Abstract
A simple method has been developed for clonal propagation of mature trees of Tecomella undulata (Sm.) Seem, a medicinally important deciduous timber tree of hot arid regions, via multiple shoot proliferation from axillary buds after examining the role of season influences and physico–chemical conditions on micropropagation. Spring season (March–April) was the best period for contamination free establishment of explants and maximum sprouting of healthy axillary buds. Shoots proliferated directly from the explant nodes cultured on Murashige and Skoog’s medium containing cytokinins, BAP supporting better growth compared to kinetin during shoot induction as well as multiplication phase. Cytokinin concentration influenced the bud induction frequency and optimal response of 2.6 buds per explant was achieved in 86.66% explants on media supplemented with 10 µM BAP. Stunted shoot buds with excessive callus were observed when cytokinin concentration was increased beyond optimal levels. Ascorbic acid (50 mg/l), arginine and citric acid (25 mg/l each) were added to proliferation and multiplication media for reducing callus proliferation and better shoot growth. Among the media (B5, MS, NN, WPM and SH) tested, SH was best for shoot multiplication. Shoot cultures were multiplied by regular subculture of axillary shoots on SH medium containing 5.0 µM each of BAP and kinetin. Shoots produced roots when cultured on ½× SH medium + 10 μM IBA. Regenerated plantlets were successfully transferred to field after hardening and acclimatization. Genetic homogeneity of tissue culture raised plants was confirmed by generation of monomorphic DNA fragments with Start codon targeted and intersimple sequence repeat (ISSR) markers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tecomella undulata (Sm.) Seem, an economically important deciduous, medicinal tree of family bignoniaceae, is designated as the state flower of Rajasthan, India. This monotypic genus originating in India and Arabia (Randhawa and Mukhopadhyay 1986) has restricted distribution in the arid regions of Arabia, southern Pakistan and northwestern India (Tewari 2007). Major population of the species is found in western parts of Rajasthan where it is locally known as rohida or Desert teak or ‘Marwar Teak of Rajasthan’. Rohida is used against infections caused by bacterial and fungal pathogens; and for anti-cancerous, cytotoxic, analgesic, anti-inflammatory, anti-obesity, and immunomodulatory activities (Kalia et al. 2014). It has also been used for backfill rehabilitation in lignite mines at Giral, Barmer in Rajasthan (Kumar et al. 2011) and for phytoremediation of soil contaminated with chromium (Mathur et al. 2010). This important component of arid zone agroforestry systems has been designated as “threatened” in Rajasthan, India, due to over-exploitation (Tripathi and Jaimini 2002). Only few young plantlets are seldom found growing in the field while the older trees sometimes have hollow trunks, due to attack by wood decaying fungi and borers, leading to its death (Kalia et al. 2014). The conventional propagation using seeds is poor due to short seed viability and improper harvest from the plant with no conservation efforts by local populations. Adding to the problem is the high rate of cross-pollination which may lead to loss of desirable silvicultural traits. Vegetative propagation methods are not available for this slow growing tree.
Micropropagation offers an alternative method for mass clonal propagation. Moreover, the quality characteristics of a species can be improved biotechnologically (transgenics) through in vitro methods only. Axillary bud proliferation is widely used for propagation in many plant species as the clones derived from axillary buds have minimum risk of appearance of somaclonal variations. A micropropagation protocol developed using mature explants has great commercial value; however, woody plants are difficult to establish under in vitro conditions when explants are obtained from mature trees. In vitro propagation of rohida using seedling explants (Varshney and Anis 2012; Singh et al. 2009; Aslam et al. 2006; Nandwani et al. 1995, 1996) and mature explants (Tyagi and Tomar 2013; Kumari and Singh 2012; Bhansali 1993; Rathore et al. 1991; Arya and Shekhawat 1986) has been reported; however, difficulties in culture establishment using mature explants, slow growth, limited rooting potential and difficulties in field establishment remain the major bottlenecks (Kalia et al. 2014). Limited number of plantlet production has been achieved in all the above studies due to inconsistency in rooting of shoots. Hardening and acclimatization of tissue-culture-raised plants to field conditions remains another limiting step in lab-to-land transition of tissue culture technology in this species (Tyagi and Tomar 2013).
