Abstract
Admission hyperglycemia (AH) is associated with worse prognosis in patients with acute myocardial infarction (AMI). Controversy remains whether the impact of AH differs among patients previously diagnosed with diabetes mellitus (DM). We retrospectively evaluated consecutive patients admitted in a coronary care unit with AMI, from 2006 to 2014. Patients were divided into 4 groups: patients without known DM with admission glycemia (AG) ≤ 143 mg/dL (group 1), patients without known DM with AG > 143 mg/dL (group 2), known DM with AG ≤ 213 mg/dL (group 3), and known DM with AG > 213 mg/dL (group 4). Primary outcome was defined as all-cause mortality during follow-up. A total of 2768 patients were included: 1425 in group 1, 426 in group 2, 593 in group 3, and 325 in group 4. After a median follow-up of 5.6 years, 1047 (37.8%) patients reached primary outcome. After multivariate analysis, group 4 was associated with the worst prognosis (HR 3.103, p < 0.001) followed by group 3 (HR 1.639, p = 0.002) and group 2 (HR 1.557, p = 0.039), when compared to group 1. When groups were stratified by type of AMI, patients in group 2 had a worse prognosis than patients in group 3 in the case of non-ST-segment elevation AMI. AH is associated with higher all-cause mortality in patients with AMI, irrespective of previous diabetic status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic heart disease, with acute coronary syndrome (ACS) as its main manifestation, is the leading cause of death worldwide. Despite the recent decline in mortality in patients with acute myocardial infarction (AMI), there is still room for further improvement in care [1].
Previous studies have shown that admission hyperglycemia (AH) is not only common in patients presenting with AMI but is also associated with a worse outcome after hospital discharge [2, 3]. While patients with DM are known to have a worse short- and long-term prognosis when compared to patients without DM [4, 5], there remains controversy whether the effect of AH is different between patients with previously known diabetes mellitus (DM) and those without the disease. To fuel this controversy, studies performed on the impact of AH in the prognosis of AMI had great heterogeneity [3, 6]. Many had varying definitions of AH, different inclusion criteria frequently excluding patients with DM, and different access to revascularization therapy. As such, disparities between studies have limited conclusions and consensus in the matter.
In this study, we sought to analyze the impact of AH on all-cause mortality of patients with and without DM admitted with AMI in a tertiary center contemporary cohort.
Methods
Study population
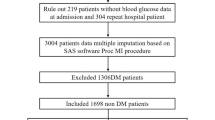
We conducted a retrospective study based on data from a single tertiary center. A total of 4792 patients with a diagnosis of AMI [ST-segment elevation myocardial infarction (STEMI) and non-ST-elevation myocardial infarction (NSTEMI)] admitted to our coronary care unit (CCU) between January 2006 and February 2017 were identified. A total of 1305 patients without admission blood glucose information, 513 repeat patients, and 244 patients without information regarding previous DM status were excluded from the analysis. All patients were admitted to the emergency department (ED) before admission to our CCU. Patients were treated with optimal medical therapy for AMI in accordance with the most recent European Society of Cardiology (ESC) ACS guidelines [7, 8]. Patients were divided into four groups according to serum glucose level on admission and previously known DM status: patients without known DM with admission glycemia (AG) ≤ 143 mg/dL (group 1), patients without known DM with AG > 143 mg/dL (group 2), patients with known DM with AG ≤ 213 mg/dL (group 3), and patients with known DM with AG > 213 mg/dL (group 4). A flowchart of the study population is shown in Fig. 1.
