Abstract
We have investigated the effects of the sulfonylurea herbicide Sekator OD 375 on common duckweed (Lemna minor L.) and its potential to recover after the herbicide exposure. The plants were exposed to Sekator OD 375 for a 7-day period at the concentrations ranging from 5 to 100 µL L−1, equivalent to 0.005–0.1 of field application rate. The treated plants were transferred to clean fresh growth medium for a 7-day period to observe the plants recovery. The exposure to herbicide significantly reduced the growth rate and the biomass of common duckweeds (L. minor), lowered the content of photosynthetic pigments and induced membrane lipid peroxidation. After the transfer to fresh, clean growth media, the plants exposed to concentrations higher than 50 µL L−1 of herbicide showed no potential to recover. L. minor exposed to relatively low levels of herbicide (below 50 µL L−1) showed a potential to recover their new fronds production: the relative growth rate in the recovery phase was higher than in the exposure period. However, after the recovery phase, the final biomass of the exposed plants was below initial values. Exposure to the herbicide-induced membrane lipid peroxidation (measured as the concentration of malondialdehyde). Thus, plants failed to recover their membranes during the recovery phase and a further increase in the concentrations of malondialdehyde was observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sulfonylurea herbicides are widely used since their discovery in 1975. As the main reasons for rapid commercial acceptance of selective post-emergence sulfonylurea herbicides are listed very low application level (10–15 g a.i. ha−1), good weed control and crop selectivity, and relatively low toxicity to animals (Brown and Cotterman 1994). The sulfonylurea herbicides strongly inhibit the synthesis of acetolactate synthase (ALS), i.e., the key enzyme of branched-chain amino acid (valine, leucine and isoleucine) biosynthesis (Schloss 1990). These herbicides can pose a risk to living organisms even at very low concentrations. It was observed that even herbicide application at a rate of 1% of the recommended field dose might adversely affect non-target plants (Boutin et al. 2000). Amidosulfuron is a broad-spectrum post-emergence herbicide used for broad-leaved weeds control in cereal crops (spring and winter wheat, barley and rye). The effects of amidosulfuron on higher terrestrial plants are quite well studied. It has been shown that it reduces plant growth, affects morphology and reproduction, and reduces the content of photosynthetic pigments (Kudsk and Mathiassen 2004; Žaltauskaitė and Brazaitytė 2013; Saja et al. 2016).
Following agricultural application herbicides can reach adjacent surface waters through spray drift, leaching and surface runoff. As sulfonylureas are highly water soluble (low Kow), after agricultural application, they can easily reach surface water bodies as well as leach to groundwater. Low sulfonylurea herbicide levels (> 1 ng L−1) were recorded in different surface waters (Battaglin et al. 2000; De Lafontaine et al. 2014) and at concentrations in the range of μL L−1 they were reported to be highly toxic to algae and periphyton (Degenhardt et al. 2010). Amidosulfuron and its degradation products were found in surface and drainage water (up to 0.31 μg L−1 of amidosulfuron) after herbicide spraying and 1 year after application in experimental barley plot in Norway (Almvik et al. 2011). The highest concentrations in water were recorded at the first significant flow event after application of herbicides. Amidosulfuron has been repeatedly detected in drainage water in Denmark (Kjær et al. 2007), catchments in Sweden (Kreuger et al. 2010) with a maximum concentration of 0.11 μg L−1.
In aquatic ecosystems the primary target of herbicide action is primary producers. In most cases aquatic plants’ physiology and reproduction are adversely affected by the sulfonylurea herbicides (Frankart et al. 2003; Eullaffroy et al. 2007). Duckweeds (Lemna sp.) are widespread, fast-growing free-floating monocot macrophytes which are widely used as standardised aquatic test organisms in toxicity testing. However, most studies were aimed to analyse herbicide-induced toxic effects and only a few studies have examined Lemna sp. recovery after exposure to heavy metals (Drost et al. 2007) and pesticides (Brain et al. 2012; Teodorović et al. 2012). Recovery is defined as the return of an impacted population or community to its pre-disturbance state or range of control systems (Gergs et al. 2016). The main determinants of recovery potential are chemical toxic mode of action, duration and magnitude of exposure, organism’s ability to metabolize and detoxify the chemical, and species tolerance. Given that herbicides are usually released to surface waters in pulses varying in duration and magnitude between which recovery can occur, it is important to understand the recovery potential of different species after their exposure to herbicides to predict the viability of populations and possible changes in communities’ structure. The aim of this research was to evaluate herbicide Sekator OD impact on the growth and physiology of common duckweed (Lemna minor L.) and to determine the recovery potential of Lemna minor after exposure to the herbicide.