Many factors like propagation methodology followed, explant source, ploidy level of the species and age of culture under in vitro conditions (Rani and Raina 2000), and use of high levels of auxins and cytokinins (Venkatachalam et al. 2007) are known to induce somaclonal variations during in vitro regeneration. Therefore, genetic homogeneity of in vitro raised plants must be assessed especially in trees having long life cycles. Morphological and physiological traits remain highly unreliable for the purpose being influenced by ecological factors. DNA markers capable to detect variations at any growth stage (Kalia et al. 2011) can supplement morphological evaluations. Many DNA-based markers (RAPD, ISSR, SCoT, AFLP, SAMPL and SSR) have been used for genetic homogeneity testing of tissue-culture-raised plants. Only RAPD markers have been used for genetic homogeneity testing of in vitro produced T. undulata plants (Kumari and Singh 2014); however, they are not very reliable being random in origin with short primers and low annealing temperatures.
Therefore, an attempt was made to clonally propagate mature trees of T. undulata through tissue culture followed by assessment of genetic homogeneity of clones using SCoT and ISSR markers.
Materials and methods
Micropropagation
Nodal segments were collected from 31-year-old trees of T. undulata growing in CAZRI campus, Jodhpur. Explants were swabbed with wet cotton, washed thoroughly in running tap water followed by Tween 20 wash prior to sterilization with 0.1% HgCl2 solution for 8–10 min in laminar airflow chamber (Chhajer and Kalia 2016). Finally these explants were washed 3-4 times with autoclaved distilled water and then cultured on MS medium (Murashige and Skoog 1962) containing plant growth regulators for axillary bud induction and proliferation. Effect of addition of 5.0–25.0 μM cytokinin (BAP or kinetin added singly) to induction medium was assessed using MS basal medium as control. To assess the seasonal influence on explant response, contamination and induction frequency, nodal segments collected every month (between dates 22nd–25th) were cultured on MS + 10 μM BAP. Effect of addition of ascorbic acid (50 mg/l), adenine sulphate (25 mg/l), citric acid (25 mg/l), arginine (25 mg/l) and 0.5% activated charcoal on callus reduction and shoot health improvement was also studied.
Shoot multiplication was standardized using SH medium containing 5.0–25.0 μM cytokinins (BAP, kinetin) alone, cytokinin-auxin combination (10.0 μM BAP and 0.5/2.5 µM NAA/IAA) and cytokinin–cytokinin combination of BAP and kinetin (5.0–15.0 μM each). Shoots were cultured on BAP and kinetin (5 μM each) enriched MS (Murashige and Skoog 1962), WPM (Lloyd and McCown 1980), NN (Nitsch and Nitsch 1969), B5 (Gamborg et al. 1968) and SH (Schenk and Hildebrandt 1972) media for selection of most suitable basal medium. To study the effect of carbon source (sucrose, table sugar, fructose and d-glucose), propagule’ size (single shoot, bunch of two, three, four and five shoots), subculture duration (1–5 weeks), and medium consistency (semi-solid vs liquid), shoots were cultured on SH medium + 5 μM BAP + 5 μM kinetin.
Shoots (2.0–2.5 cm) were transferred to 0.6% agar gelled SH medium (without additives) containing 5–25 μM NAA, IBA, IAA or NOA added singly and 2.0% sucrose for rooting, basal medium without growth regulators was used as control. In another set ferulic acid, choline chloride, ascorbic acid and activated charcoal were added singly in SH medium containing 10 µM IBA to study their role in root induction. For hardening, the rooted plantlets were washed thoroughly in autoclaved water to remove agar and transferred to liquid SH medium (¼× salts, 1% sucrose) containing Whatman’s filter paper bridge as support. These hardened plantlets were then established in soilrite in magenta boxes (kept for 2 weeks) and fed with 1/4× SH medium, followed by transfer to earthen pots containing equal proportions of soil and farmyard manure.
