Abstract
The purpose of this investigation was to investigate the association between first attempt success and intubation-related complications in the Intensive Care Unit after the widespread adoption of video laryngoscopy. We further sought to characterize and identify the predictors of complications that occur despite first attempt success. This was a prospective observational study of consecutive intubations performed with video laryngoscopy at an academic medical Intensive Care Unit. Operator, procedural, and complication data were collected. Multivariable logistic regression was used to examine the relationship between the intubation attempts and the occurrence of one or more complications. A total of 905 patients were intubated using a video laryngoscope. First attempt success occurred in 739 (81.7 %), whereas >1 attempt was needed in 166 (18.3 %). One or more complications occurred in 146 (19.8 %) of those intubated on the first attempt versus 107 (64.5 %, p < 0.001) of those requiring more than one attempt. Logistic regression analysis shows that >1 attempt is associated with 6.4 (95 % CI 4.4–9.3) times the adjusted odds of at least one complication. Pre-intubation predictors of at least one complication despite first attempt success include vomit or edema in the airway as well as the presence of hypoxemia or hypotension. There are increased odds of complications with even a second attempt at intubation in the Intensive Care Unit. Complications occur frequently despite a successful first attempt, and as such, the goal of airway management should not be simply first attempt success, but instead first attempt success without complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The “difficult airway” is one in which mask ventilation, laryngoscopy or tracheal tube placement, supraglottic airway placement, or front-of-neck access is challenging [1–3]. While this is a fairly qualitative definition, it is not inconsequential. The cornerstone of all airway management is performing laryngoscopy to place a tracheal tube, and the only option to performing oral laryngoscopy has historically been with a direct laryngoscope. These laryngoscope blades are designed to compress the soft tissues of the upper airway to allow the operator a direct line-of-sight to the glottic opening. Thus, a “difficult intubation” refers to those requiring repeated attempts (usually 3 or more) at obtaining a laryngeal view or placing the tracheal tube with a direct laryngoscope [1–5], and has a high incidence of complications in the Intensive Care Unit (ICU) [4, 6]. Video laryngoscopy (VL) has been developed to overcome these anatomic challenges to laryngoscopy, yet despite these advances, complications with airway management still occur.
Many strategies attempt to predict the difficult intubation, all of which are based on anatomic characteristics that may predict difficulty with airway visualization and tube placement with direct laryngoscopy, and have had only little to moderate success [7–10]. In critically ill patients requiring intubation in the ICU, the difficult intubation as it is currently defined is associated with increased complications. Mort showed that more than two attempts with direct laryngoscopy are associated with higher odds of hypoxemia, esophageal intubation, aspiration, and cardiac arrest, among others [11]. However, newer evidence from the emergency department (ED) shows that the odds of complications increases with each successive attempt [12], suggesting that the current definition does not account for the reduced tolerance for repeated attempts in critically ill patients. In addition, many critically ill patients have physiologic disturbances prior to intubation, which increases the risk of deterioration after intubation [13–16]. The goals of this investigation are to address the remaining gaps in knowledge, including the incidence of complications with successive attempts despite improved laryngoscopic technology, the odds of a complication with more than one attempt at intubation in the ICU, and the predictors of complications despite first attempt success.
Methods
Study design, patient population, and setting
This was an observational study of all VL intubations at an academic referral center between January 2012 and January 2016 using prospectively collected data. Patients were excluded if they were intubated with a device other than a video laryngoscope or intubated by a medical student or attending physician. This medical ICU service is staffed by two teaching teams, which includes residents from medicine and emergency medicine, pulmonary and critical care fellows, and board certified intensivists from pulmonary and critical care medicine. Occasionally, fellows from anesthesiology or surgical critical care fellowships rotate through the medical ICU service. All intubations are performed under direct supervision by faculty skilled in airway management. All fellows participate in an ongoing didactic airway curriculum consisting of lectures and a monthly simulation experience [17]. This study is approved by the University of Arizona Institutional Review Board (#1601327187).
Procedure and data collection
Immediately following each intubation, the operator completed a data collection form, which included the following information: patient and operator demographics, indication for intubation, pharmacologic agents and device(s) used, presence of certain difficult airway characteristics (DACs), pre-oxygenation methods, the Cormack–Lehane (CL) view and Percentage of Glottic Opening (POGO) score of the airway, number of attempts at intubation, and the outcome of each attempt, including complications. A biweekly report available from the electronic health record ensured that all intubations were captured.
