Abstract
Sodium cardanol sulfonate surfactant was synthesized from cardanol from cashew nut shell liquid. Surfactant properties of cardanol sulfonate were determined and compared with dodecylbenzene sulfonate. Surface tension values were determined to be 32.25 mN/m for cardanol sulfonate at 20% w/v and 28.00 mN/m for dodecylbenzene sulfonate at 15% w/v. Critical micelle concentrations of dodecylbenzene sulfonate and cardanol sulfonate were found to be 0.435 and 0.372 mol/L, respectively. In comparison with dodecylbenzene sulfonate, the relative detergency of cardanol sulfonate was calculated to be 93.7% compared to dodecylbenzene sulfonate. Results suggest that cardanol sulfonate can be used as alternative anionic surfactant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cashew nut shell liquid (CNSL), which is extracted from the nut shell of the cashew tree Anacardium occidentale, occurs as a reddish brown viscous liquid in the soft honeycomb structure of the shell of the cashew nut. The cashew nut is attached to the cashew apple and is grey colored, kidney shaped and 2.5–4.0 cm in length. The shell is about 0.3 cm thick, having a soft leathery outer skin and a thin hard inner skin. Between these skins is the honeycomb structure containing the phenolic material popularly called CNSL. Inside the shell is the kernel wrapped in a thin brown skin, known as the testa. The nut thus consists of the kernel (20–25%), the shell liquid (20–25%), and the testa (2%), the rest being the shell.
CNSL is classified into two types, solvent-extracted CNSL and technical CNSL. A typical solvent-extracted CNSL contains anacardic acid (60–65%), cardol (15–20%), cardanol (10%) and traces of methyl cardol (Fig. 1). Technical CNSL is obtained by roasting shells and contains mainly cardanol (60–65%), cardol (15–20%), polymeric material (10%), and traces of methyl cardol [1].
Cardanol is a mixture of a four components: saturated (~5%), monoene (~49%), diene (l6.8%) and triene (29.3%) [1, 2]. Typical applications of cardanol include brake linings, paints and varnishes, foundry core oil, and distilled cardanol for epoxy resins and laminates. The other minor uses of cardanol are, for example, in chemically resistant cements, oil tempered hardboard, and waterproofing compounds and resins [1, 3, 4].
Nowadays, in the chemical industries, anionic surfactants such as sodium dodecylbenzene sulfonate are used extensively. The structure of cardanol is similar to dodecylbenzene in the aromatic and the hydrocarbon side chain. Cardanol obtained from CNSL, possesses a typical lipid structure with a hydrocarbon hydrophobic group and a phenolic end group. Therefore, it is expected that the products obtained from sulfonation of aromatic ring of cardanol, will have similar properties to dodecylbenzene sulfonate.
In this research, the surfactant from cardanol was prepared (Fig. 2). The different parameters such as reaction time and reaction temperature were varied to obtain the highest yield. Finally, its physicochemical properties such as solubility in hard water, surface tension, critical micelle concentration (CMC) and detergency were determined.
Experimental Procedures
Materials
Cashew nut shell liquid (CNSL) was obtained from the local market. Sodium sulfate (anhydrous), diethylenetriamine, ethyl acetate, 20% oleum, sodium hydroxide, ethanol, methanol, and acetone were purchased from Merck & Co., Inc. Thailand. Formaldehyde solution hexane and petroleum ether (40–60 °C) were obtained from Fluka Industriestrasse, Thailand, and dodecylbenzene sulfonate was obtained from the Kao Commercial (Thailand) Co. Ltd.
Synthesis of Sodium Cardanol Sulfonate
Separation of Cardanol from CNSL [2, 5]
CNSL (100 g) was added to a 800-mL beaker and then the constituent anacardic acids were decarboxylated by heating at 135–140 °C for 2–3 h. The decarboxylated CNSL (60 g), 40% formaldehyde solution (19.4 g) and diethylenetriamine (2.57 g) were mixed in methanol (200 mL). After mixing the reactants for thirty minutes, a phase separation occurred into an upper, slightly reddish solution, and a lower phase which solidified and was dark in color. The upper phase was decanted and treated with water (40 mL) followed by extraction with petroleum ether. After distillation of the solvent a reddish residue of cardanol was obtained (48 g, 80% w/w).
Sulfonation
Cardanol (10 g) was dissolved in methanol (100 mL) in a 250-mL Erlenmeyer flask and then the solution was cooled in an ice-water bath to about 10 °C. To this solution, the oleum (10.5 g) in a dropping funnel was added slowly over 2 h. The reaction mixture was allowed to reach room temperature over a period of 3 h. Then the reaction mixture was neutralized with 60 mL of 5 M aqueous sodium hydroxide at room temperature. After stirring for an hour, the mixture was extracted with hexane. The solid residue was separated from the aqueous phase and then washed with acetone. An insoluble portion was filtered off, and this solid obtained was dried to give the sodium cardanol sulfonate (11.5 g, 86.47%).
