Abstract
Understanding factors influencing the time of weed seedling establishment can contribute to developing predictive models for control measures at early growth stages. Non-linear regression models (Dent-like and Quadratic polynomial) and hydrothermal time models were considered for estimating cardinal temperature and predicting the emergence time of the Setaria species (S. viridis, S. verticillata, and S. glauca) at different constant temperatures and water potentials. Field experiments were also conducted, in which seeds of the species were sown and seedling emergence was recorded daily. The optimum temperature for germination was 27.7, 30.2, and 30.5 °C as estimated by a Dent-like model at 0 MPa water potential for S. glauca, S. viridis and S. verticillata, respectively. According to the hydrotime model, the minimum amount of base water potential (Ψb) was observed at the optimum temperatures, while it reached its highest value at temperatures exceeding the optimum. Overall, at sub-optimal temperatures, with the decrease in water potential, the thermal time (TT) constant increased linearly until − 0.6 MPa, but this trend was downward at supra-optimal temperatures. The hydrothermal time constant (θHT) was 213.5, 228.8, and 318.8 MPa °C h for S. viridis, S. verticillata, and S. glauca, respectively. Non-linear regression and hydrothermal models showed that S. glauca can emerge earlier than other species because of lower base temperature and a higher hydrothermal time constant. Setaria species did not show a significant difference in their tolerance to water stress by similar base water potential (Ψb(50) ~ − 0.5).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foxtail species (Setaria spp.), belonging to the Poaceae family, are among the most problematic summer annual weeds in global agricultural lands and disturbed areas (Holm et al. 1991, 1997). Setaria species cause numerous problems to many crops such as maize (Zea mays L.), wheat (Triticum aestivum L.), soybean (Glycine max L.), sugarcane (Saccharum officinarum L.) and potato (Solanum tuberosum L.) (Amini et al. 2015; Blackshaw et al. 1981a).
Genetic and phenotypic diversity helps Setaria spp. in successful colonization, invasion, and adaptation to new and disturbed areas with diverse climatic conditions, such as temperate, tropical, and sub-tropical (Dekker 2003). Due to competitive ability and their synchrony with summer crop emergence, three Setaria species, including S. viridis (L.) P. Beauv., S. glauca (L.) P. Beauv. and S. verticillata (L.) P. Beauv. are considered to be the most problematic weeds in the fields and orchards in Iran (Amini et al. 2015). These species have a rapid life cycle and high reproductive potential (Forcella et al. 1996; Nadeau and Morrison 1986). Dekker (2003) reported that seeds of S. glauca, S. viridis and S. verticillata can survive for an extended period (13–39 years) in soil. Setaria species have after-ripening seed dormancy; therefore, their seeds are almost entirely dormant immediately after harvest (Acharya et al. 2017; Dekker 2003; Dekker et al. 1996; Taylorson 1986).
The major factors that can affect seed germination are temperature and soil water (Baskin and Baskin 2001). Thermal requirements have the most significant impact on the start of germination, as well as the percentage and rate of germination; determining the success or failure of the weed’s establishment (Al-Ahmadi and Kafi 2007). Cardinal temperatures are the specific temperature range for seed germination which includes the minimum (base), optimum, and maximum (ceiling) temperatures (Baskin and Baskin 1988). The germination rate within temperature below base temperature (Tb) and above ceiling temperature (Tc) is zero, whereas at the optimum temperature the germination rate is maximum (Baskin and Baskin 1988). Cardinal temperatures can be estimated by different non-linear models (Soltani et al. 2006). These modelling methods can predict critical growth stages of germination and emergence by using cardinal temperatures as the model input (Guillemin et al. 2013; Trudgill et al. 2005). In addition to non-linear models, the use of a thermal time model is a common approach for predicting seed germination and the emergence of weeds by considering the interactive effect of temperature and time (Trudgill et al. 2005). Despite the wide application of thermal time models, limitations for factoring soil water availability leave a gap in determining seed emergence data.