Materials and methods
Common duckweed (Lemna minor L.) was originally collected in the pond at Kaunas Botanical Garden and grown in laboratory at Vytautas Magnus University. The stock culture of L. minor was grown at 24 °C ± 2 °C (16:8 h light:dark cycle) in a modified Steinberg medium (3.46 mM KNO3, 1.25 mM Ca(NO3)2, 0.66 mM KH2PO4, 0.072 mM K2HPO4, 0.41 mM MgSO4, 1.94 µM H3BO3, 0.63 µM ZnSO4, 0.18 µM Na2MoO4, 0.91 µM MnCl2, 2.81 µM FeCl3, 4.03 µM EDTA (ISO 20079).
Herbicide toxicity study was performed using a commercially available Sekator OD 375 (Bayer CropScience) herbicide. Sekator OD 375 is composed of two active compounds [amidosulfuron (9%) and iodosulfuron methyl, sodium salt (2.2%)] and herbicide safener mephenpyr diethyl (22%). Both active compounds (amidosulfuron and iodosulfuron methyl, sodium salt) act by inhibiting the synthesis of acetolactate synthase (ALS). The plants were exposed to 5, 10, 25, 50, 75, 100 µL L−1 of herbicide (i.e., 0.005–0.1 field application rate) in the growth medium of L. minor. The experiment was performed in three replicates per concentration.
The bioassays with L. minor were conducted in accordance with OECD Guideline 221 (2004); the experiment consisted of 7 days of exposure phase and 7 days of recovery phase. For the exposure phase, 40 double-fronded healthy L. minor colonies were placed to each test beaker with different concentrations of herbicide. After the exposure phase (7 days), 20 double-fronded L. minor colonies from each beaker were transferred to new test beakers with fresh clean Steinberg growth medium. The recovery phase lasted 7 days. Both the exposure and the recovery phases were conducted under static conditions (without medium renewal) and in the same environmental conditions at 24 °C ± 2 °C with 16:8 h light:dark cycle.
Herbicide toxicity to L. minor was evaluated using the following endpoints: fronds number, dry weight, content of photosynthetic pigments (chlorophyll a, b and carotenoids), and lipid peroxidation. Fronds were counted every day, counting all visible new fronds. All other parameters were measured two times: at the end of the exposure (after 7 days) and recovery phases (after 14 days). The plants were considered to have recovered when the values of endpoint reached the level of the control.
The relative growth rate (during the exposure and recovery phases) was calculated with measured fronds number (N) at day t1 (last day) and at day t0 (start of the experiment) according to the following equation:
The relative growth rate was calculated for the exposure and recovery phases (7 days duration), and day-to-day growth rate (every 24 h) was calculated for the recovery phase to investigate the fronds production dynamics.
For the determination of the dry weight, the plants were dried at 60 °C for 48 h up to a constant weight. The content of the photosynthetic pigments in plant tissue extract (in 100% acetone) was measured spectrophotometrically according to Von Wettstein (1957). Lipid peroxidation was evaluated as the concentration of malondialdehyde (MDA), the by-product of lipid peroxidation, and it was determined according to Buege and Aust (1978).
A one-way ANOVA was used to assess the herbicide concentration effect on estimated endpoints. Significant differences between controls and herbicide treatments were determined by the Dunnett’s test. The differences between the treatments were determined by Student’s t test. All the differences were considered to be significant at p < 0.05. Effective concentrations (EC) values were calculated using three parameter log-logistic dose–response model (Ritz and Streibig 2005). Regression analysis was used to detect the relationship between herbicide concentration and estimated endpoints. R version 2.15.2 (R Development Core Team 2004) and Statistica software were used for the statistical analysis.
Results
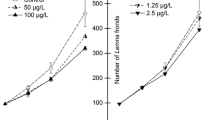
The control plants growth rate ranged from 0.275 to 0.314 day−1 during the exposure and recovery phases, i.e. the validity criteria was met. A highly significant effect of the herbicide on the relative growth rate of L. minor was observed (F = 1123.95, p < 0.001), and L. minor grew slower in all the treatments with herbicide compared to control plants (Dunnett’s test, p < 0.01) (Fig. 1a). The exposure to low levels of herbicide (5–10 µL L−1) resulted in 9.6–11.1% lower growth rate of L. minor compared with the growth of control plants (p < 0.05). The increase in herbicide concentration up to 50 µL L−1 evoked a very sharp decrease in the growth rate compared to control (by 81.8%). An inverse linear relationship between 7-day L. minor growth rate and herbicide exposure concentration was detected (r = − 0.96, p < 0.001). Estimated value of 7-day EC50 for growth rate was 28.87 ± 1.30 µL L−1.