Data collection and variables
All patients performed a 12-lead electrocardiogram on admission to the ED. We collected and analyzed the following patient data: demographic and clinical characteristics (age, gender, previous medical history, and previous drug use), clinical and analytical findings (complete blood count, creatinine, lipid profile, glucose, and troponin I) on admission in the ED and during hospitalization, drug use during hospitalization and at discharge, interventions during hospitalization, reperfusion therapy and coronary angiography characteristics, echocardiographic findings, and other interventions during hospitalization. All patients met criteria for AMI according to the fourth Universal Definition of Myocardial Infarction [9]: clinical evidence of acute myocardial ischemia and detection of a rise and/or fall of cardiac troponin values with at least one value above the 99th percentile upper reference limit and at least one of the following: (1) symptoms of myocardial ischemia; (2) new ischemic ECG changes; (3) development of pathological Q waves; (4) imaging evidence of new loss of viable myocardium or new wall motion abnormality consistent with ischemic etiology; (5) identification of a coronary thrombus by angiography or autopsy.
Previous DM was defined as having a previous history of DM treated with diet only, oral antidiabetic drugs, and/or insulin, based on previous clinical history, medication, and analytical parameters obtained from hospital and primary care physicians records. DM diagnosis during hospitalization was made when the patient fulfilled 1 of the following criteria: (1) fasting plasma glucose levels ≥ 126 mg/dL, 2-h plasma glucose levels ≥ 200 mg/dL after oral glucose tolerance test (at 2 h after drinking 75 g of glucose, in patients without known DM), or glycosylated hemoglobin (HbA1c) ≥ 6.5%, in accordance with the most recent American Diabetes Association consensus [10]. All laboratory criteria were repeated to confirm a diagnosis.
AH was defined as an admission glycemia ≥ 143 mg/dL for patients without known DM and ≥ 213 mg/dL for patients with known DM, based on receiver-operating characteristic (ROC) curve analysis and Youden index, performed to assess the discriminative power of the different values of AG in estimating the probability of death during follow-up (Table S1 and Figure S1 of Supplemental material).
Study endpoint
The primary objective of our study was to evaluate the impact of different AH levels on all-cause mortality during follow-up. All patients were followed until February 2017. Follow-up information was obtained by reviewing hospital records and the official national vital registry. Time to the occurrence of death was defined from the date of admission.
Statistical analysis
Both the study population and the four groups were characterized regarding categorical variables using absolute frequencies and relative frequencies; for continuous variables, the central tendency of data was characterized by sample mean or median; for this type of variables, data dispersion was characterized by standard deviation, in the case of sample mean, or by interquartile range, in the case of median. Normal distribution was assessed from histograms, P–P plots, and Kolmogorov–Smirnov test.
A comparison was made between the four groups. Regarding categorical variables, the comparison was performed using the χ2 test or Fisher’s exact test when the assumptions of the former were not met. Continuous variables were compared concerning means using the T test for independent samples and regarding medians using the non-parametric Mann–Whitney test. The survival functions of the four groups to be compared were estimated using Kaplan–Meier method and represented in the form of Kaplan–Meier curves and compared using the Log-Rank test. Additionally, to identify a possible association of AH with all-cause death during follow-up, we adjusted Cox regression models, first only including AG (univariable model). Afterward, multivariable Cox regression models were adjusted for possible confounders (variables with a P value < 0.05 between groups—all covariates are shown in Table 3). In the Cox model, we considered the Stepwise Forward variable selection method with likelihood ratio test, and the effect of the variables was evaluated by estimating the hazard ratio and its confidence interval. Group 1 was the reference in all comparisons. In every Cox model, we tested for first-order interactions between covariables and each prognosis variable being tested. Risk was expressed as hazard ratio (HR) and their 95% confidence interval (CI).
All statistical analysis was performed at the 5% significance level. The analysis was conducted using IBM® SPSS® Statistics, version 26.0 software. The authors had full access to data and take full responsibility for its integrity.
Results
Baseline characteristics
A total of 2768 patients were included in the final analysis, of which 33.2% (n = 918) were patients with previously known DM. The distribution of patients among groups was as follows: 1424 patients in group 1, 426 in group 2, 593 in group 3, and 325 patients in group 4 (Fig. 1). The median (interquartile range) AG levels were 127 (67) mg/dL for the entire cohort, 107 (25) mg/dL for patients in Group 1, 167 (49) mg/dL for patients in Group 2, 146 (59) mg/dL for patients in Group 3, and 286 (98) mg/dL for patients in Group 4. A total of 18.2% (n = 504) were diagnosed with DM during hospitalization, 18.8% (n = 267) in group 1 and 55.6% (n = 237) in group 2.