Water availability is an important factor for controlling seed dormancy state and germination percentage (Chauhan and Johnson 2008). Any change in soil water can change the soil water potential (Ψ) and consequently affect seed germination and emergence timing (Hegarty 1978). Base water potential (Ψb) is the minimum water potential that seeds need to start the germination process and is a required parameter for predicting germination periods (Dahal and Bradford 1994). The hydrotime model (MPa–time) is another model that predicts the emergence process by considering water potential and seed germination rates (Bradford 1990; Gummerson 1986). Through the integration of thermal time and hydrotime models, hydrothermal time models (MPa-°C-time) can be developed, which have the ability to quantify the effect of both temperature and soil water on seed dormancy, germination and seedling emergence (Bradford 2002; Colbach et al. 2006; Grundy et al. 2000; Gummerson 1986; Roman et al. 1999). These models can be used to compare the seed germination time between different species or the same species under different climatic conditions (Steinmaus et al. 2000; Trudgill and Perry 1994).
Information about germination time and the emergence of weeds is crucial for designing effective weed management strategies (Forcella et al. 2000; Ogg and Dawson 1984). Germination phenomenon can occur when seeds are non-dormant under different environmental factors such as light, temperature, soil pH, and water conditions (Del Monte and Dorado 2011; Sabila et al. 2012). As seed heterogeneity and dormancy of Setaria species change under different environmental conditions, there is no single optimal hydrothermal condition that can be applied for all these species (Dekker 2003). This diversity in required conditions for emergence resulting in a different emergence time between species (Guillemin et al. 2013) resulting in management difficulties.
Summer annual weeds are persistent issues in summer crops, as they can successfully compete for light, soil water, nutrients, and space, consequently reducing crop yield (Lindquist et al. 1996; Radosevish et al. 1997). Knowledge of weed emergence time relative to crops is valuable in order to evaluate a weed’s competitive ability (Blackshaw et al. 1981a; Shurtleff and Coble 1985). Predicting emergence time and pattern is also useful for the timely use of herbicides and non-chemical weed control methods. Research assessing germination potential against different hydrothermal environments in Setaria spp. exists (Amini et al. 2015; Blackshaw et al. 1981b) but to our knowledge, there is no work on interspecific variation responses of key Setaria species to changes in hydrothermal conditions and the comparison of seed germination time between Setaria species. The objectives of this study were to determine: (1) the germination response of the three Setaria species’ to variations in temperature and water potential, (2) the cardinal temperatures, (3) the hydrothermal model for predicting emergence time, and (4) the seedling emergence pattern in the field.
Materials and methods
Seed collection and preparation
Mature seeds of three Setaria spp. (S. viridis, S. glauca, and S. verticillata) were collected from the research field of Ferdowsi University of Mashhad, Khorasan Razavi Province, Iran (latitude 36° 18′ S, longitude 59° 36′ E and altitude 995 m above sea level). The average minimum and maximum air temperature (°C), and the annual rainfall (mm) in the past three-decades at the seed collection area are presented in Fig. 1. All the seeds were cleaned and kept in paper bags and stored in darkness at room temperature (23 ± 2 °C). The collected seeds were after-ripened for one year in the Weed Science Laboratory of the Department of Agrotechnology, Ferdowsi University of Mashhad to release dormancy (Amini et al. 2015; Born 1971; Sebastian et al. 2014). Seed dormancy was investigated for all three species before experiments and no dormancy was observed.
Germination experiments
Laboratory experiments were carried out in 2017 at the Weed Science Laboratory of Ferdowsi University of Mashhad. All germination tests were conducted in incubators, which were set at eight constant temperatures (10, 15, 20, 25, 30, 35, 40 and 45 °C) and for 12-h photoperiod and four water potentials (0, − 0.3, − 0.6, − 1.2, and − 1.6 MPa). Before planting, seeds were surface-sterilised using sodium hypochlorite (NaOCl) 1% for 1 min and were rinsed afterward with distilled water. Twenty-five seeds were placed in 9-cm-diameter Petri dishes with two layers of sterilised filter papers (Whatman No. 1). Filter papers were moistened with 5 ml distilled water or water potential solutions. Seeds were considered germinated when the radicle emerged (at least 2 mm in length). Germination was recorded every day and germinated seeds were removed. The counting of germinated seeds was continued until all germination ceased (approx. 28 days). Different water potential solutions (MPa) were prepared by using Polyethylene glycol (PEG) 8000. The solutions of PEG were prepared and adapted to each germination temperature according to Michel and Radcliffe (1995).