MS/SH medium containing 3% sucrose, ascorbic acid (50 mg/l), arginine and citric acid (25 mg/l each), 0.7% agar adjusted to pH 5.8 was used in the experiments. The 100 ml conical flasks containing 30 ml medium were autoclaved at 1.06 kg cm−2 for 15 min at 121 °C. The cultures were provided an irradiance of 30 μmol m−2 s−1 for 16 h daily and maintained at 25 ± 2 °C temperature.
Genetic fidelity testing
For genetic fidelity testing total genomic DNA of five tissue culture raised plants and the mother plant was extracted using the protocol of Doyle and Doyle (1990). DNA yield and purity was checked in 0.8% agarose gel and by using Biophotometer (D30, Eppendorf, Germany). Eight SCoT [designed by Collard and Mackill (2009)] and nine ISSR primers out of fifteen each screened were used for the assessment of genetic fidelity. PCR reaction (25 µl) containing 50–70 ng genomic DNA, 1× PCR buffer (Sigma Aldrich), 0.2 mM dNTP mix (Thermo Scientific), 1.5 mM MgCl2, 1 µM primer (ISSR-Life-Technologies, India Pvt. Ltd; SCoT-IDT Inc.) and 0.6 U Taq DNA polymerase (Sigma Aldrich) was run in an Eppendorf thermocycler (Mastercycler Nexus GSX1). PCR program used was-Initial denaturation at 94 °C (4 min); 40 cycles of denaturation at 94 °C (45 s), annealing at Ta °C (1 min) and extension at 72 °C (1 min), and final extension at 72 °C (10 min). The 1× TAE gels (2% agarose, Amresco, USA) containing ethidium bromide (1.0 µg/ml gel; Sigma Aldrich) were run at 110 V for 2 h and captured using an Alphaimager EC (Protein Simple, California, USA) gel documentation system. GeneRuler 100 bp plus DNA ladder (Thermo Scientific, EU, Lithuania) was used in all agarose gels. All reactions were repeated once to ensure consistency in results and the well-resolved unambiguous and consistently produced fragments were scored manually.
Data analysis
Each experiment having 20 explants per treatment was repeated twice. Fortnightly observations quantified the treatment effects based on number of responsive explants, number and length of shoots/explant, callus growth and overall health of shoots. Percent rooting, root number and length were measured in rooting experiments. The results expressed as mean ± SE of three independent experiments were subjected to SPSS v.17 (SPSS, Chicago, USA) for calculation of one-way analysis of variance (ANOVA). Duncan’s multiple range tests (DMRT) at P 0.05 was used to calculate the significance of differences among the means.