Median AG was higher in STEMI patients when compared to NSTEMI patients [138 interquartile range (IQR) 72 vs. 118 IQR 61, mg/dL, p < 0.001]. Median follow-up time was 5.6 (IQR 4.3) years. Mean cohort age was 68 ± 13 years, and 68.2% were male and had a mean body mass index of 27.6 ± 4.5 kg/m2.
Compared with those of group 1, patients in groups 3 and 4 had a higher incidence of arterial hypertension, dyslipidemia, previous AMI, previous PCI, previous coronary artery bypass grafting (CABG), previous stroke/TIA, and peripheral artery disease. By contrast, patients in groups 1 and 2 had a higher incidence of active smoking, being predominantly male (Table 1).
Compared with groups 1 and 3, patients in groups 2 and 4 registered a higher rate of Killip–Kimball (KK) class ≥ 2, higher release of troponin I, higher CRP, and lower LVEF values at admission. Creatinine values were higher in groups 3 and 4, as well as HbA1c at admission. Patients in the same groups presented with lower hemoglobin, high-density lipoprotein, and low-density lipoprotein (Table 1).
Different groups registered some differences regarding cardiovascular drug use during stay. Although there was no difference regarding the use of aspirin or P2Y12 inhibitors, the need for use of glycoprotein IIb/IIIa inhibitors and inotropic/vasopressors was higher in groups 2 and 4, compared to other groups. Also, beta-blocker and angiotensin-converting enzyme inhibitor prescription were lower in groups 2 and 4, when compared to groups 1 and 3. Diuretic use was higher in groups 2, 3, and 4 when compared with group 1 (Table 2).
There was no difference in access to coronary angiography between groups. Left anterior descending artery lesions were more common in groups 2 and 4, and 3-vessel disease was more common in group 4 when compared to other groups. Percutaneous coronary intervention was performed more often in groups 2 and 4 when compared to other groups (Supplementary Material, Table S2).
Primary outcome
All-cause mortality was recorded in 37.8% (n = 1047) of patients. Unadjusted all-cause mortality during follow-up, when compared to group 1, was the highest in group 4 [Hazard ratio (HR) 2.145, CI 95% 1.790–2.570, p < 0.001], followed by group 3 (HR 2.045, 95% CI 1.758–2.380, p < 0.001) and group 2 (HR 1.757, 95% CI 1.478–2.088, p < 0.001). After multivariate analysis, group 4 was still associated with the worst prognosis (HR 3.103, 95% CI 2.000–4.814, p < 0.001) followed by group 3 (HR 1.639, 95% CI 1.191–2.255, p = 0.002) and finally group 2 (HR 1.557, CI 95% 1.024–2.369, p = 0.039). As we found a significant interaction between groups and type of AMI (NSTEMI or STEMI), we plotted the impact of different AG and diabetes status stratified by type of AMI. In patients with NSTEMI, AG and diabetic status were more potent predictors of all-cause mortality than in patients with STEMI. When compared to group 1, group 4 remained associated with the worst prognosis (HR 3.457, 95% CI 2.143–5.575, p < 0.001), followed by Group 2 (HR 2.177, 95% CI 1.334–3.553, p = 0.002) and Group 3 (HR 1.888, 95% CI 1.353–2.636, p < 0.001). In the case of patients admitted with STEMI, group 4 presented the worse prognosis (HR 2.132, 95% CI 1.226–3.710, p = 0.007); however, the prognosis of patients in group 3 (HR 1.461, 95% CI 0.878–2.431, p = 0.144) and group 2 (HR 1.282, 95% CI 0.797–2.062, p = 0.306) was not significantly different than patients in group 1. When analyzing fasting plasma glucose as a continuous variable, adjusted for the same variables entered in the main analysis, we observed that an increase in 50 mg/dL was associated with a 1.10-fold increase in the hazard of reaching primary outcome (HR 1.002, CI 95% 1.001–1.004, p = 0.008). Performing the same analysis for admission glucose as a continuous variable, we observed that an increase in 50 mg/dL of admission glucose was associated with a 1.16-fold increase in the hazard of reaching the primary outcome (HR 1.003, CI 95% 1.002–1.005, p < 0.001).