Field experiment
The field experiments were conducted during 2016 and 2017, at the Research Field of Ferdowsi University of Mashhad to evaluate the emergence time. Daily maximum and minimum air temperature of the field experiment site was collected from the nearest weather station and is presented in Fig. 2. Maximum seedling emergence of Foxtail species (S. glauca, S. viridis and S. verticillata) occurs at depths between 1 and 5 cm (Amini et al. 2015; Dekker 2003). Therefore; 100 seeds of the Setaria spp. were manually planted at 1 cm soil depth in 0.5 m × 0.5 m quadrats. All trials were sown on the 5th of May based on the standard planting date for maize in Mashhad, Khorasan Province, Iran (Moradi et al. 2013). After planting, the emergence rate was observed on a daily basis. The counting of emerged seedlings was continued until no emergence was observed for consecutive 45 days.
Statistical analysis
Two sets of statistical analysis were performed, the first was conducted using a completely randomized design (CRD) with four replications for germination experiments and the second analysis was designed as a randomized complete block design (RCBD) with three replications for field experiments. Both experiments were repeated twice. Before analyses, the homogeneity and normality of the data were checked and data were pooled as there was no time by treatment interaction. Analysis of variance (ANOVA) was performed using the SAS software (version 9). Differences amongst treatment means were evaluated by Fisher’s protected Least Significant Differences (LSD, p ≤ 0.05), test.
Calculation of the rate of germination
For calculating the rate of germination (S) Eq. (1) was adopted from Maguire (1962),
where En is the number of seeds germinated in the nth daily counting, and Nn is the number of days after starting.
Calculation of cardinal temperatures
To determine the cardinal temperatures at different water potentials, nonlinear regression models: dent-like and quadratic polynomial models were used by Eqs. 2 and 3, respectively
where T is the actual temperature, Tb is the base temperature, To1 is the lower optimum temperature, To2 is the upper optimum temperature, and Tc is the ceiling temperature (°C).
Hydrothermal model
The hydrothermal time model was assessed through the combination of the thermal and hydrotime models (Bradford 1995) mentioned below.
A thermal time model design was employed according to Eqs. 4 and 5 at suboptimal and supra optimal temperatures, respectively. The thermal time model has several criteria: thermal time constant, TT (°C-time) and t(g) is the time for particular germination percentage, g(%)
Since the germination rate (GR) is the reverse of the radicle emergence time, the Eqs. 4 and 5 can be written as Eq. 6:
here we can use the hydrotime model (MPa-time) to predict the emergence time by considering water potential and time using Eq. 7 (Bradford 1990; Gummerson 1986).
where θH is hydrotime constant and Ψb(g) is the water potential for the particular germination percentage, g(%), If Ψb variation has a normal distribution in the seed population, we write Eq. 7 in the form of a probit equation:
where Ψb (50) is the Ψb of 50% germination and σΨb is the standard deviation in Ψb.
The hydrothermal time model can be explained by Eqs. 9 and 10 through combining thermal time and hydrotime models (Bradford 1995, 2002)
where θHT is the hydrothermal time constant to germination, (MPa °C time) and ψb (g) is water potential for a particular germination percentage, g(%). The probit equation can be defined as:
Field emergence time
A three-parameter sigmoid model was fitted to the seedling emergence data obtained (Chauhan and Johnson 2009) at different days after sowing the seeds in the field.
Gmax is the maximum seedling emergence (%), X is the day after sowing, X50 is the time to reach 50% of seedling emergence, and Grate is seedling emergence rate.
Results
Evaluation of cardinal temperature under different water potentials
Setaria germination was significantly (p < 0.01) affected by the interactive effect of different water potentials and temperatures in our models (Table 1 and Fig. 3). As no germination was observed at − 1.6 MPa across all species, this water potential was not shown in the figures and tables. The germination rate of all species increased rapidly in the temperatures ranging between (Tb) and (To) under all the water potential treatments. Estimating the cardinal temperatures at 0 MPa using a quadratic model showed that the maximum germination rate could happen at 27.5, 30.2 and 30.5 °C (To) for S. glauca, S. viridis and S. verticillata, respectively. However, the range of optimum temperatures (To1–To2) estimated by the Dent-like model were 24.5–34.9, 22.9–35.9 and 23.1–40.1 °C at 0 MPa for S. glauca, viridis, and verticillata, respectively. According to the Dent-like model, Tb was 7.7 for S. glauca, 10.2 for S. verticillata, and S. viridis at 0 MPa. The base temperature required for germination did not have significant variation when water potential decreased from 0 to − 0.6 MPa, in S. viridis and S. verticillata but it showed an increase (approx. 1–3° C) from 0 to − 0.3 MPa in S. glauca. The models were not fitted for S. glauca at − 1.2 MPa. Water stress also caused a reduction in To and Tc for all examined species except S. glauca. Comparing the non-linear regression models (Dent-like and quadratic model) exhibited that both models have an acceptable accuracy (R2) to evaluate germination response to temperature and water potential [R2 > 0.88 by Dent-like model and R2 > 0.58 by quadratic model except at water potential − 1.2 (MPa)]. However, the Dent-like model showed a greater performance for estimating the cardinal temperature with the lowest root-mean-square error (RMSE) and a high R2.