Results and discussion
Micropropagation
Axillary bud induction: It is always desirable to clone selected elite trees in silviculturally important species; however, this cloning is usually difficult due to decrease in competence of mature explants to dedifferentiate. Differentiation potential and plasticity of cells and tissues usually decline with their progressive specialization over the years (Abdullah et al. 1987). Proliferating axillary meristems usually result in progenies free from somaclonal variations, as has also been proved using molecular markers (Singh et al. 2013a). In the absence of vegetative propagation methods for this endangered timber tree, nodal explants were collected from 31-year-old trees for culture establishment. Establishment of mature nodal explants as well as their response was influenced by the season of explant collection. HgCl2 was more suitable for explant sterilization compared to sodium hypochlorite, 0.1% HgCl2 treatment for 8–10 min resulted in 70 to 72% aseptic and viable cultures. Explants collected during spring season (March–April) showed lesser contamination, early shoot initiation and better percent bud break with more shoots per explant (Fig. 1). Spring season marks the end of dormancy and commencement of period of increased vegetative growth. Fresh sprouts start to appear on existing branches in natural conditions in T. undulata in the months of Feb–March (Kalia et al. 2014). Explants collected during active growth period are more responsive towards bud break (Singh et al. 2012a, b). More auxins are produced in young buds during spring season which stimulate cell division in the cambium thus supporting increased cell division and growth (Funada et al. 2001). Contrary are the reports of Tyagi and Tomar (2013) and Rathore et al. (1991) who reported maximum bud break in Jan–Feb and Aug–Sept respectively in rohida. The winter months (Dec–Jan) were highly unsuitable for in vitro establishment of nodal explants due to dormant growth. Singh et al. (2012a) observed similar trend in Dendrocalamus asper. Maximum contamination was recorded in rainy season (July–Aug) when high moisture supports luxuriant growth of microbes on branches and in shoot axils. Similar reports of seasonal effects on contamination percentage and bud break in explants collected from mature trees are also available in many other plant species (Arya et al. 2005; Bhatt and Dhar 2005; Tyagi and Tomar 2013). As reported by Tyagi and Tomar (2013) phenolic leaching was not a major problem in this species.
Multiple shoots were induced on the mature nodal segments cultured on growth regulator supplemented media; however, nodes cultured on basal medium without growth regulators showed no axillary bud break. Cytokinins induced development of multiple shoots in node axils within 2–3 weeks of culture. BAP was more effective compared to kinetin, showing optimal response of 2.6 buds in 86.66% explants at 10 μM concentration (Fig. 2). In both the cytokinins tested, length of shoot buds increased with increase in concentration up to the optimal levels and showed a decline thereafter. Profuse callusing and fewer shoot buds per explant were observed at higher BAP (20 and 25 μM) concentrations. These results are in conformity with those of Tyagi and Tomar (2013) in T. undulata. Moreover, these buds were stunted and vitrified which could not be subcultured further. Higher levels of cytokinin are known to induce leaf yellowing and root mass reduction in intact plants, and programmed cell death in cell cultures (Carimi et al. 2003). Cytokinins are also known to enhance biosynthesis of ethylene (Abeles et al. 1992) thereby adversely affecting the growth of cultures. Shoot buds were also seen developing in the leaf axils on primary and secondary shoots (secondary and tertiary buds respectively) in some explants cultured on BAP supplemented medium. Kinetin supplemented media supported more elongation of the fewer axillary buds induced in explant axils (Fig. 2). Addition of TDZ, a non-purine cytokinin known for improving multiplication response in woody plants was found unsuitable in T. undulata as it led to proliferation of excessive callus with negligible shoot proliferation. However, Varshney and Anis (2012) reported maximum shoot proliferation on TDZ supplemented medium in Tecomella. Therefore, BAP was more potent compared to kinetin and TDZ. Critical role of cytokinin type and its dosage in affecting shoot organogenesis is well documented. BAP can be metabolized more readily compared to other synthetic growth regulators and can also induce production of other growth regulators like zeatin in the tissues (Zaerr and Mapes 1982), thus supporting better bud induction rates. Efficiency of BAP in inducing multiple shoots and its toxicity at supraoptimal concentrations has also been reported by Kalia et al. (2004, 2007), and Khalafalla and Daffalla (2008). Induction of secondary and tertiary buds at higher BAP concentrations was also observed in Pinus roxburghii (Kalia et al. 2007). Use of liquid medium was not effective during axillary bud break in the present study.