When trying different group cut-offs of AH in the multivariate analysis such as the cut-offs suggested by Capes et al. [6] (144 mg/dL for patients without DM and 180 mg/dL for patients with DM), the cut-offs suggested by Wahab et al. [11] (198 mg/dL for patients with or without previous DM) and the cut-offs suggested by Scott et al. [12] (5.6 mmol/L and 11 mmol/L), the same relationships between groups and similar survival curves were observed, with group 4 having the worse prognosis, followed by group 3 and finally group 2, when compared to group 1.
Regarding patients with a new diagnosis of DM during hospitalization, unadjusted all-cause mortality during follow-up was higher when compared to patients without DM (HR 1.298, 95% CI 1.089–1.547, p = 0.004). However, after multivariate analysis, all-cause mortality of the patients with a new diagnosis of DM was no longer different than patients without DM (HR 1.085, 95% CI 0.722–1.632, p = 0.694).
Univariate and multivariate analysis are depicted in Table 3, and Kaplan–Meier curves stratified by AMI subtype can be seen in Fig. 2. Adjusted survival curves of different groups are shown in Fig. 3.
Adjusted survival curves of patients admitted with acute myocardial infarction, stratified by previous diabetic status and admission glycemia. a General cohort. b NSTEMI patients. c STEMI patients. Adjustment variables can be seen in Table 3
Discussion
In the present study, we analyzed a sample with one of the highest age-adjusted comparative prevalence of DM in Europe and we focused on the impact of AG on the prognosis of patients admitted with AMI, grouped by two AG cut-point values (143 mg/dL and 213 mg/dL) and previously known diabetic status. We found that AG is a strong predictor of all-cause mortality, both in patients with and without DM. In our study, patients with known DM and AG > 213 mg/dL had the highest mortality after hospital discharge, followed by patients with known DM and AG ≤ 213 mg/dL and patients without known DM and AG > 143 mg/dL, with both group 2 and 3 having a similar prognosis. This effect of AG on prognosis remained true after adjustment for other variables known to affect the prognosis of AMI patients, such as age, LVEF, kidney function, and troponin release measured at admission. The impact of AG remained significant in NSTEMI but only in patients with known DM in STEMI. Therefore, in the contemporary era of percutaneous revascularization, AH may be a stronger predictor of long-term mortality than a previous history of DM.
Multiple studies have shown the effect of hyperglycemia on ischemic myocardium. It has been shown that acute hyperglycemia abolishes the cardioprotective effect of ischemic preconditioning by inhibiting Akt phosphorylation in mice [13]. The same phenomenon was also observed in dogs after a prolonged coronary artery occlusion and modest degrees of hyperglycemia. In the same study, profound hyperglycemia not only abolished preconditioning but also increased infarct size [14]. Hyperglycemia was also associated with elevated systolic and diastolic blood pressures as well as with QT interval prolongation, changes reverted after glycemia correction [15]. Timmer et al. [16] have associated hyperglycemic STEMI patients with a lower rate of spontaneous reperfusion and Iwakura et al. [17] showed a higher incidence of the no-reflow phenomenon in patients with hyperglycemia after successful reperfusion therapy. Also, Torella et al. [18] found that myocardial carbonic anhydrase is significantly elevated in human diabetic ischemic cardiomyopathy, which is associated with the hyperactivation of the Na+/H+ exchanger 1 to promote cardiomyocyte hypertrophy, myocardial dysfunction, apoptosis, and failure. Several studies have also suggested that hyperglycemia is associated with a prothrombotic state, through lower tissue plasminogen activity [19], increased platelet aggregation [20], and increased vascular inflammation [21]. Recently, it has been found that thromboxane-dependent platelet activation was at least as high in impaired glucose tolerance as in patients with DM and further increased over time [22]. This finding can potentially explain the similar prognosis of patients in group 2 and group 3. However, a question arises whether hyperglycemia is a real mediator of adverse outcomes or a marker of high-risk AMI. As found in a recent study, hyperglycemia was more common in patients with DM, older patients who arrived at the ED with STEMI [23]. Stubbs et al. [24] found that plasma cortisol and insulin levels were both positively correlated with glucose concentrations, and in the specific case of cortisol, the hormone concentrations were also correlated to the infarct size. Likewise, in the 2015 meta-analysis performed by Singh et al. [2] which analyzed 12 studies, the authors found that AH occurred in patients who had larger infarcts, multivessel disease, anterior myocardial infarction, and history of previous AMI. These findings are in line with ours, as we also found that patients with AH had similar markers of increased risk, except for previous AMI.