The germination response of Setaria viridis, S. verticillata and S. glauca to temperature and the water potential with non-linear regression models [Dent-like (a) and quadratic polynomial (b)]. Parameters are shown in Table 1
Evaluation of hydrotime model
The estimated values for θH, Ψb(50), and σΨb varied at different temperatures. The highest θH was observed at 15, 15 and 10 °C for S. verticillata, S. viridis and S. glauca, respectively. The values of θH presented a declining trend by an increase in temperature (except an increase in 30 °C) in all the species. In S. verticillata, the value of Ψb(50) was reduced by the rise of temperature from 10 to 35 °C, while increasing the temperature from 35 to 45 °C led to an increase in Ψb(50). A similar trend was observed in S. viridis and S. glauca at Ψb(50), but the minimum value of Ψb(50) was observed at 30 °C. The estimated values of σΨb were between 0.56 and 0.89. The hydrotime model showed R-squared values at acceptable levels (~ 80–90%) in all examined temperatures except 10 °C for S. verticillata and S. viridis and 45 °C S. glauca (Table 2).
Evaluation of thermal time model
Overall, the percentage of maximum germination (Gmax) decreased by reducing water potential in all species. S. glauca and S. verticillata had the highest and lowest Gmax, respectively, at all water potential treatments.
The estimated values of the thermal time constant (TT) changed under different water potentials. At sub-optimal temperatures, thermal time constant (TTsub) showed an upward trend by decreasing water potential from 0 to − 0.6. In supra-optimal temperatures, the thermal time constant (TTsupra) did not show a meaningfultrend with decreasing water potential (Table 3). TTsub had greater values in comparison with TTsupra for all observed water potentials. S. verticillata showed higher TT at both sub and supra optimal temperatures at 0 MPa than the other species.
The thermal time model showed higher R-squared values at sub-optimal temperatures than supra optimal temperatures and the lower TTsub was observed for S. glauca at all water potentials compared with other species.
Evaluation of the hydrothermal time model
The hydrothermal time constant (ƟHT) was 213.5, 228.8, and 318.8 (Mpa °C h) for S. viridis, S. verticillata, and S. glauca respectively (Table 4). The estimated Ψb(50) value was − 0.49, − 0.52 and − 0.53 (MPa) for S. viridis, S. glauca, and S. verticillata, respectively (Table 4). S. glauca showed the maximum ƟHT. The standard deviation coefficient (σΨb) was almost the same in all species (~ 1). Cumulative germination fraction by hydrothermal time model showed that the higher percentage of germination can happen at the 0 MPa water potential treatment at 30, 30 and 35 °C for S. viridis, S. glauca, and S. verticillata seeds, respectively (Table 4 and Figs. 4, 5, 6). The values of R-squared for the hydrothermal time model (0.78, 0.85 and 0.87 for S. glauca, S. verticillata and S. viridis, respectively) indicated that the model was able to predict seed germination of the species.
Cumulative germination fraction for Setaria viridis seeds at the range of water potentials and temperature. The circles indicate the interpolation of observed germination data and the lines indicate the cumulative germination fraction predicted by the hydrothermal time model, based on parameter estimates in Table 2
Cumulative germination fraction for Setaria verticillata seeds at the range of water potentials and temperatures. The circles indicate the interpolation of observed germination data and the lines indicate the cumulative germination fraction predicted by the hydrothermal time model, based on parameter estimates in 2
Cumulative germination fraction for Setaria glauca seeds at the range of water potentials and temperature. The circles indicate the interpolation of observed germination data and the lines indicate the cumulative germination fraction predicted by the hydrothermal time model, based on parameter estimates in Table 2
Field emergence time and sequence
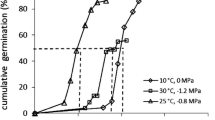
Seedling emergence data exhibited that the Setaria species started to emerge from 7 to 40 days after sowing (Fig. 7). Comparing the time required for 50% emergence (X50) of examined species indicated that S. verticillata germinated more rapidly than the other species. A greater emergence rate was observed by 71.2 and 68.4% at 40 days after sowing for S. verticillata and S. glauca, respectively. The total emergence of S. viridis was 56.2% at the end of the experiment (Fig. 7 and Table 5).