The standard preoperative difficult airway predictors are challenging to apply under emergent conditions and with uncooperative patients [8, 9]. Furthermore, these prediction schemes were derived for DL, and not validated for use with VL. As a result, we utilize a list of DACs that are practical under urgent and emergent circumstances by simple examination of the patient prior to intubation. DACs are divided into anatomic and physiologic DACs. The anatomic DACs are those that make the visualization of the vocal cords more difficult, and include the presence of blood or vomit in the airway, cervical immobility, facial or neck trauma, airway edema, small mandible, obesity, large tongue, short neck, limited mouth opening, or secretions. Limited mouth opening and the presence of secretions were added to the data collection instrument 8 months after the start of the study, and therefore, these data are unavailable for the first 133 intubations. Physiologic DACs are those that reduce the tolerance for apnea or transition to positive pressure ventilation, and include hypoxia and hemodynamic instability. There is no institutional intubation protocol, and operators determine their intubation plan based on their pre-intubation assessment.
For the duration of the study period, the following video laryngoscopes were available: GlideScope (GVL) (Verathon, Bothell, WA) with both reusable and disposable hyperangulated blade configurations with blade sizes 3 and 4, and the C-MAC (Karl Storz, Tuttlingen, Germany) with Macintosh-type blade sizes 3 and 4. More recently, the McGrath MAC (Covidien, Mansfield, MA) with Macintosh-type blade sizes 2–4 became available and we have trialed the King Vision (King Systems, Nobelsville, IN). Figure 1 illustrates total intubations, exclusions, and types of devices used during the study period.
Definitions
All definitions were developed prior to the start of data collection, and remained constant throughout the study period. An intubation attempt was defined as insertion of the laryngoscope blade into the oropharynx regardless of whether an attempt was made to pass the endotracheal tube (ETT). Successful intubation was defined as correct placement of the ETT in the trachea as confirmed by standard means. Any placement, and then removal and replacement of the ETT due to uncertain placement, is considered an esophageal intubation. First attempt success (FAS) is defined as successful tracheal intubation on the initial laryngoscope insertion. When the C-MAC or McGrath MAC was used as DL, the attempt is considered a VL attempt, as the supervising physician is able to view the monitor.
Complication data collected included: esophageal intubation, desaturation, aspiration, hypotension, airway trauma, mainstem intubation, and “other.”. Desaturation was defined as a >10 % drop in oxygen saturation from baseline as measured by pulse oximetry during the intubation. Oxygen saturation was continuously monitored by pulse oximetry. We performed a post hoc analysis to assess the oxygen saturation nadir, defining moderate as an oxygen saturation <80 % and severe as <70 %. Aspiration was defined as any witnessed aspiration of oropharyngeal or gastric contents during laryngoscopy. Hypotension was defined as any drop in blood pressure within 5 min of the intubation requiring clinical intervention, such as a fluid bolus, initiation of, or titration of vasopressors. Blood pressure was monitored either continuously by arterial line or by non-invasive cuff every 2 min. Airway trauma included any lacerations, dental injury, or edema of the airway caused by the intubation attempt. “Other” included a space on the data collection form for the operator to record the nature of the complication.
Outcome measures and statistical analysis
The primary outcome was the occurrence of at least one intubation-related complication. A secondary analysis was performed to determine DACs associated with at least one complication despite successful intubation on the first attempt.
Descriptive statistics were performed for measured variables as means and standard deviations, medians and interquartile ranges (IQR), or proportions using the “exact” method, as appropriate. A multivariable logistic regression analysis was performed for the dependent variable of at least one complication using backwards elimination with a p value threshold for retention in the model of <0.20. Predictor variables included the number of DACs, the need for more than one attempt and the potential confounders of operator post-graduate year (PGY), the reason for intubation, the particular video laryngoscope used during the first attempt, and the method of intubation (sedation only vs. use of a paralytic). Similarly, in the subgroup analysis, the occurrence of a complication despite FAS was modeled using the same technique, but with each individual DAC added to the model in place of the number of DACs. A univariate logistic regression was performed for the dependent variable, the occurrence of at least one complication, and each individual predictor variable that was significant in the final multivariable model. We calculated model diagnostic statistics for the logistic regression models (leverage residuals, deviance, and Pregibon’s Delta–Beta). We used the Hosmer–Lemeshow goodness-of-fit test using ten groupings, and calculated the area under the receiver operator characteristic curve (AUC) as measures of model discrimination. All statistical analyses were performed with Stata Version 13 (StataCorp, College Station, TX).