After transferring L. minor fronds to the fresh growth medium for recovery, recovery in new fronds production was apparent in the treatments with 5–50 µL L−1 of herbicide (Fig. 1b). The plants pre-exposed to the highest levels of tested herbicide (75–100 µL L−1) produced no fronds indicating irreversible toxicity to fronds production. Herbicide had a significant effect on L. minor relative growth rate in the post-exposure period, i.e. recovery period (F = 246.38, p < 0.01) and duckweeds in all the treatments grew slower than in control (Dunnett’s test, p < 0.01). The growth rate of L. minor during the recovery phase significantly decreased along with herbicide concentration to which the duckweeds were exposed (R2 = 0.94, p < 0.001).
When herbicide pre-exposed plants were transferred to fresh medium, L. minor started to produce new fronds more effectively than in the exposure phase. The growth rate of L. minor previously treated with 5–50 µL L−1 of herbicide within the 7-day post-exposure period was by 4–63% higher than during the exposure phase, though the significant difference was only observed in the treatment with 25 µL L−1 (p < 0.05). However, L. minor growth rate during the recovery phase did not manage to reach the growth rate level of control.
Seeking to examine the dynamics of fronds production within the recovery period in more details, the day-to-day growth rate—every day in comparison with the previous day, i.e., for every 24 h, was analysed (Fig. 2). A significant effect of the herbicide concentration during the exposure phase on the day-to-day growth rate during the recovery phase was found (F > 7.70, p < 0.01). The most intense growth was observed at the second day of recovery period (between 24 and 48 h), and this was apparent in all the treatments. Thereafter, the growth rate began to decrease, and the growth rate of L. minor exposed to low herbicide concentration reached the level of the control. The biggest differences between the growth of control and herbicide pre-exposed plants were observed at the beginning of the recovery phase. During the first 48 h of the recovery period, the relative day-to-day growth rate of control plants exceeded the growth rate of plants pre-exposed to herbicide, however, the differences were insignificant in the case of the lowest concentrations (5–10 µL L−1) (Dunnett’s test, p > 0.05). The 24 h and 48 h day-to-day growth rate of duckweeds previously exposed to 25 and 50 µL L−1 was lower by 36.00–64.12 and 86.30–91.45%, respectively, as compared with the control plants. The differences between the day-to-day growth rates of control and pre-exposed plants declined with the increasing duration of the recovery period, and no statistical differences were observed after 120 h of the recovery period (p > 0.05). Such a trend indicated a recovery potential.
Lemna minor exposure to low herbicide concentrations (5–25 μL L−1) led to a slight increase in dry biomass during the exposure phase (Fig. 3), although the changes were statistically insignificant (p > 0.05). Our observations indicate that L. minor exposed to low herbicide concentrations produced fewer fronds, although they were of higher biomass. A further increase in herbicide concentration resulted in a decrease in dry biomass of L. minor, the highest tested concentration frond biomass being by 17.87% lower than in the control.
During the recovery phase, it was observed that the biomass of pre-exposed L. minor remained lower than the biomass of control plants (p < 0.05), excluding the lowest concentration treatment. Moreover, at the end of the recovery period, the weight of plants pre-exposed to 10–100 μL L−1 of herbicide was significantly lower than the weight after the exposure. So, after transferring the colonies to fresh medium, a further decrease in biomass was observed despite the recovery of new fronds production (Fig. 1). Additionally, dry biomass of L. minor decreased along with herbicide concentration (R2 = 0.66, p < 0.001) during the recovery phase and the biomass of L. minor pre-exposed to the highest tested concentration (100 μL L−1) was twofold lower that of the control plants.
Herbicide treatment significantly affected the content of chlorophyll a (ANOVA, F = 19.22, p < 0.05) (Fig. 4). Low herbicide concentrations (5–25 μL L−1) induced a slight increase in the content of chlorophyll a, while a sharp decrease in chlorophyll a content was recorded in the treatments 50–100 μL L−1. The content of chlorophyll a in L. minor exposed to 100 μL L−1 herbicide decreased by 38.55% compared to the control level. Chlorophyll a content decreased along with the concentration of herbicide (R2 = 0.80, p < 0.001).