Discussion
The decrease and inhibition of germination at a temperature range above To obsereved in all three species may be due to the thermal denaturation of proteins and altering membrane composition and mobility (Bradford 2002). Thermoinhibition belongs to an increased sensitivity of germination to water deficiency (Bradford and Somasco 1994) and in our study also, an increase in temperatures (at supra optimum) caused an increase in Ψb(50) values.
Stresses such as osmotic stress and low/high temperatures may cause the development of secondary seed dormancy which can affect the germination rate (Baskin and Baskin 2001; Gulden et al. 2004; Taylorson and Brown 1977). Taylorson (1986) reported that high temperature and soil moisture could induce secondary dormancy in germinable S. faberi seeds. Therefore, induced secondary dormancy could also prove useful for inhibiting seed germination at high temperatures and under moisture stress conditions. We also observed that water stress results in a significant decrease in germination and an increase in TT at sub-optimal temperatures. Reducing water availability can reduce the turgor pressure required for cell expansion, thereby impacting seed germination (Bradford and Somasco 1994; Bradford and Still 2004; Mundree et al. 2002). Furthermore, water stress can result in drying seeds and the inability to initiate embryo growth and germination, ultimately decreasing germination rate and percentage (Bradford 2002).
Tribouillois et al. (2016) found that decreasing temperature from 35 to 10 °C resulted in reduced germination from 60 to 5% in S. italica. Blackshaw et al. (1981b) mentioned germination rates of S. viridis declined by reducing water potential from 0 to − 0.8 MPa, and they estimated Ψb for S.viridis at − 0.8 MPa. Amini et al. (2015) observed that germination of S. viridis was inhibited at − 0.5 MPa water potential, while this inhibition happened at − 1 MPa water potential for S. glauca and S. verticillata; however, our research estimated Ψb(50) ~ − 0.5 MPa by hydrothermal time model for all the three species. In our study, seed germination of Setaria species was slightly tolerant to water stress; however, earlier studies reported a high level of tolerance to different water potentials (Blackshaw et al. 1981b; Taylorson 1986). The Ψb(50) value is a correlated parameter that represents the seedling emergence ability of a species under water stress and it also indicates that genotypes with more negative values of Ψb(50), have more tolerance to water-deficient (Soltani et al. 2017). In our study, the results of hydrothermal time showed that Ψb(50) ranged between − 0.49 and − 0.53 MPa, and any significant difference was observed between the Setaria species in water stress tolerance in this study. However, the results of hydrotime model represented − 0.88, − 0.99 and − 1 MPa for Ψb(50) at 30 °C for S. verticillata, S. viridis and S. glauca, respectively. This can be considered as evidence for slightly better tolerance of S. viridis and S. glauca than S. verticillata under water stress.
The estimated values of Ψb(50) in our examined species decreased in T < To and then increased (positive) in T > To. In other studies, the Ψb(50) have also been reported to be at a minimum amount at TO and increased at T > To (Bradford and Somasco 1994; Kebreab and Murdoch 1999). The thermal time model showed lower TT for S. glauca at both sub and supra temperatures at − 0.6 MPa compared with other species, suggesting lower thermal requirements of this species for initiating germination in the field in water deficit conditions.