Results
Patient characteristics
During the study period, there were a total of 1138 intubations performed. Of these, 233 were excluded and 905 intubations ultimately met criteria for inclusion in this analysis (Fig. 1). There were few baseline differences between those in which FAS occurred and cases in which additional attempts were required (Table 1). FAS cases more frequently reported the absence of DACs (24 vs 13.3 %, p = 0.003). Intubations with a failed first attempt more commonly had blood in the airway (22.3 vs 11.4 %, p < 0.001), cervical immobility (6 vs 2.2 %, p = 0.017), airway edema (12.1 vs 4.6 %, p = 0.001), obesity (38 vs 26.3 %, p = 0.003), large tongue (15.7 vs 9.5 %, p = 0.025), short neck (31.3 vs 20.2 %, p = 0.003), limited mouth opening (17.3 vs 10 %, p = 0.018), and the presence of secretions (30.2 vs 18 %, p = 0.002).
Operator and technical characteristics
Operator characteristics significantly differed between groups with relatively higher levels of post-graduate training, and trainees from emergency medicine, critical care medicine, and pulmonary-critical care performing a higher proportion of the intubations that resulted in first attempt success (Table 1). The VL used did not differ between groups, with the C-MAC being the most frequently used. Neuromuscular blocking agents were used more frequently in cases with FAS (80.5 vs 65.6 %, p < 0.001).
Complications
At least one intubation-related complication occured in 28 % of intubations. Complications occured significantly less frequently when FAS was achieved (19.8 vs 64.5 %, p < 0.001) (Table 2), and increased with each successive attempt (Fig. 2). Patients requiring more than one attempt had 7.4 (95 % CI 5.1–10.6) times the unadjusted odds of at least one complication than patients intubated on first attempt (Table 3). After controlling for potential confounders, the failure to achieve FAS was associated with 6.4 (95 % CI 4.4–9.3) times the adjusted odds of at least one complication (Table 3). Increasing numbers of DACs were associated with increasing odds of at least one complication, with the presence of one DAC increasing the adjusted odds of at least one complication by 1.8 times (95 % CI 1.04–3.0). The reason for intubation was not statistically associated with the odds of at least one complication, but met the criteria to be retained in the model. Method, operator PGY and specialty, and device used did not meet the pre-specified p < 0.2 criterion for retention in the model.
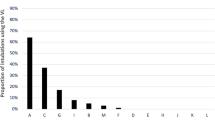
Complications by the number of attempts and DACs associated with first attempt complications: intubation-related complications increased 41.7 % after the first attempt, and by the 5th attempt, all patients experienced at least one complication. Nearly, 20 % of patients had an intubation-related complication on first attempt. After controlling for potential confounders, hypoxemia, hemodynamic instability, airway edema, and the presence of vomit were the difficult airway characteristics significantly associated with higher odds of a first attempt complication
The most common complication was desaturation, which occured more frequently when FAS was not achieved (53 vs 11.5 %, p < 0.001). Failure to achieve FAS was also associated with higher rates of aspiration (7.8 vs 1.6 %, p < 0.001), airway trauma (5.4 vs 0.4 %, p < 0.001), and “other” (6 vs 1 %, p < 0.001) (Table 2). The reported “other” complications consisted of cardiac arrest, bradycardia, and laryngospasm (Table 2).
Complications occurred in 19.8 % of cases despite FAS. The presence of hypoxemia (aOR 2.0, 95 % CI 1.3–3.2), hemodynamic instability (aOR 1.7, 95 % CI 1.1–2.6), vomit (aOR 2.9, 95 % CI 1.4–5.9), and airway edema (aOR 2.6, 95 % CI 1.2–5.6) were associated with increased odds of at least one complication despite FAS, adjusted for the method of intubation, short neck, large tongue, and cervical immobility, which were not statistically significant, but met criteria for inclusion in the model (Fig. 2). Model diagnostics revealed several outliers, which were checked for coding errors, then removed, and the modeling repeated as a sensitivity analysis with no change in the model components or their significance.