Addition of arginine, ascorbic acid and citric acid improved shoot health with reduction in callus content. Addition of adenine sulphate on the other hand increased callusing while activated charcoal reduced callusing with deterioration of shoot health. Therefore, ascorbic acid (50 mg/l), citric acid and arginine (25 mg/l each) were routinely added to induction and multiplication media. Supplementation of adsorbents like activated charcoal and antioxidants such as cysteine and ascorbic acid is known to improve shoot health (Sanyal et al. 2005; Arditti and Ernst 1993). Agarwal et al. (2015) also reported that medium containing adenine sulphate, ascorbic acid, citric acid and arginine improved shoot induction, multiplication and growth in Alhagi maurorum. Ascorbic acid and citric acid are anti-oxidants, while l-arginine provides reduced nitrogen (Patel et al. 2014). Adenine sulphate shows cytokinin-like activity due to base structure similarity to cytokinins (Gaspar et al. 1996) therefore its addition may have supplemented the effect of BAP and produced response similar to supra-optimal concentration of BAP in the medium. However, Singh et al. (2012a) reported enhanced multiplication rates in Dendrocalamus asper when adenine sulphate was incorporated in the medium. Agar concentration was also reduced from routinely used 0.8 to 0.7% during induction and multiplication experiments to reduce callusing at explant base as hard medium leads to radial growth of callus.
Shoot multiplication: The elongating axillary shoots were excised from nodal explants and cultured on multiplication media supplemented with cytokinin, cytokinin–cytokinin or cytokinin–auxin combination. Shoots cultured on basal medium without growth regulators failed to multiply and died within 3–4 weeks. BAP supplemented medium supported a better multiplication rate (2.32) with more callus proliferation and shorter shoots (1.03–1.56 cm) compared to kinetin which supported development of fewer (maximum multiplication rate 1.12) but longer shoots (2.03–3.79 cm) with less callus. The bud to shoot conversion rate declined with increasing BAP concentration as was also observed by Malik et al. (2005) in Garcinia indica. Addition of NAA or IAA in the medium did not improve the regeneration response (Table 1). BAP in combination with NAA/IAA was deleterious and significant decline was observed in percent response, average bud number (1.70–2.65) and bud length (0.20–0.51 cm). Average bud number induced on these media combinations was much less compared to medium containing only BAP (2.75–6.95) or kinetin (2.85–3.35) or combination of BAP and kinetin (2.55–9.05). Moreover, shoot cultures showed callus development and also enhanced vitrification in NAA or IAA supplemented media. On the contrary many reports on T. undulata document regular use of IAA or NAA during establishment and multiplication phase (Robinson et al. 2005; Kumari and Singh 2012; Tyagi and Tomar 2013). Deleterious effects of NAA on shoot multiplication rates have been recorded in Pinus roxburghii (Kalia et al. 2007) and Garcinia indica (Malik et al. 2005) also. Kalia et al. (2004) reported that responsiveness of in vitro cultures towards added NAA was genotype dependent, six clones of Dalbergia sissoo (from Rajasthan and Haryana), showed enhanced growth while another six clones (from Uttranchal and Uttar Pradesh) showed decline in response when NAA was added to the multiplication medium. Further, the multiple bud induction capacity declined with increase in auxin level. The seasonal variation in endogenous levels of auxins in different species and genotypes will control their variable response on auxin-supplemented media. The auxins accumulating at the base of explants are known to inhibit their shoot forming capacity (Marks and Simpson 1994). Combination of BAP (5 µM) and kinetin (5 µM) supported maximum multiplication rates (3.02) as well as shoot length (3.10 cm) and health, and was therefore selected for all further experiments. Combination of two or more cytokinins has also been used in previous studies (Bhansali 1993).