The (σΨb) value quantifying seed germination uniformity and the lower amount (σΨb) indicates uniform germination (Bradford and Still 2004). The (σΨb) values showed average uniformity in seed germination of the Setaria species in our findings. Comparing the hydrothermal time constant between the species illustrated the maximum ƟHT for S. glauca; therefore, S. glauca can germinate earlier than the other species. The minimum Tb estimated by non-linear regression models also represents the ability of S. glauca to germinate earlier. The result of seedling emergence in the field experiment is also consistent with the estimated hydrothermal time constant (Figs. 4, 5, 6, and Tables 1, 2, 3). Our results suggest that the Setaria species can germinate from the middle of March until the end of October in Mashhad, Khorasan, Iran as the daily mean air temperatures during this period are similar to the Setaria spp. cardinal temperatures. Geographical distribution and ecological niche can affect germination responses to temperatures and water availability (Baskin and Baskin 2001). These varying results across studies of seedling emergence can differ between species from different areas according to differences in temperature, soil water potential, soil and air quality, and light requirements (Forcella et al. 2000). This type of study is beneficial in field management strategies based on predicting the emergence time of weed species via their thermal and hydro requirements and daily mean air temperatures. Knowing emergence time and sequence of weeds is valuable for management strategies, via comparing non-synchrony or synchrony with crop emergence and avoiding crop-weed competition with management programs. Although application of pre-emergence herbicides is a popular method for weed control in agricultural systems, they can be replaced by post-emergence herbicide use informed by emergence time, density and competitive ability of weeds (Battla and Benech-Arnold 2007; Lemieux et al. 2003). It was also found that S. viridis was more competitive with late-planted wheat (Rahman and Ashford 1972) and maize (Nieto and Staniforth 1961) at low water availability, due to its tolerance to higher soil temperatures and lower water potentials than wheat and maize, respectively. Species that emerge earlier than crops can be managed using different weed control methods such as cultivation, pre-emergence herbicides, etc., compared with species that emerge after the crop. Furthermore, seed germination of Setaria spp. can inhibit in Ψ < − 1 MPa and supra optimal temperatures. This response to water stress could be used in the management of this annual weed especially in crops which germinate at Ψ < − 1 MPa. Management of planting date is also another important method in weed control strategies.
Hydrothermal time models can present mathematical and physiological explanations for seed germination and cardinal temperatures (Alvarado and Bradford 2002). These models have the potential to quantify seed germination and seedling emergence responses to the thermal and hydric environment (Bradford 2002). The ability to determine germinability patterns between seed populations and consequently forecast the future development of weeds under different environmental conditions.
Conclusion
The response of the germination rate to different conditions of temperature and water potential was species-dependent. Comparing the parameters of hydrothermal time models, Tb and Ψb between the Setaria species showed variation in germination requirements in these species. Cardinal temperatures for S. glauca were lower than the other two species, suggesting that this species can germinate earlier than the other species in summer. The Setaria spp seeds, especially of S. verticillata, were slightly tolerant to low osmotic potential but highly tolerant to high temperatures, indicating that these species may have a competitive advantage against other plant and weed species under water-deficient conditions. The optimum temperature threshold for the Setaria spp. germination was 27–30 °C when water was not limiting; however, it decreased by osmotic stress and inhibited at Ψ < − 1 MPa. Thus, considering water management with other management practices can be effective in the weed control process. Using the hydrothermal time models verified that seed germination phenomena are adaptive to environmental conditions and are affected by hydrothermal availability. Knowing seedbed patterns, germination requirements and predicting the time of emergence can optimise weed management scenarios.
Author contribution statement
Conceptualization and methodology: EID; Analyzed and interpreted data: MM; Writing-orginal draft: MM; Writing-review & editing: BSC, EID and MBA; The experiments were performed by MM with the supervision of EID and MBA; All authors have read and approved the final manuscript.