The changes in the content of chlorophyll a during the recovery phase were in the same manner as in the exposure phase (Fig. 4). There was a highly significant effect of herbicide concentration on the chlorophyll a content during the post-exposure period (F = 62.79, p < 0.001), and the chlorophyll a content decreased as a function of herbicide concentration during the exposure phase (R2 = 0.83, p < 0.001). In addition, after the entire recovery phase, the chlorophyll a content in herbicide pre-exposed plants was lower than that during the exposure period. These findings indicate that L. minor did not fully recover chlorophyll a content within 7 days after the exposure period had ended.
The data of the concentrations of chlorophyll b and carotenoids after the plants treatment with herbicide were very scattered and there was no clear dependence on the herbicide concentration (ANOVA, chlorophyll b F = 16.28, carotenoids F = 4.10, p < 0.05) (Fig. 4). The content of chlorophyll b and carotenoids during the recovery phase reached the level of untreated plants, which indicated a considerable recovery.
Membrane lipid peroxidation was evoked due to plants’ exposure to herbicide (Table 1). A significant effect of herbicide on MDA concentration was found (F = 4.06, p < 0.05), and MDA concentration in plant tissues increased along with herbicide concentration (R2 = 0.56, p < 0.05). During the recovery phase, the MDA concentration further significantly increased (by 1.7-fold) indicating irreversible changes in membranes. Moreover, the significant increase in MDA level within the recovery period implies that harmful reactive oxygen species are present in the cells. Induced oxidative damage may lead to the reduction of growth, biomass, productivity or may evoke the death of plants. A significant relationship was detected between MDA content in the tissues and dry weight of L. minor both for exposure (r = − 0.78, p < 0.05) and recovery (r = − 0.75, p < 0.05) phases suggesting that induced oxidative stress inhibits plant growth and may lead to reduced reproduction or yield.
Discussion
Common duckweed (L. minor L.) showed a strong response after the treatment with herbicide Sekator OD375. The exposure to herbicide significantly reduced L. minor growth rate, plant biomass and the content of photosynthetic pigments. New fronds production (as growth rate) was the most sensitive endpoint. Amidosulfuron (the main component of Sekator OD375) toxicity towards aquatic plants is very high; EFSA reported 7-days EC50 for relative growth rate and the biomass of L. gibba to be at 9.2 µg L−1 (EFSA 2007). Reported EC50 for relative growth rate for iodosulfuron methyl, sodium salt was even lower, i.e., 1.34 µg L−1 (EFSA 2016). However, the sensitivity of L. minor and L. gibba to these compounds may differ as these species exhibit different sensitivity to other herbicides. L. gibba showed higher sensitivity to urea herbicide diuron than L. minor (Burns et al. 2015). Our data suggest that formulated Sekator OD is only slightly less toxic than its active substances. Comparison of aquatic toxicity of ten different technical and formulated herbicides, showed that herbicide formulation did not enhance their toxicity to aquatic plants by more than a factor of approximately two (Cedergreen and Streibig 2005).
Common duckweeds pre-exposed to low levels of herbicide showed a potential to recover, however, their growth rate has not reached the control’s growth rate. In general, the plants need more time to recover with higher exposure concentrations or a longer exposure period. As the day-to-day growth rates after 5 days of the post exposure (recovery) period were close to that of control plants (Fig. 2), it clearly indicates the evident recovery of new fronds production. However, at the end of the recovery phase, the relative day-to-day growth rate slightly decreased, which may be related to the increased L. minor density (Driever et al. 2005). We may presume that a 7-day recovery period is insufficient to fully recover the production of new fronds and the plants need more time to recover their physiology. It was reported that exposure to higher sulfonylurea herbicides concentrations (continuous or pulse) have caused longer lag periods for recovery of new fronds production (Mohammad et al. 2006, 2010; Rosenkratz et al. 2013). Burns et al. (2015) observed that L. minor and L. gibba were able to restore their new fronds production and biomass after the exposure to low levels of diuron. However, even after a relatively short term of exposure, plants need a more prolonged period to recover their growth. It was recorded that L. minor plants exposed for 10 days to herbicide norflurazon recovered their growth rates (10–500 µg L−1, 6-day EC50 24.9 ± 4.1 µg L−1) only after 28 days (Wilson and Koch 2013).