AH has been commonly recorded in patients presenting with AMI. The incidence of AH ranges from 11.3 to 65.6%, depending on the cut-off used for diagnosis. This variability of the cut-off for the diagnosis of AH is a limitation to the generalization of study findings. In 2008, The American Heart Association Diabetes Committee defined acute hyperglycemia in the context of AMI as an admission glycemia of > 140 mg/dL [25]. On the other hand, the American Diabetes Association recommends that insulin therapy should be initiated in persistent hyperglycemia starting at a threshold of ≥ 180 mg/dL [9]. As the definition of AH is not clear, the methodological considerations of previous studies were not coherent. As such, different cut-offs were used, ranging from 110 to 306 mg/dL [2]. The cut-off values used in our study were considered after the meta-analysis of 19 articles by Capes et al. [10], which stated that in patients without DM, glucose concentrations more or equal to range 110–144 mg/dL were associated with a higher risk of death than in patients without DM and concentrations higher than values in the range of 144–180 mg/dL on admission were associated with increased risk of congestive heart failure or cardiogenic shock in patients without DM. Also, the same meta-analysis found that in patients with DM, glucose concentrations more than or equal to the range 180–198 mg/dL were associated with an increased risk of death. Our cut-offs were similar to a recent study which found that 178 mg/dL was the optimal cut-off for patients with DM and 145 mg/dL for patients without DM [26]. However, it is important to note that changing between different threshold values for AH did not significantly change our results.
Previous studies have found AH to be associated with a worse prognosis. In a prospective study performed in the thrombolytic era, Wahab et al. [11] found hyperglycemia upon admission in 8.1% of patients without known diabetes and in 16.9% of patients with known diabetes, being associated with poor outcome, even in patients without known diabetes. In another study that analyzed 834 consecutive STEMI patients, the majority treated with fibrinolytic agents, the incidence of admission hyperglycemia was 79.8% in patients with DM and 42.8% in patients without DM. However, despite being found a predictor of mortality and arrhythmias, outcomes were not affected by diabetic status [27]. In the classic DIGAMI study, hyperglycemia was associated with a higher mortality rate in patients treated with fibrinolytic agents [28]. In a more contemporary setting, other studies reached similar conclusions. Ishihara et al. [29] found that, in a population of AMI patients vastly treated by PCI, admission hyperglycemia was still associated with short-term mortality. Recently, Hao et al. [26] found that, in NSTEMI patients undergoing PCI, AH may be used to predict 30-day and 3-year MACEs, irrespective of diabetic status. In a larger retrospective study with 1698 AMI patients, Ding et al. [30] found that the all-cause in-hospital mortality risk increased in patients without DM with AH, especially in patients with AG ≥ 180 mg/dL.
We found that patients with AH were more likely to be active smokers, presented more frequently with STEMI and KK class ≥ 2, had lower LVEF during hospitalization, showed more frequently left anterior descending artery lesions, and had larger troponin release. The same patients also registered more frequently a no-reflow phenomenon after PCI, with the need for rescue therapy with Gp IIb/IIIa inhibitors and were more frequently prescribed diuretics and inotropic/vasopressor drugs during hospitalization. Our findings go in line with other studies that found AH to be associated with cardiogenic shock [31, 32], heart failure [33], STEMI [34, 35], larger infarcts [32], atrial fibrillation [35], and no-reflow after PCI [16].