References
Acharya BR, Roy Choudhury S, Estelle AB, Vijayakumar A, Zhu C, Hovis L, Pandey S (2017) Optimization of phenotyping assays for the model monocot Setaria viridis. Front Plant Sci 8:2172–2173
Al-Ahmadi JM, Kafi M (2007) Cardinal temperatures for germination of Kochia scoparia (L.). J Arid Environ 68(2):308–314
Alvarado V, Bradford KJ (2002) A hydrothermal time model explains the cardinal temperatures for seed germination. Plant Cell Environ 25(8):1061–1069
Amini V, Zaefarian F, Rezvani M (2015) Effect of pre-chilling and environmental factors on breaking seed dormancy and germination of three foxtail species. Acta Agric Slov 105(2):269–278
Baskin CC, Baskin JM (2001) Seeds, ecology, biogeography and evolution of dormancy, and germination. Plant Ecol 152(2):204–205
Baskin JM, Baskin CC (1988) Role of temperature in regulating the timing of germination in Portulaca oleracea. Can J Bot 66(3):563–567
Batlla D, Benech-Arnold RL (2007) Predicting changes in dormancy level in weed seed soil banks: implications for weed management. Crop Prot 26(3):189–197
Blackshaw RE, Stobbe EH, Shaykewich CF, Woodbury W (1981a) Influence of soil temperature and soil moisture on green foxtail (Setaria viridis) establishment in wheat (Triticum aestivum). Weed Sci 29(2):179–184
Blackshaw RE, Stobbe EH, Sturko ARW (1981b) Effect of seeding dates and densities of green foxtail (Setaria viridis) on the growth and productivity of spring wheat (Triticum aestivum). Weed Sci 29(2):212–217
Born WV (1971) Green foxtail: seed dormancy, germination and growth. Can J Plant Sci 51(1):53–59
Bradford KJ (1990) A water relations analysis of seed germination rates. Plant Physiol 94(2):840–849
Bradford KJ (1995) Water relations in seed germination. In: Kigel J, Galili G (eds) Seed development and germination. Marcel Dekker, Inc., New York, pp 351–396
Bradford KJ (2002) Applications of hydrothermal time to quantifying and modeling seed germination and dormancy. Weed Sci 50(2):248–260
Bradford KJ, Somasco OA (1994) Water relations of lettuce seed thermoinhibition. I. Priming and endosperm effects on base water potential. Seed Sci Res 4(1):1–10
Bradford KJ, Still DW (2004) Applications of hydrotime analysis in seed testing. Seed Technol 26:75–85
Chauhan BS, Johnson DE (2008) Influence of environmental factors on seed germination and seedling emergence of eclipta (Eclipta prostrata) in a tropical environment. Weed Sci 56(3):383–388
Chauhan BS, Johnson DE (2009) Influence of tillage systems on weed seedling emergence pattern in rainfed rice. Soil Till Res 106(1):15–21
Colbach N, Dürr C, Roger-Estrade J, Chauvel B, Caneill J (2006) AlomySys: Modelling black-grass (Alopecurus myosuroides Huds.) germination and emergence, in interaction with seed characteristics, tillage and soil climate: I. Construction. Eur J Agron 24(2):95–112
Dahal P, Bradford KJ (1994) Hydrothermal time analysis of tomato seed germination at suboptimal temperature and reduced water potential. Seed Sci Res 4(2):71–80
Dekker J (2003) The foxtail (Setaria) species-group. Weed Sci 51(5):641–656
Dekker J, Dekker B, Hilhorst H, Karssen C (1996) Weedy adaptation in Setaria spp. IV. Changes in the germinative capacity of S. faberii (Poaceae) embryos with development from anthesis to after abscission. Am J Bot 83(8):979–991
Del Monte JP, Dorado J (2011) Effects of light conditions and after-ripening time on seed dormancy loss of Bromus diandrus Roth. Weed Res 51(6):581–590
Forcella F, Arnold RLB, Sanchez R, Ghersa CM (2000) Modeling seedling emergence. Field Crops Res 67(2):123–139
Forcella F, Peterson DH, Barbour JC (1996) Timing and measurement of weed seed shed in corn (Zea mays). Weed Technol 10(3):535–543
Grundy AC, Phelps K, Reader RJ, Burston S (2000) Modelling the germination of Stellaria media using the concept of hydrothermal time. New Phytol 148(3):433–444
Guillemin JP, Gardarin A, Granger S, Reibel C, Munier-Jolain N, Colbach N (2013) Assessing potential germination period of weeds with base temperatures and base water potentials. Weed Res 53(1):76–87
Gulden RH, Thomas AG, Shirtliffe SJ (2004) Secondary dormancy, temperature, and burial depth regulate seedbank dynamics in canola. Weed Sci 52(3):382–388
Gummerson RJ (1986) The effect of constant temperatures and osmotic potentials on the germination of sugar beet. J Exp Bot 37(6):729–741
Hegarty TW (1978) The physiology of seed hydration and dehydration, and the relation between water stress and the control of germination: a review. Plant Cell Environ 1(2):101–119