Decapitation of longer shoots of the explant led to faster elongation of the remaining smaller buds. Parasharami et al. (2003) also reported similar trend in Pinus roxburghii. This response is attributed to release of apical dominance due to removal of auxins accumulated in the apical region; therefore elongated shoots were regularly removed. This hedging technique is usually employed in tissue culture for continuous production of shoots (Aitken-Christie and Jones 1987). Optimal shoot multiplication rate were achieved using 1.5–2.0 cm long shoots; shorter/longer shoots multiplied at a slower rate. Factors affecting shoot-forming capacity in vitro included (1) cytokinin type and concentration, (2) shoot length, (3) propagule size and (4) subculture duration. Single shoots when cultured on multiplication medium had more vitrified appearance compared to bunch of two or more shoots. A bunch of 3-4 shoots supported optimal multiplication rates compared to sparse or more dense bunch (Fig. 3). Similar reports are also available in many trees (Arya et al. 1999; Kalia et al. 2004). Sub-culturing was done periodically every 3 weeks so as to maintain healthy shoot cultures. Longer sub-culture durations of 4–5 weeks led to yellowing of shoots instead of enhancing the multiplication rate further while optimal multiplication rates were not achieved in shorter subculture durations of 1–2 weeks (Fig. 4). Further, the shoots subcultured after 4–5 weeks had a reduced multiplication potential compared to those subcultured after 3 weeks. The nutrient availability becomes a limiting factor during longer subculture cycles thus hampering the shoot health. Rathore et al. (1991) also reported extensive leaching, senescence and deterioration of cultures when maintained beyond 25 days in the same culture vessel/medium. Bisht et al. (2010) and Singh et al. (2012b) also reported similar results in Gigantochloa atroviolaceae and Dendrocalamus hamiltonii respectively.
Many combinations of nutrients have been formulated by different research groups for different plants over a period of time. Many plant species have shown differential preference for specific medium during explant establishment and growth (Sokolov et al. 2014; Singh et al. 2012a, b). The in vitro raised Tecomella shoots gave significantly higher response in SH medium (7.01 shoots per propagule; 2.34-fold multiplication rate) compared to MS, B5, WPM or NN (Fig. 5). Shoots maintained on WPM and NN medium showed poor health with yellowing of leaves. Therefore, composition of the basal medium remained an important factor determining the physiological performance of shoots in vitro. The media differ in the type of salts and concentration of macro- and microelements, and vitamins. High ammonium and nitrate content in B5 medium can lead to inhibition of shoot growth and multiplication (Constabel 1984), while better axillary growth in forest trees has been reported on media like WPM, GD, NN etc. containing weaker salt formulations (McCown and Sellmer 1987). However, contrasting results have been recorded in the present study as also by Singh et al. (2012a) in Dendrocalamus asper. Shoots cultured in liquid medium had lower multiplication rates as compared to agar gelled medium, and these shoots had pale vitrified appearance (Table 2). Similarly, Agarwal et al. (2015) and Singh et al. (2012a) reported decline in response in liquid medium; however, contrary are the results in D. hamiltonii (Singh et al. 2012b) and Acacia nilotica (Rathore et al. 2014).
Multiplication rates were not significantly affected when sucrose was replaced with table sugar (Fig. 6). Micropropagation studies on Pogostemon cablin (Swamy et al. 2010) and Chlorophytum borivilianum (Singh and Goyal 2008) reportedly used table sugar instead of sucrose during in vitro studies.
Therefore, SH medium + 3% sucrose/table sugar + 5 μM BAP + 5 μM kinetin + 50 mg/l ascorbic acid + 25 mg/l arginine + 25 mg/l citric acid + 0.7% agar was optimized as multiplication medium using 3-4 shoots as propagule with regular sub-culturing at 3 week interval.
Rooting and hardening: Induction of adventitious roots in tree species is highly variable in vivo as well as in vitro, therefore, is a major bottleneck in plantlet regeneration. Rooting potential of cuttings becomes a limiting factor with increase in mother tree age due to progressive specialization of tissues and reduction in dedifferentiation potential of cells (Abdullah et al. 1987). Rooting remained the major bottleneck during in vitro propagation of T. undulata also where no method of vegetative propagation is available due to negligible rooting potential of cuttings in nature. Preliminary experiments indicated that 0.8% agar gelled medium led to excessive callus formation at shoot base with negligible rooting therefore, agar concentration was reduced to 0.6% during rooting experiments. Previously, Kalia et al. (2007) also reported that softer (0.6% agar gelled) medium supported better rooting in Pinus roxburghii compared to harder medium (0.7 and 0.8% agar gelled) due to better root penetration and lesser radial growth of callus on medium surface. Similarly, Mohammed and Vidaver (1988) reported that impediment of gaseous exchange in agar gelled medium led to poor root quality.