Previous studies have reported a high prevalence of undiagnosed DM in AMI patients. Giraldez et al. [36] reported that, in a population high-risk NSTEMI, the prevalence of undiagnosed diabetes was 12.2%. Another study found that when AMI patients with AG < 200 mg/dL and without previous DM were tested by an oral glucose tolerance after discharge, 40% turned out to be glucose intolerant and 25% had undiagnosed diabetes [37]. In our study, 18.2% of patients had a new diagnosis of diabetes during hospitalization, particularly in patients without known DM and AG > 143 mg/dL where 55.6% had a new diagnosis of DM. However, after multivariate analysis, the prognosis of this group of patients was not significantly different from patients without DM. This finding can be partially explained by the shorter course of the disease in these patients and exposure to lower glycemia, as expressed by HbA1c levels during hospitalization. As a group, patients with a new diagnosis of DM during hospitalization showed some similar clinical characteristics to patients without DM, reflected in no significant differences regarding age, gender, GRACE score, creatinine at admission, and days of hospitalization. On the other side, they show more significant coronary lesions, higher troponin release at admission, have a higher incidence of atrial fibrillation during hospitalization, and present to the ED with a higher Killip–Kimball class. Further research on this issue is warranted.
Study limitations
Our study has a retrospective design and, as such, has the inherent limitations of such studies. Thus, despite using multivariable analysis with adjustment for potential confounders, we cannot exclude some degree of residual confounding. First, because we defined groups as previous or current diagnosis of DM at the time of ED admission, we did not discriminate in the main analysis patients with undiagnosed DM. As such, this specific group of patients might influence our results. However, in a sub-analysis of those patients, we found that the long-term prognosis of patients with non-diagnosed DM was not significantly different than patients with euglycemic levels at admission and no previous DM diagnosis. Also, the prevalence of patients with undiagnosed DM was similar to other studies. Second, we did not include in the analysis the in-hospital management of hyperglycemia, which may have an impact on long-term mortality, as found in other studies [28, 38]. In particular, Marfella et al. [39] found that in hyperglycemic patients with STEMI, an intensive peri-procedural glycemic control may improve the outcome after PCI, reducing oxidative stress and inflammation levels with a lower rate of in-stent restenosis. However, the clinical practice in our unit meets the latest guidelines for the treatment of hyperglycemia in the context of acute myocardial infarction, so we have no reason to suspect a bias created by the lack of this information. Third, our study was conducted on the Portuguese population which, in November 2017, had the third-highest age-adjusted comparative prevalence of DM in Europe [40]. This can lead to different results in other populations with different DM prevalence and may limit the reproducibility of our findings.
Conclusion
In patients with a high prevalence of DM hospitalized with AMI, admission hyperglycemia is associated with higher long-term all-cause mortality, irrespective of previous diabetic status and type of AMI, and is a strong predictor of all-cause mortality after AMI.
Data availability
The data sets generated during and/or analyzed during this study are available on request.