Holm LG, Holm L, Holm E, Pancho JV, Herberger JP (1997) World weeds: natural histories and distribution. Wiley, New York, pp 756–768
Holm LG, Plucknett DL, Pancho JV, Herberger JP (1991) The world's worst weeds. Distribution and biology. University Press of Hawaii, Hawaii
Kebreab E, Murdoch AJ (1999) Modelling the effects of water stress and temperature on germination rate of Orobanche aegyptiaca seeds. J Exp Bot 50(334):655–664
Lemieux C, Vallee L, Vanasse A (2003) Predicting yield loss in maize fields and developing decision support for post-emergence herbicide applications. Weed Res 43(5):323–332
Lindquist JL, Mortensen DA, Clay SA, Schmenk R, Kells JJ, Howatt K, Westra P (1996) Stability of corn (Zea mays)-velvetleaf (Abutilon theophrasti) interference relationships. Weed Sci 44(2):309–313
Maguire JD (1962) Speed of germination—aid in selection and evaluation for seedling emergence and vigor 1. Crop Sci 2(2):176–177
Michel BE, Radcliffe D (1995) A computer program relating solute potential to solution composition for five solutes. Agron J 87(1):126–130
Moradi R, Koocheki A, Mahallati MN, Mansoori H (2013) Adaptation strategies for maize cultivation under climate change in Iran: irrigation and planting date management. Mitig Adapt Strateg Glob Chang 18(2):265–284
Mundree SG, Baker B, Mowla S, Peters S, Marais S, Vander Willigen C, Thomson JA (2002) Physiological and molecular insights into drought tolerance. Afr J Biotechnol 1(2):28–38
Nadeau LB, Morrison IN (1986) Influence of soil moisture on shoot and root growth of green and yellow foxtail (Setaria viridis and S. lutescens). Weed Sci 34(2):225–232
Nieto H, Staniforth DW (1961) Corn-foxtail competition under various production conditions 1. Agron J 53(1):1–5
Ogg AG, Dawson JH (1984) Time of emergence of eight weed species. Weed Sci 32(3):327–335
Radosevish S, Holt J, Ghersa C (1997) Weed ecology, 2nd edn. Wiley, New York
Rahman A, Ashford R (1972) Control of green foxtail in wheat with trifluralin. Weed Sci 20(1):23–27
Roman ES, Thomas AG, Murphy SD, Swanton CJ (1999) Modeling germination and seedling elongation of common lambsquarters (Chenopodium album). Weed Sci 47(2):149–155
Sabila MH, Grey TL, Webster TM, Vencill WK, Shilling DG (2012) Evaluation of factors that influence Benghal dayflower (Commelina benghalensis) seed germination and emergence. Weed Sci 60(1):75–80
Sebastian J, Wong MK, Tang E, Dinneny JR (2014) Methods to promote germination of dormant Setaria viridis seeds. PLoS ONE 9(4):e95109
Shurtleff JL, Coble HD (1985) The interaction of soybean (Glycine max) and five weed species in the greenhouse. Weed Sci 33(5):669–672
Soltani A, Hammer GL, Torabi B, Robertson MJ, Zeinali E (2006) Modeling chickpea growth and development: phenological development. Field Crops Res 99(1):1–13
Soltani E, Adeli R, Akbari GA, Ramshini H (2017) Application of hydrotime model to predict early vigour of rapeseed (Brassica napus L.) under abiotic stresses. Acta Physiol Plant 39(11):252–263
Steinmaus SJ, Prather TS, Holt JS (2000) Estimation of base temperatures for nine weed species. J Exp Bot 51(343):275–286
Taylorson RB (1986) Water stress-induced germination of giant foxtail (Setaria faberi) seeds. Weed Sci 34(6):871–875
Taylorson RB, Brown MM (1977) Accelerated after-ripening for overcoming seed dormancy in grass weeds. Weed Sci 25(6):473–476
Tribouillois H, Dürr C, Demilly D, Wagner MH, Justes E (2016) Determination of germination response to temperature and water potential for a wide range of cover crop species and related functional groups. PLoS ONE 11(8):1–16
Trudgill DL, Perry JN (1994) Thermal time and ecological strategies—a unifying hypothesis. Ann Appl Biol 125(3):521–532
Trudgill DL, Honek A, Li D, Van Straalen NM (2005) Thermal time—concepts and utility. Ann Appl Biol 146(1):1–14
Acknowledgements
The authors wish to acknowledge the support provided to this work by the Ferdowsi Universityof Mashhad, Iran through the research program (Project No. 3/40529).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
No conflicts of interest have been declared.
Additional information
Communicated by F. Araniti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mollaee, M., Darbandi, E.I., Aval, M.B. et al. Germination response of three Setaria species (S. viridis, S. verticillata, and S. glauca) to water potential and temperature using non-linear regression and hydrothermal time models. Acta Physiol Plant 42, 149 (2020). https://doi.org/10.1007/s11738-020-03133-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03133-w