Mohammad et al. (2008) examined Lemna sp. ability to recover their growth rate after the treatment with herbicides with different modes of action. They found that sulfonylureas showed the highest toxicity among other herbicides (such as triazine, urea, thiocarbamate, etc.), but plants recover more quickly than from other types of herbicides. Boxall et al. (2013) found that pulsed (2 and 4 days) and continuous (42 days) L. minor exposure to metsulfuron-methyl resulted in a very similar effect, while pulse exposure to isoproturon (photosystem II(PSII)-inhibiting herbicide) had a less adverse effect than the continuous ones. This phenomenon was explained by the fact that the restoration of amino acid synthesis and cell division (the targets of sulfonylureas) needs a lag period while the recovery of plants from PSII-inhibiting herbicides is relatively rapid. A very similar L. minor growth rate response to 3 h pulsed and 4-day metsulfuron-methyl exposure was recorded by Cedergreen et al. (2005), the recovery L. minor after pulsed exposure being observed only after 4 days. Despite a relatively slow L. minor growth rate recovery after the exposure to ALS inhibitors, it can be presumed that after a longer recovery period, herbicide pre-exposed L. minor could show growth rates close to control level.
The changes in the biomass and other endpoints during the post-exposure period were in different manner than that of the growth rate. After transferring the colonies to fresh medium, a further decrease in biomass was observed. This indicates that after the exposure to herbicide Sekator OD375 L. minor did not manage to gain their biomass and recover, despite a slight recovery of new fronds production. L. minor plants were shown to be able to recover their biomass after short (3 days) and longer (7 days) exposure to atrazine, and a recovery was recorded after the 6 days of the recovery period (Teodorović et al. 2012). Sulfosulfuron (0–10 μL L−1) had mostly no significant effect on Glyceria maxima, Lagarosiphon major and Myriophyllum spicatum dry weight measured 70 days after the exposure period had ended (Davies et al. 2003). The studies with terrestrial non-crop plants showed that plants were able to recover their biomass after the treatment with herbicides, although the recovery potential was species, development stage and environmental conditions dependent (Riemens et al. 2009).
Notwithstanding that sulfonylureas are ALS inhibitors, and photosystem is not primary site of their action, herbicide significantly affected the content of photosynthetic pigments during the exposure phase (Fig. 4). Low Sekator OD concentrations induced some increase in chlorophyll a concentrations and it might be explained by the hormetic response. Induction of hormesis by sulfonylureas in L. minor was recorded by Cedergreen et al. (2007), though the frequency and magnitude of hormesis is species, endpoint- and contaminant-dependent (Calabrese and Blain 2005). Chlorophyll a exhibited the highest sensitivity to Sekator OD compared to chlorophyll b and carotenoids, and L. minor did not recover chlorophyll a content during the post-exposure period. In contrast, chlorophyll b and carotenoids content during the recovery phase reached the level of untreated plants, which shows that a considerable recovery process was taking place. A different pattern of the chlorophyll a and b recovery might be explained by the reactions of the chlorophyll cycle (Rüdiger 2002). The decrease in chlorophyll content in Lemna spp. exposed to various herbicides was recorded in numerous studies (Geoffroy et al. 2004; Olette et al. 2008). The data on the impact of ALS inhibitors on the content of photosynthetic pigments in plants are very controversial. Cedergreen et al. (2004) reported that chlorophyll a content in aquatic plants was not impaired by metsulfuron-methyl. Turgut et al. (2003) reported that four different sulfonylureas herbicides had reduced the content of chlorophyll a, b and carotenoids in Myriophyllum aquaticum plants. Chlorophyll a content was also adversely affected in the submerged macrophytes C. demersum, V. natans and E. nuttallii after their exposure to bensulfuron-methyl (Huiyun et al. 2009). Wheat Triticum aestivum treatment with chlorimuron-ethyl caused significant damage to chlorophyll accumulation in seedlings (Wang and Zhou 2006). The impact of ALS inhibitors on the photosystem could be explained by the fact that these compounds induce soluble carbohydrates and starch accumulation which in turn inhibits photosynthesis via sugar feedback mechanism (Zabalza et al. 2013). However, the effect of ALS inhibitors on the photosynthesis could only be seen within several days (Cobb and Reade 2010). Our results support the data of other studies reporting that the exposure to ALS inhibitors severely reduces the growth rate of L. minor, whose recovery is long, though the impact on the content of photosynthetic pigments is less pronounced and the recovery is quite fast (Cedergreen et al. 2005). As most studies analyzing the recovery of Lemna spp. from herbicides exposure focus only on the recovery of new frond production or biomass, we cannot compare them to our results regarding the changes in photosynthetic pigments concentrations. However, Wilson et al. (2006) examined norflurazon effect on aquatic macrophyte Vallisneria americana and reported the restoration of leaf greenness to control levels, which indicates that a recovery process was in progress. As no clear recovery in chlorophyll a (except for the low concentrations) was observed, it is consistent with the results of biomass, which did not recover either. The recovery in chlorophyll a in the treatment with the lowest level of herbicide was translated to biomass growth. However, a slight recovery in photosynthetic activity in other concentrations was insufficient to recover the biomass.
