutions at concentrations of 1.0 and 1.5%.
The results of foam volumes produced by surfactants dissolved in deionized, hard and hypersaline water showed that at the studied concentrations, the water salinity does not seem to influence the foamability of the three types of surfactant solutions.
References
Saint-Jalmes A, Durian DJ, Weitz DA (2005) Foam. In: Kirk-Othmer RE (eds) Kirk-Othmer encyclopedia of chemical technology, vol 12, pp 1–28
Physics and Astronomy Department, University of California, Los Angeles Foam. http://www.physics.ucla.edu/~dws/foam.html. Accessed Nov 2004
Bikerman JJ (1973) Foams. Springer, New York
Wilson AJ (1996) Experimental techniques for the characterization of foams. In: Prud’Homme RK, Khan SA (eds) Foams: theory, measurements, and applications, surfactant science series, vol 57. Marcel Dekker, New York, pp 243–274
Huang D, Nikolov A, Wasan D (1986) Foams: basic properties with applications to porous media. Langmuir 2:672–677
Ross S (1947) Foaming volume and foam stability. NACA-tn-1153, University of Stanford
Pandey S, Bagwe RP, Shah DO (2003) Effect of counterions on surface and foaming properties of dodecyl sulfate. J Colloid Interface Sci 267:160–166
Shrestha LK, Acharya DP, Sharma SC, Aramaki K, Asaoka H, Ihara K, Tsunehiro T, Kunieda H (2006) Aqueous foam stabilized by dispersed surfactant solid and lamellar liquid crystalline phase. J Colloid Interface Sci 301:274–281
Joseph DD (1997) Understanding foams and foamability. J Fluids Eng 5:1–8
Kibbe AH (2000) Handbook of pharmaceutical, excipients. American Pharmaceutical Association and the Washington DC and Pharmaceutical Press, London
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Amaral, M.H., das Neves, J., Oliveira, Â.Z. et al. Foamability of Detergent Solutions Prepared with Different Types of Surfactants and Waters. J Surfact Deterg 11, 275–278 (2008). https://doi.org/10.1007/s11743-008-1088-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-008-1088-0