Discussion
These data show that the odds of an intubation-related complication increase with more than one attempt. The incidence of complications increased by 42 % between the first and second attempts, compared to only an increase of 10 % between the second and third attempts. This is consistent with Sakles et al. who reported that more than one attempt in the ED was associated with a 33 % increase in complications [12]. In addition, we found a nearly 20 % complication rate despite FAS. Hypoxemia, hemodynamic instability, the presence of vomit in the airway, and airway edema were significantly associated with an intubation-related complication despite FAS.
Intubation-related complications are common, largely due to the high incidence of the “difficult intubation,” which has been reported between 10 and 22.5 % [4–6, 11]. Mort reported that when emergency intubation required more than two attempts, serious complications increased dramatically [11]. In patients who required more than two attempts, aspiration (22 vs 2 %), hypoxemia (70 vs 12 %), and cardiac arrest (11 vs 1 %), all occurred more frequently. Jaber showed an overall severe complication rate of 28 %, and in the 12 % of difficult intubations requiring 3 or more attempts or another skilled operator, 40 % had severe hypoxemia [5]. Griesdale et al. reported an overall complication rate of 39 % in the ICU, with 21 % of intubations involving technical complications related to laryngoscopy [6]. Using a propensity score adjusted regression analysis, they found two or more attempts to be associated with a 3.31 (95 % CI 1.30–8.40) times higher odds of a severe complication (O2 sat <80 % or SBP <70 mmHg during or within 5 min of intubation). In contrast, Schwartz et al. found 27 % of intubations required more than one attempt and despite an 8 % difficult intubation rate, multiple attempts were not associated with a complication, although they were only assessing seizures and cardiac arrest as complications [18].
The majority of the existing literature on difficult laryngoscopy has been with direct laryngoscopy. Similarly, methods of predicting difficult laryngoscopy have met minimal success, and are not validated for the use of VL [7–10, 19]. In the Mort study, all intubations were performed with DL and were all out-of-operating room intubations, with 12 % being difficult [11]. In the study by Scwhartz, 90 % of the intubations were performed using DL, and the remaining with nasal intubation, lighted stylets, or flexible fiberoptic scopes [18]. In the study by Griesdale, operators were classified as “expert” or “non-expert,” which led to a difference in device selection. They found that compared to non-experts, experts tended to use the GlideScope (23 vs 7 %, p < 0.01) or perform awake fiberoptic intubations (19 vs 2.4 %) rather than DL (56 vs 91 %); yet, despite increased VL use among experts, most patients were still intubated using DL. This resulted in an improved FAS (experts 77 % vs non-experts 61 %), but no difference in complication rates (experts 37 % vs non-experts 40 %) [6]. In our study, utilizing VL, only 2.7 % of intubations required 3 or more attempts, yet despite this improved laryngoscopic technology, we find that more than even one attempt is associated with higher odds of a complication. Furthermore, the greatest increase in complications occurred when a second attempt is required.
Physiologic DACs present a significant problem in critically ill patients. In the Schwartz study, patients with a pre-intubation BP of <90 died within 30 min of intubation in 15 % vs <1 % for patients with a BP >90 mmHg prior to intubation [18]. Others have also found pre-intubation hypotension and increased shock index to be associated with severe cardiovascular collapse and death after intubation [13–16, 20]. Interestingly, Jaber found that none of the patients with shock [5] requiring intubation had a difficult intubation, yet the presence of shock was associated with increased odds of a serious complication. They also found that acute respiratory failure as the indication for intubation was associated with higher odds of a serious complication. We confirm that hemodynamic instability is associated with intubation-related complications. However, we find that hemodynamic instability and hypoxemia are associated with complications not only with more than one attempt, but also despite first attempt success. These results indicate that the physiologic DACs are signs of a difficult airway despite unchallenging laryngoscopy, suggesting that the definition of the difficult airway should be revisited in the ICU lexicon. These physiologic DACs may represent targets for enhancing the safety of tracheal intubation by implementing strategies to minimize or eliminate them prior to intubation [21].