During the 7-day recovery phase, a further increase in MDA content was observed. MDA is a breakdown product from membrane’s lipid peroxidation induced by reactive oxygen species (ROS). Thus, the increase in MDA level in L. minor tissues might indicate the presence of harmful reactive oxygen species and oxidative damage. An evoked oxidative stress may lead to the reduction of the growth rate, biomass, reproduction or may cause the death of plants. This was proved by a significant relationship between MDA content and L. minor dry weight.
Our results indicate that different endpoints have a diverse potential to recover. Sobrero et al. (2007) reported that after the treatment with glyphosate, L. gibba recovered its growth rate, though the effects on the other parameters (frond growth, frond number per colony, root length and total chlorophyll content) remained the same or even decreased. Thus, the measurement of potential recovery cannot be based on a single particular endpoint.
Conclusions
The study examined the herbicides’ impact and recovery potential of non-target aquatic macrophyte Lemna minor. The herbicide exposure reduced the growth rate (measured as frond number) and dry weight of L. minor, impaired the content of photosynthetic pigments and induced lipid peroxidation. Our results indicate that duckweeds exposed to low levels of herbicide have a potential to recover their new frond production, although the growth rate did not fully reach the control level during the 7-day post-exposure period. The results demonstrate that the recovery of the growth rate was initiated, and after a more prolonged recovery period the growth rate may reach the growth rate of untreated plants. After the treatment with herbicide L. minor plants did not manage to recover their biomass and lipid peroxidation. The response of photosynthetic pigments was different—the content of chlorophyll a was not recovered, although the chlorophyll b and carotenoids showed a moderate recovery. However, in this study the time needed for a full recovery was not determined.
The study shows that aquatic plants potential to recover from the herbicides exposure should be studied deeper. A further analysis in the impact of ALS inhibitors on the oxidative stress and recovery patterns, in terms of the length of exposure and recovery phases, might be very useful for a more realistic environmental hazard assessment. The results show that recovery studies could help to predict more accurately possible ecological effects. As well, the recovery studies could be included in an environmental risk assessment.
Author contribution statement
Authors equally contributed to the present work: experiments, text writing, discussions.
References
Almvik M, Riise G, Bolli R, Børresen T, Christiansen A, Odenmarck SR, Holten R (2011) Multi-year transport studies of sulfonylurea herbicides from a barley field in Norway, 2007–2010—including development of LC–MS/MS methods for quantitative analysis of sulfonylurea herbicides and degradation products. Bioforsk Rep 6(10):44
Battaglin WA, Furlong ET, Burkhardt MR, Peter CJ (2000) Occurrence of sulfonylureas, sulfonamide, imidazolinone, and other herbicides in rivers, reservoirs and ground water in the Midwestern United States, 1998. Sci Total Environ 248:123–133
Boutin C, Lee H-B, Peart ET, Batchelor PS, Maquire RJ (2000) Effects of the sulfonylurea herbicide metsulfuron methyl on growth and reproduction of five wetland and terrestrial plant species. Environ Toxicol Chem 19(10):2532–2541
Boxall ABA, Fogg LA, Ashauer R, Bowles T, Sinclair CJ, Colyer A, Brain RA (2013) Effects of repeated pulsed herbicide exposures on the growth of aquatic macrophytes. Environ Toxicol Chem 32(1):193–200
Brain RA, Hosmer AJ, Desjardins D, Kendall TZ, Krueger HO, Wall SB (2012) Recovery of duckweed from time-varying exposure to atrazine. Environ Toxicol Chem 31(5):1121–1128