Instruments
IR spectra were recorded on a Nicolet model Impact 410 instument. NMR spectra were measured using a Bruker ACF-200 spectrometer (200 MHz for 1H and 40 MHz for 13C). The stability of the detergent in hard water was determined according to ISO 10636. The surface tension was determined using a Kruss Tensiometer Model K6 carried out according to ISO 3047. The percentage of detergency was determined by ICS 578 using a Terg-o-meter model Ueshima No. 890342 and a Spectro-colour-meter SE 2000 Nippon Denshoku.
Physicochemical Testing
The solubility in hard water, the surface tension by drawing up liquid films and critical micellization concentration were determined using international standard ISO1063 [6], international standard ISO304 [7] and an international standard ISO3411 [8], respectively.
The Thai Industrial Standard ICS578-2540 [9] was used for the determination of the detergency of the products. This method is used for evaluation of the ability of a detergent or formulation to remove carbon powder as an artificial soil deposited on cotton cloth or a clay soil deposit on cotton cloth.
For preparation of sample solutions and determination of detergency, the percentages of detergency of the finished products were in the range of 30–35%. The concentration of the solution was 0.08% w/w, therefore, 22.85 g of dodecylbenzene sulfonate or cardanol sulfonate was weighed. Then water was added to each sample to make a total weight of 1,000 g. The mixture was stirred for 10 min. About 100 mL of each sample solution was placed in the terg-o-meter and the solution was diluted with water to make a total volume of 1,000 mL. The process was carried out at temperature of 30 °C, a spinning rate of 100 rpm and a process time of about 10 min. Before washing, the cottons with the clay soil of 5 pieces per sample were test to detect their reflectance by a spectro-colour-meter SE 2000. After washing, the cottons were dried and tested for reflectance once again.
Results and Discussion
Cardanol was obtained by decarboxylation of CNSL at 135–140 °C for 3 h and the product was investigated by IR and NMR spectroscopy. The infrared spectrum of decarboxylated CNSL showed no absorption signal of C=O stretching of carboxylic acid at 1,660 cm−1. The 13C-NMR spectrum of decarboxylated CNSL also showed no carbonyl signal of the carboxylic group at δ C 174.2 ppm.
Cardanol was isolated from the decarboxylated residue using Tyman’s method [5] in 80% w/w from the decarboxylated CNSL. It was identified using spectroscopic techniques. The infrared spectrum of cardanol showed absorption peaks at 3,390 cm−1 (O–H stretching of phenol), 3,006 cm−1 (=C–H stretching of aromatic), 2,940 and 2,846 cm−1 (C–H stretching of aliphatic), 1,592 cm−1 (C=C ring stretching of aromatic), and 1,160 cm−1 (C–O stretching). The 1H-NMR spectrum of cardanol showed signals at δH 6.73, 6.81, 7.18 and 6.71 ppm, which belonged to protons at c, e, f and g (Fig. 3), respectively. In addition, the spectrum also dispayed signals of methylene protons adjacent to the aromatic at δH 2.66 ppm, olefinic protons of side chain at δH between 5.09 and 5.98 ppm, allylic methylene protons at δH 2.13, diallylic methylene protons at δH 2.92 and methyl protons at δH 1.01 ppm. The 13C-NMR spectrum of cardanol showed signals at δC 155.7, 115.7, 144.3, 121.4, 129.9 and 112.9 ppm, which are belonged to carbons at b, c, d, e, f and g, respectively (Fig. 3). Moreover, the spectrum also demonstrated signals of sp 3 methylene and methyl carbons at δC 14.6–37.0 and sp 3 carbons of the side chain at δC 115.1–137.1 ppm. The spectroscopic data can be confirmed that separation of the decarboxylated CNSL gave cardanol.
Sulfonation of Cardanol
Cardanol was treated with oleum to give cardanol sulfonate in 86% and cardanol sulfonate was analyzed using 1H-NMR, 13C-NMR and IR spectroscopy. The spectral data of cardanol sulfonate suggested that the sulfonation occurred at the ortho and para positions of a phenolic group of cardanol. The infrared spectrum of cardanol sulfonate showed absorption peaks at 1,174 cm−1 (SO2 stretching). The 1H-NMR spectrum of cardanol sulfonate showed signals at δH 6.7, 7.1 and 6.8 ppm, which belonged to protons at c, f and g, respectively. In addition, the spectrum also demonstrated signals of methylene protons adjacent to the aromatic at δH 2.3–2.8 ppm, olefinic protons in side chain at δH 4.9–5.8 ppm, methyl protons at δH 0.71–0.85 ppm and proton of sulfonic group at 6.32 ppm. The 13C-NMR spectrum of cardanol sulfonate showed signals at δC 157, 116, 130, 121, 130 and 116 ppm, which are belonged to carbons at b, c, d, e, f and g, respectively (Fig. 3). Also, the spectrum also demonstrated signals of methylene and methyl groups at δC 22–36 ppm and double bonds in the aromatic ring and side chain at δC 127–137 ppm. It can be confirmed that cardanol was sulfonated to cardanol sulfonate.