There are several limitations when considering the results of this study. First, this is an observational study, where the details of the intubation are recorded by the operator immediately following the procedure, and thus, there is the potential for self-report bias. This bias may overestimate the risk for each DAC, as the operator may be more likely to report DACs in the event of a failed first attempt. Second, this is a single-center study in an academic ICU, where the utilization of VL and neuromuscular blocking agents for intubation is quite high, where there is no standard protocol, and where all airway procedures are directly supervised by an attending physician. Third, there are baseline differences between the comparison groups; however, this is to be expected given inherent differences in comparing more than one attempt to intubations limited to one attempt. We tried to control for these differences with multivariable analyses; however, the generalizability of our results may be limited given the exposure, airway curriculum, experience of trainees, and definitions used of both DACs and complications at our institution. We used multivariable regression to correct for potential confounders and computed model diagnostics to ensure a good fit to the data; however, there may be important confounders that remain unmeasured. Fourth, we use several different video laryngosocopes with both hyperangulated and traditional blade designs. However, the device type does not meet criteria for retention in the final regression model. Unfortunately, certain variables are not available, such as the duration of each intubation attempt, and whether the intubation occurred during regular working hours or off hours. Finally, the starting and lowest oxygen saturation and occurrence of hypotension are self-reported, as our institution does not import continuous data from the monitoring system into the electronic health record.
Conclusion
Our results suggest that the current definition of difficult intubation is poorly applicable to the critically ill population. Despite significant improvements in technology available to perform laryngoscopy, there are increased odds of complications with even a second attempt. Even when first attempt success is achieved, many patients have complications, where physiologic DACs are significant predictors. Thus, the goal in the ICU should not be simply first attempt success but instead first attempt success without complications, and the “difficult airway” is one in which that cannot be accomplished.
References
Law JA, Broemling N, Cooper RM, Drolet P, Duggan LV, Griesdale DE, Hung OR, Jones PM, Kovacs G, Massey S, Morris IR, Mullen T, Murphy MF, Preston R, Naik VN, Scott J, Stacey S, Turkstra TP, Wong DT (2013) The difficult airway with recommendations for management–part 1–difficult tracheal intubation encountered in an unconscious/induced patient. Canadian journal of anaesthesia. J Can Anesth 60(11):1089–1118. doi:10.1007/s12630-013-0019-3
Frerk C, Mitchell VS, McNarry AF, Mendonca C, Bhagrath R, Patel A, O’Sullivan EP, Woodall NM, Ahmad I (2015) Difficult airway society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth 115(6):827–848. doi:10.1093/bja/aev371
Apfelbaum JL, Hagberg CA, Caplan RA, Blitt CD, Connis RT, Nickinovich DG, Hagberg CA, Caplan RA, Benumof JL, Berry FA, Blitt CD, Bode RH, Cheney FW, Connis RT, Guidry OF, Nickinovich DG, Ovassapian A, American Society of Anesthesiologists Task Force on Management of the Difficult A (2013) Practice guidelines for management of the difficult airway: an updated report by the American society of anesthesiologists task force on management of the difficult airway. Anesthesiology 118(2):251–270. doi:10.1097/ALN.0b013e31827773b2
Le Tacon S, Wolter P, Rusterholtz T, Harlay M, Gayol S, Sauder P, Jaeger A (2000) Complications of difficult tracheal intubations in a critical care unit. Ann Fr Anesth Reanim 19(10):719–724
Jaber S, Amraoui J, Lefrant J, Arich C, Cohendy R, Landreau L, Calvet Y, Capdevila X, Mahamat A, Eledjam J (2006) Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Crit Care Med 34:2355–2361
Griesdale DE, Bosma TL, Kurth T, Isac G, Chittock DR (2008) Complications of endotracheal intubation in the critically ill. Intensive Care Med 34(10):1835–1842. doi:10.1007/s00134-008-1205-6