Brown HM, Cotterman JC (1994) Recent advances in sulfonylurea herbicides. In: Stetter J (ed) Chemistry of plant protection, vol 10. Herbicides inhibiting branched chain amino acid biosynthesis. Springer, Berlin
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzym 52:302–310
Burns M, Hanson ML, Prosser RS, Crossan AN, Kennedy IR (2015) Growth recovery of Lemna gibba and Lemna minor following a 7-day exposure to the herbicide diuron. Bull Environ Contam Toxicol 95:150–156
Calabrese EJ, Blain R (2005) The occurrence of hermetic dose-responses in the toxicological literature, the hormesis database: an overview. Toxicol Appl Pharmacol 202:289–301
Cedergreen N, Streibig JC (2005) The toxicity of herbicides to non-target aquatic plants and algae: assessment of predictive factors and hazard. Pest Manag Sci 61:1152–1160
Cedergreen N, Streibig JC, Spliid NH (2004) Sensitivity of aquatic plants to the herbicide metsulfuron-methyl. Ecotoxicol Environ Saf 57:153–161
Cedergreen N, Anderson L, Olesen CF, Spliid NH, Streibig JC (2005) Does the effect of herbicide pulse exposure on aquatic plants depend on Kow or mode of action? Aquat Toxicol 71:261–271
Cedergreen N, Streibig JC, Kudsk P, Mathiassen SK, Duke SO (2007) The occurrence of hormesis in plants and algae. Dose-Response 5:150–162
Cobb AH, Reade JPH (2010) The inhibition of amino acid biosynthesis, in herbicides and plant physiology, 2nd edn. Wiley-Blackwell, Oxford
Davies J, Honegger JL, Tencalla FG, Meregalli G, Brain P, Newman JR, Pitchford HH (2003) Herbicide risk assessment for non-target aquatic plants: sulfosulfuron—a case study. Pest Manag Sci 59:231–237
De Lafontaine Y, Beauvais C, Cessna AJ, Gagnon P, Hudon C, Poissant L (2014) Sulfonylurea herbicides in agricultural catchment basin and its adjacent wetland in the St. Lawrence River basin. Sci Total Environ 479–480:1–10
Degenhardt D, Cessna AJ, Raina R, Pennock DJ, Farenhorst A (2010) Trace level determination of selected sulfonylurea herbicides in wetland sediment by liquid chromatography electrospray tandem mass spectrometry. J Environ Sci Health Part B 45:11–24
Driever SM, van Nes EH, Roijackers RMM (2005) Growth limitation of Lemna minor due to high plant density. Aquat Bot 81:245–251
Drost W, Matzke M, Backhaus T (2007) Heavy metal toxicity to Lemna minor: studies on the time dependence of growth inhibition and the recovery after exposure. Chemosphere 67:36–43
EFSA (2016) Peer review of the pesticide risk assessment of the active substance iodosulfuron-methyl-sodium (approved as iodosulfuron). EFS J 14(4453):111
EFSA Scientific Report (2007) 116, 1–86. Conclusions on the peer review of amidosulfuron
Eullaffroy P, Frankart C, Biagianti S (2007) Toxic effect assessment of pollutant mixtures in Lemna minor by using polyphasic fluorescence kinetics. Toxicol Environ Chem 89:683–696
Frankart C, Eullaffroy P, Vernet G (2003) Comparative effects of four herbicides on non-photochemical fluorescence quenching in Lemna minor. Environ Exper Bot 49:159–168
Geoffroy L, Frankart C, Eullaffroy P (2004) Comparison of different physiological parameter responses in Lemna minor and Scenedesmus obliquus exposed to herbicide flumioxazin. Environ Pollut 131:233–241
Gergs A, Classen S, Strauss T, Ottermanns R, Brock TCM, Ratte HT, Hommen U, Preuss TG (2016) Ecological recovery potential of freshwater organisms: consequences for environmental risk assessment of chemicals. Rev Environ Contam Toxicol 236:259–294
Huiyun P, Xiaolu L, Shixiang G (2009) Phytotoxicity of four herbicides on Ceratophyllum demersum, Vallisneria natans and Elodea nuttallii. J Environ Sci 21:307–312
Kjær J, Olsen P, Barlebo HC, Henriksen T, Plauborg F, Grant R, Nygaard P, Gudmundsson L, Rosenbom A (2007) The Danish pesticide leaching assessment programme. Monitoring results May 1999–June 2006. Geological Survey of Denmark and Greenland
Kreuger J, Graaf S, Patring J, Adielsson S (2010) Pesticides in surface water in areas with open ground and greenhouse horticultural crops in Sweden 2008, p. 49