References
Dégano IR, Salomaa V, Veronesi G et al (2015) Twenty-five-year trends in myocardial infarction attack and mortality rates, and case-fatality, in six European populations. Heart 101(17):1413–1421 (published correction appears in Heart. 2018 Aug;104(16):e2)
Angeli F, Reboldi G, Poltronieri C et al (2015) Hyperglycemia in acute coronary syndromes: from mechanisms to prognostic implications. Ther Adv Cardiovasc Dis 9(6):412–424
Singh K, Hibbert B, Singh B et al (2015) Meta-analysis of admission hyperglycaemia in acute myocardial infarction patients treated with primary angioplasty: a cause or a marker of mortality? Eur Heart J Cardiovasc Pharmacother 1(4):220–228 (published correction appears in Eur Heart J Cardiovasc Pharmacother. 2016 Oct;2(4):217)
Mukamal KJ, Nesto RW, Cohen MC et al (2001) Impact of diabetes on long-term survival after acute myocardial infarction: comparability of risk with prior myocardial infarction. Diabetes Care 24(8):1422–1427
Abbott RD, Donahue RP, Kannel WB, Wilson PW (1988) The impact of diabetes on survival following myocardial infarction in men vs women. The Framingham Study. JAMA 260(23):3456–3460 (published correction appears in JAMA 1989 Apr 7;261(13):1884)
Capes SE, Hunt D, Malmberg K, Gerstein HC (2000) Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 355(9206):773–778
Ibanez B, James S, Agewall S et al (2018) 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39(2):119–177
Roffi M, Patrono C, Collet JP et al (2016) 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 37(3):267–315
Thygesen K, Alpert JS, Jaffe AS et al (2018) Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 72(18):2231–2264
American Diabetes Association (2020) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care 43(Suppl 1):S14–S31
Wahab NN, Cowden EA, Pearce NJ et al (2002) Is blood glucose an independent predictor of mortality in acute myocardial infarction in the thrombolytic era? J Am Coll Cardiol 40(10):1748–1754
Scott AR, Cheng A, Greenacre M et al (2007) Implications of hyperglycaemia and ethnicity in patients with acute coronary syndromes in New Zealand. Diabetes Obes Metab 9(1):121–126
Yang Z, Tian Y, Liu Y, Hennessy S, Kron IL, French BA (2013) Acute hyperglycemia abolishes ischemic preconditioning by inhibiting Akt phosphorylation: normalizing blood glucose before ischemia restores ischemic preconditioning. Oxid Med Cell Longev 2013:329183
Kersten JR, Schmeling TJ, Orth KG, Pagel PS, Warltier DC (1998) Acute hyperglycemia abolishes ischemic preconditioning in vivo. Am J Physiol 275(2):H721–H725
D’Amico M, Marfella R, Nappo F et al (2001) High glucose induces ventricular instability and increases vasomotor tone in rats. Diabetologia 44(4):464–470
Timmer JR, Ottervanger JP, de Boer MJ et al (2005) Hyperglycemia is an important predictor of impaired coronary flow before reperfusion therapy in ST-segment elevation myocardial infarction. J Am Coll Cardiol 45(7):999–1002
Iwakura K, Ito H, Ikushima M et al (2003) Association between hyperglycemia and the no-reflow phenomenon in patients with acute myocardial infarction. J Am Coll Cardiol 41(1):1–7
Torella D, Ellison GM, Torella M et al (2014) Carbonic anhydrase activation is associated with worsened pathological remodeling in human ischemic diabetic cardiomyopathy. J Am Heart Assoc 3(2):e000434
Pandolfi A, Giaccari A, Cilli C et al (2001) Acute hyperglycemia and acute hyperinsulinemia decrease plasma fibrinolytic activity and increase plasminogen activator inhibitor type 1 in the rat. Acta Diabetol 38(2):71–76
Gresele P, Guglielmini G, De Angelis M et al (2003) Acute, short-term hyperglycemia enhances shear stress-induced platelet activation in patients with type II diabetes mellitus. J Am Coll Cardiol 41(6):1013–1020
Pechlivani N, Ajjan RA (2018) Thrombosis and vascular inflammation in diabetes: mechanisms and potential therapeutic targets. Front Cardiovasc Med 5:1
Santilli F, Zaccardi F, Liani R et al (2020) In vivo thromboxane-dependent platelet activation is persistently enhanced in subjects with impaired glucose tolerance. Diabetes Metab Res Rev 36(2):e3232
Cinar H, Avci A, Gulen M, Avci BS, Comertpay E, Satar S (2019) Does stress hyperglycemia affect mortality? Acute myocardial infarction—case control study. Arch Med Sci Atheroscler Dis 4:e201–e207