In vitro rooting efficiency is influenced by several factors like basal medium used and its strength, auxin type and concentration, and additives incorporated (Yokawa et al. 2013). Half strength SH medium proved better for in vitro root induction compared to slow and delayed rooting response on 1/4× and 1× SH medium. Half strength of basal salts has been suitable for in vitro rooting in earlier reports (Agarwal et al. 2015; Singh et al. 2012b). Auxin was found essential for rooting as no roots developed in shoots cultured on 1/2× SH medium without auxins. An average of 3.74 roots developed in 20% shoots on ½× SH medium containing 10 μM IBA (Table 3). IBA was found better than NAA for root induction, while IAA and NOA failed to induce roots at any concentration. Addition of ascorbic acid improved the rooting potential marginally (3.97 roots in 21.66% shoots); however, addition of ferulic acid, choline chloride or activated charcoal could not improve rooting response. Other methods used to improve the rooting percentage included: (a) transferring the shoots to basal medium for 7 days, before shifting to rooting medium, to reduce the carry-over effect of cytokinins, (b) culturing shoots on high IBA (50 ppm) supplemented medium for 3 days followed by transfer to basal medium, and (c) treatment of shoot base with commercial rooting powder (Veradix, Evergreen Chemicals, Bhiwadi, India) or (d) 500 ppm IBA for 30 min before transferring them to sterile Soilrite for rooting. However, none of these methods could improve the rooting percentage. In contrast are the reports of Kumari and Singh (2014) and Tyagi and Tomar (2013) who reported 63.8% (nodal explants derived from 2–3-year-old tree) and 43.4% (nodal explants derived from 15-year-old tree) rooting when the shoots were pulse treated with auxin for 48 h (5 µM NAA) or 15 min (492.1 µM IBA) followed by transfer to ½ MS and ½ B5 basal medium, respectively. In our studies shoots shriveled within 7–10 days of transfer on to basal medium and were not able to remain healthy during multiplication as well as rooting experiments. Increase in mother tree age which is known to reduce its competence to produce adventitious roots (Abdullah et al. 1987) probably led to such lesser rooting percentage in 31-year-old tree derived explants. Adding to this is the fact that vegetative propagation of Tecomella remains a big challenge in absence of its ability to produce roots in soft or hard cuttings even after hormone treatments.
Plantlets developed in vitro grow heterotrophically on a medium containing ample sugar and nutrients, lower light intensity and under high humidity levels within the culture vessels. These micropropagated plantlets having a weak root system and poorly developed cuticle showed high mortality when transferred to ex vitro conditions without proper hardening and acclimatization. Gradual hardening and acclimatization helped the plants to develop phototrophic growth. The roots proliferated further in liquid ½× SH medium containing filter paper bridges as support (Fig. 7). These plantlets were further transferred to autoclaved soilrite in magenta boxes, fed with 1/4× SH medium, and kept under culture room conditions for another three weeks. Supplementation of reduced mineral salts during hardening forces the regenerates to strengthen their own photosynthetic system (Kozai et al. 1988). The in vitro developed plants hardened for 4–6 weeks could survive under normal conditions. Finally these hardened plantlets were transferred to pots containing soil and FYM in 1:1 ratio, fed with 1/4× SH salts and covered with perforated polybags. Duration of exposure to ambient conditions was increased from 1 to 2 h daily to permanent removal of perforated polybag covers within three weeks. During hardening and acclimatization to ambient conditions, leaf size and thickness gradually increased with development of cuticle. The properly hardened plantlets had 100% survival in the field, resumed growth and showed normal morphological characteristics. Tyagi and Tomar (2013) had reported that 50% of the rooted shoots (43.4%) having necrotic shoot tips were not suitable for hardening; however, such tip necrosis was not recorded in the present study. Further, they reported 35% survival during hardening in contrast to 100% survival observed here. Singh et al. (2012b) also reported better survival and vigorous growth of in vitro raised plants in dune sand (3 parts) and vermi-compost (1 part) combination.