Kudsk P, Mathiassen KS (2004) Joint action of amino acid biosynthesis-inhibiting herbicides. Weed Res 4:313–322
Mohammad M, Itoh K, Suyama K, Yamamoto H (2006) Recovery of Lemna sp. after exposure to sulfonylurea herbicides. Bull Environ Contam Toxicol 76:256–263
Mohammad M, Itoh K, Suyama K (2008) Comparative effects of different families of herbicides on recovery potentials in Lemna sp. J Pest Sci 33(2):171–174
Mohammad M, Itoh K, Suyama K (2010) Effects of herbicides on Lemna gibba and recovery from damage after prolonged exposure. Arch Environ Contam Toxicol 58:605–612
OECD 221 (2004) Guideline for the testing of chemicals. Lemna sp. growth inhibition test. Organisation for Economic Co-operation and Development, Paris
Olette R, Couderchet M, Biagianti S, Eullaffroy P (2008) Toxicity and removal of pesticides by selected aquatic plants. Chemosphere 70:1414–1421
Riemens MM, Dueck T, Kempenaar C, Lotz LAP, Kropff MJJ (2009) Sublethal effects of herbicides on the biomass and seed production of terrestrial non-crop plant species, influenced by environment, development stage and assessment date. Environ Pollut 157:2306–2313
Ritz C, Streibig JC (2005) Bioassay analysis using R. J Stat Softw 12:1–22
Rosenkratz RT, Baun A, Kusk KO (2013) Growth inhibition and recovery of Lemna gibba after pulse exposure to sulfonylurea herbicides. Ecotoxicol Environ Saf 89:89–94
Rüdiger W (2002) Biosynthesis of chlorophyll b and the chlorophyll cycle. Photosynth Res 74:187–193
Saja D, Rys M, Stawoska I, Skoczowski A (2016) Metabolic response of cornflower (Centaurea cyanus L.) exposed to tribenuron-methyl one of the active substances of sulfonylurea herbicides. Acta Physiol Plant 38:168
Schloss JV (1990) Acetolactate synthase, mechanism of action and its herbicide binding site. Pest Sci 29(3):283–292
Sobrero MC, Rimoldi F, Ronco AE (2007) Effects of the glyphosate active ingredient and a formulation on Lemna gibba L. at different exposure levels and assessment end-points. Bull Environ Contam Toxicol 79:537–543
Teodorović I, Knežević V, Tunić T, Čučak M, Nikolić Lečić J, Leovac A, Ivančev Tumbas I (2012) Myriophyllum aquaticum versus Lemna minor: sensitivity and recovery potential after exposure to atrazine. Environ Toxicol Chem 31(2):417–426
Turgut C, Grezichen A, Fomin A (2003) Toxicity of sulfonylurea herbicides to dicotyledonous macrophyte Myriophyllum aquaticum in a 14 day bioassay. Fresen Environ Bull 12:619–622
Von Wettstein D (1957) Chlorophyll-letale und der submik roskopische Formwechsel der Plastiden. Exp Cell Res 12:427–506
Wang M, Zhou Q (2006) Effects of herbicide chlorimuron-ethyl on physiological mechanisms in wheat (Triticum aestivum). Ecotoxicol Environ Saf 64:190–197
Wilson PC, Koch R (2013) Influence of exposure concentration and duration on effects and recovery of Lemna minor exposed to the herbicide norflurazon. Arch Environ Contam Toxicol 64:228–234
Wilson PC, Wilson SB, Haunert D (2006) Toxicity of the norflurazon to the aquatic macrophyte Vallisneria Americana (Michx). J Toxicol Environ Health Part A 69:1167–1179
Zabalza A, Zulet A, Gil-Monreal M, Igal M, Royuela M (2013) Branched-chain amino acid biosynthesis inhibitors: herbicide efficacy is associated with an induced carbon-nitrogen imbalance. J Plant Physiol 170:814–821
Žaltauskaitė J, Brazaitytė V (2013) Assessment of the effects of sulfonylureas herbicide amidosulfuron application on target and non-target organisms. Fresen Environ Bull 22:1977–1982
Acknowledgements
The authors thank Aurelija Dapkutė for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by S. Weidner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zaltauskaite, J., Kaciene, G. Lemna minor recovery potential after short-term exposure to sulfonylurea herbicide. Acta Physiol Plant 42, 35 (2020). https://doi.org/10.1007/s11738-020-3022-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-3022-7