Physical Properties of Cardanol Sulfonate Compared with Dodecylbenzene Sulfonate
Determination of Solubility in Hard Water
Solubility in hard water for dodecylbenzene sulfonate (DDBS) and cardanol sulfonate (CDS) was determined using ISO 1063. The results are shown in Table 1. It can be seen that the score of solubility in hard water for dodecylbenzene sulfonate and cardanol sulfonate were compared. The results suggested that the toleration ability against calcium salts of DDBS and CDS are exceptionally similar.
Determination of Surface Tension
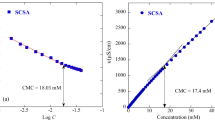
The surface tensions of DDBS and CDS were determined by ISO 304. The results are shown in Fig. 4. Surface tension values of dodecylbenzene sulfonate and cardanol sulfonate decreased when the concentration of the surfactant solution increased. DDBS gave the minimum surface tension (28 mN/m) at −0.361 (15% w/v) while CDS gave the minimum surface tension (32.25 mN/m) at −0.304 (20% w/v) (Fig. 4). The results demonstrate that the surface tension reduction of cardanol sulfonate was not significantly different from dodecylbenzene sulfonate and it was acceptable to be used for commercial application like common anionic surfactants.
Determination of the Critical Micelle Concentration (CMC)
The critical micelle concentrations of DDBS and CDS were determined using ISO 3411. From a surface tension of detergent—log C plot illustrating the surface tension reduction, the result suggested that the surface tension of a CDS solution decreased steadily with increasing CDS concentration until the concentration reached a value known as the critical micelle concentration. The surface tension of cardanol sulfonate at this concentration is therefore very close to the minimum surface tension. From the surface tension of the two cases, the C.M.C. value of DDBS and CDS were determined to be 0.435 and 0.372 mol/L, respectively (Fig. 4). The results indicated that the CMC of the two cases was not so different from dodecylbenzene sulfonate. Therefore it may be used in place of dodecylbenzene sulfonate.
Determination of the Percentage Detergency
Detergency values of dodecylbenzene sulfonate and cardanol sulfonate were determined by the ICS method. The results are shown in Table 2. Reflectance of dodecylbenzene sulfonate and cardanol sulfonate were determined to be 78.1 and 73.2%, respectively. Using the dodecylbenzene sulfonate as reference, the percentage detergency of CDS was calculated to be 93.7%. These results were in agreement with the ICS standard, which specifies that washing powder should have over 80% detergency when compared with the reference. Therefore, cardanol sulfonate can be accepted for use as a detergent.
In conclusion, the surfactant properties of cardanol sulfonate were found to be similar to those of dodecylbenzene sulfonate; therefore, cardanol sulfonate may be used as a raw material for commercial detergent production. Cardanol sulfonate may probably be produced on an industrial scale because of sulfonation of cardanol gave 86.47% and the production process of cardanol sulfonate was not complicated because there is no difficult step of alkylation of benzene before sulfonation like common alkylbenzene sulfonate. Production of cardanol sulfonate on an industrial scale is of low cost in comparison with the production of common detergents.
References
Phani PK, Paramashivappa R, Vithayathil PJ, Subba Rao PV, Srinivasa Rao A (2002) Process for isolation of cardanol from technical cashew (Anacardium occidentale L.) nut shell liquid. J Agric Food Chem 50:4705
Menon ARR, Pillai CKS, Sudha JD, Mathew AG (1985) Cashew nut shell liquid. Its polymeric and other industrial products. J Sci Ind Res 44:324
Nieckarz G, Carlson F, Detlefsen W (1998) Wood-product laminated composites. Borden Chemical, Inc. U.S. Patent.6(132)549
Mizunuma T, Tanaka S, Tamaki R, Funada H, Taniguchi T, Sasaki H (1996) Asphalt additive and asphalt composition J.P. 5,721,296
Tyman, JHP (1985) Purification of cardanol G.B. 2,152,925 A
International Organization for Standardization (1969) Specification for determination of stability in hard water. ISO1063
International Organization for Standardization (1963) Specification for determination of surface tension and interfacial tension. ISO304
International Organization for Standardization (1979) Specification for determination of the critical micelle concentration-method by measuring surface tension with a plate, stirrup or ring. ISO3411
Thai Industrial Standard (1997) Methods of analysis and test for laundry detergent powder ICS578-2540
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Peungjitton, P., Sangvanich, P., Pornpakakul, S. et al. Sodium Cardanol Sulfonate Surfactant from Cashew Nut Shell Liquid. J Surfact Deterg 12, 85–89 (2009). https://doi.org/10.1007/s11743-008-1082-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-008-1082-6