De Jong A, Clavieras N, Conseil M, Coisel Y, Moury PH, Pouzeratte Y, Cisse M, Belafia F, Jung B, Chanques G, Molinari N, Jaber S (2013) Implementation of a combo videolaryngoscope for intubation in critically ill patients: a before-after comparative study. Intensive Care Med 39(12):2144–2152. doi:10.1007/s00134-013-3099-1
Bair A, Caravelli R, Tyler K, Laurin E (2010) Feasibility of the preoperative Mallampati airway assessment in emergency department patients. J Emerg Med 38:677–680
Levitan R, Everett W, Ochroch E (2004) Limitations of difficult airway prediction in patients intubated in the emergency department. Ann Emerg Med 44:307–313
Norskov AK, Rosenstock CV, Wetterslev J, Astrup G, Afshari A, Lundstrom LH (2015) Diagnostic accuracy of anaesthesiologists’ prediction of difficult airway management in daily clinical practice: a cohort study of 188 064 patients registered in the Danish Anaesthesia Database. Anaesthesia 70(3):272–281. doi:10.1111/anae.12955
Mort T (2004) Emergency tracheal intubation: complications associated with repeated laryngoscopic attempts. Anesth Analg 99:607–613
Sakles J, Chiu S, Mosier J, Walker C, Stolz U (2013) The importance of first pass success when performing orotracheal intubation in the emergency department. Acad Emerg Med Off J Soc Acad Emerg Med 20:71–78
Heffner AC, Swords D, Kline JA, Jones AE (2012) The frequency and significance of postintubation hypotension during emergency airway management. J Crit Care 27:417.e9–417.e13. doi:10.1016/j.jcrc.2011.08.011
Heffner AC, Swords DS, Nussbaum ML, Kline JA, Jones AE (2012) Predictors of the complication of postintubation hypotension during emergency airway management. J Crit Care 27(6):587–593. doi:10.1016/j.jcrc.2012.04.022
Smischney NJ, Demirci O, Diedrich DA, Barbara DW, Sandefur BJ, Trivedi S, McGarry S, Kashyap R (2016) Incidence of and risk factors for post-intubation hypotension in the critically Ill. Med Sci Monit Int Med J Exp Clin Res 22:346–355
Green RS, Turgeon AF, McIntyre LA, Fox-Robichaud AE, Fergusson DA, Doucette S, Butler MB, Erdogan M, Canadian Critical Care Trials G (2015) Postintubation hypotension in intensive care unit patients: a multicenter cohort study. J Crit Care 30(5):1055–1060. doi:10.1016/j.jcrc.2015.06.007
Mosier JM, Malo J, Sakles JC, Hypes CD, Natt B, Snyder L, Knepler J, Bloom JW, Joshi R, Knox K (2015) The impact of a comprehensive airway management training program for pulmonary and critical care medicine fellows. A three-year experience. Annals Am Thorac Soc 12(4):539–548. doi:10.1513/AnnalsATS.201501-023OC
Schwartz D, Matthay M, Cohen N (1995) Death and other complications of emergency airway management in critically ill adults. A prospective investigation of 297 tracheal intubations. Anesthesiology 82:367–376
De Jong A, Molinari N, Terzi N, Mongardon N, Arnal JM, Guitton C, Allaouchiche B, Paugam-Burtz C, Constantin JM, Lefrant JY, Leone M, Papazian L, Asehnoune K, Maziers N, Azoulay E, Pradel G, Jung B, Jaber S, AzuRea Network for the Frida-Rea Study G (2013) Early identification of patients at risk for difficult intubation in the intensive care unit: development and validation of the MACOCHA score in a multicenter cohort study. Am J Respir Crit Care Med 187(8):832–839. doi:10.1164/rccm.201210-1851OC
Perbet S, De Jong A, Delmas J, Futier E, Pereira B, Jaber S, Constantin JM (2015) Incidence of and risk factors for severe cardiovascular collapse after endotracheal intubation in the ICU: a multicenter observational study. Crit Care 19:257. doi:10.1186/s13054-015-0975-9
Mosier JM, Joshi R, Hypes C, Pacheco G, Valenzuela T, Sakles JC (2015) The physiologically difficult airway. West J Emerg Med 16(7):1109–1117. doi:10.5811/westjem.2015.8.27467
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Sakles serves on the scientific advisory committee for Verathon Medical.
Statement of human and animal rights
This study was reviewed and approved by the University of Arizona Institutional Review Board.
Informed consent
This study was granted exemption from informed consent.
Rights and permissions
About this article
Cite this article
Hypes, C., Sakles, J., Joshi, R. et al. Failure to achieve first attempt success at intubation using video laryngoscopy is associated with increased complications. Intern Emerg Med 12, 1235–1243 (2017). https://doi.org/10.1007/s11739-016-1549-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-016-1549-9