Stubbs PJ, Laycock J, Alaghband-Zadeh J, Carter G, Noble MI (1999) Circulating stress hormone and insulin concentrations in acute coronary syndromes: identification of insulin resistance on admission. Clin Sci (Lond) 96(6):589–595
Deedwania P, Kosiborod M, Barrett E et al (2008) Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 117(12):1610–1619 (published correction appears in Circulation. 2010 Jun 15;121(23):e444)
Hao Y, Lu Q, Li T, Yang G, Hu P, Ma A (2017) Admission hyperglycemia and adverse outcomes in diabetic and non-diabetic patients with non-ST-elevation myocardial infarction undergoing percutaneous coronary intervention. BMC Cardiovasc Disord 17(1):6
Sanjuán R, Núñez J, Blasco ML et al (2011) Prognostic implications of stress hyperglycemia in acute ST elevation myocardial infarction. Prospective observational study. Rev Esp Cardiol 64(3):201–207
Malmberg K, Rydén L, Efendic S et al (1995) Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol 26(1):57–65
Ishihara M, Kagawa E, Inoue I et al (2007) Impact of admission hyperglycemia and diabetes mellitus on short- and long-term mortality after acute myocardial infarction in the coronary intervention era. Am J Cardiol 99(12):1674–1679
Ding XS, Wu SS, Chen H, Zhao XQ, Li HW (2019) High admission glucose levels predict worse short-term clinical outcome in non-diabetic patients with acute myocardial infraction: a retrospective observational study. BMC Cardiovasc Disord 19(1):163
Zeller M, Cottin Y, Brindisi MC et al (2004) Impaired fasting glucose and cardiogenic shock in patients with acute myocardial infarction. Eur Heart J 25(4):308–312
Eitel I, Hintze S, de Waha S et al (2012) Prognostic impact of hyperglycemia in non-diabetic and diabetic patients with ST-elevation myocardial infarction: insights from contrast-enhanced magnetic resonance imaging. Circ Cardiovasc Imaging 5(6):708–718
Puymirat E, Riant E, Aissaoui N et al (2016) β blockers and mortality after myocardial infarction in patients without heart failure: multicentre prospective cohort study. BMJ 354:i4801 (published correction appears in BMJ. 2016 Oct 17;355:i5602)
Schiele F, Descotes-Genon V, Seronde MF et al (2006) Predictive value of admission hyperglycaemia on mortality in patients with acute myocardial infarction. Diabet Med 23(12):1370–1376
Petursson P, Herlitz J, Caidahl K et al (2007) Admission glycaemia and outcome after acute coronary syndrome. Int J Cardiol 116(3):315–320
Giraldez RR, Clare RM, Lopes RD et al (2013) Prevalence and clinical outcomes of undiagnosed diabetes mellitus and prediabetes among patients with high-risk non-ST-segment elevation acute coronary syndrome. Am Heart J 165(6):918-925.e2
Norhammar A, Tenerz A, Nilsson G et al (2002) Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet 359(9324):2140–2144
Sasso FC, Rinaldi L, Lascar N et al (2018) Role of tight glycemic control during acute coronary syndrome on CV outcome in type 2 diabetes. J Diabetes Res 2018:3106056
Marfella R, Sasso FC, Siniscalchi M et al (2012) Peri-procedural tight glycemic control during early percutaneous coronary intervention is associated with a lower rate of in-stent restenosis in patients with acute ST-elevation myocardial infarction. J Clin Endocrinol Metab 97(8):2862–2871
International Diabetes Federation (2019) IDF Diabetes Atlas, 9th edn. International Diabetes Federation, Brussels
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Human and animal rights statement
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. As this is an observational study, the local ethics committee has confirmed that no ethical approval is required.
Informed consent
Consent was not obtained as procedures were part of patient standard care and there was no concern about identifying information.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ferreira, J.A., Baptista, R.M., Monteiro, S.R. et al. Admission hyperglycemia and all-cause mortality in diabetic and non-diabetic patients with acute myocardial infarction: a tertiary center analysis. Intern Emerg Med 16, 2109–2119 (2021). https://doi.org/10.1007/s11739-021-02693-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-021-02693-0