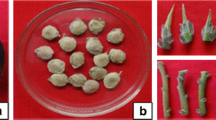
Rooting of shoots and hardening of in vitro raised plantlets of T. undulata (a) Rooting on 1/2× SH + 10 µM IBA, (b) Hardening in 1/2× SH liquid medium with filter paper bridge, (c) In vitro raised plantlet. Plantlet growing in (d) sterile soilrite (e) Pot containing soil and FYM in 1:1 ratio after 1 month and (f) 4 months
Genetic fidelity analysis
Axillary bud proliferation circumvents the dedifferentiation of pre-existing meristems into callus, therefore usually avoid genetic changes (Negi and Saxena 2010). However, testing of genetic homogeneity of visually uniform tissue culture raised plants is important (Lakshmanan et al. 2007) in perennial trees and woody shrubs having long rotation cycles. The DNA-based molecular marker techniques can precisely assess genetic fidelity of micropropagated plants (Agarwal et al. 2015; Rathore et al. 2014). Assessment of genetic stability of tissue culture raised shoots of T. undulata using RAPD markers was reported by Kumari and Singh (2014). According to Singh et al. (2013a) use of multiple marker systems will ensure a wider coverage of genome and allow better identification of genetic variations. Therefore, two marker sets namely ISSR and SCoT were used in the present study (Table 4). The semi-arbitrary, dominant and more stringent ISSR markers having medium to high reproducibility are capable to scan the whole genome quickly and randomly (Singh et al. 2013b) while the ATG translation start codon based SCoT markers designed from its short conserved flanking regions (Collard and Mackill 2009) specifically targets the coding regions. The 9 ISSR primers, selected after preliminary screening of 15 primers, amplified 49 distinct, unambiguous, and all monomorphic amplicons ranging in size from 200 to 1500 bp. A total of 41 DNA fragments, 300 to 3000 bp long, were amplified by 8 SCoT primers (out of 15 tested). The number of amplicons varied from 2 to 8 with ISSR and 2–10 with SCoT markers. A representative monomorphic profile with ISSR 12 and SCoT 14 is presented in Fig. 8. SCoT markers have been used in genetic homogeneity testing in some recent reports (Agarwal et al. 2015). The arbitrary markers like RAPD and ISSR usually target the non-coding regions of DNA while SCoT primers being derived from the flanking sequences of start codon usually generate information based on coding regions. Choice of one primer targeting the coding region and other targeting non-coding regions ensured a wider coverage of genome during the study. The 100% monomorphic profiles of the mother plant and regenerated plantlets with ISSR and SCoT markers confirmed the clonal nature of tissue culture raised plants.
Conclusions
A simple procedure for in vitro multiplication and plantlet regeneration of Tecomella undulata, an endangered medicinally important agroforestry tree of hot arid regions of western Rajasthan, India has been standardized. Direct proliferation of pre-existing axillary meristems ensured clonal multiplication of mother trees. Clonal identity of the regenerated plantlets has been established using two simple PCR amenable marker systems, ISSR and SCoT which produced all monomorphic amplicons among mother tree and regenerated plants.
Author contribution statement
The experiments were conducted by SC, and RKK designed the experiments, arranged the research grant and prepared the final manuscript.
Abbreviations
- BAP:
-
6-Benzylaminopurine
- FYM:
-
Farmyard manure
- IBA:
-
Indole-3-butyric acid
- ISSR:
-
Inter simple sequence repeat
- MS:
-
Murashige and Skoog’s medium (1962)
- NAA:
-
α-Napthaleneacetic acid
- NOA:
-
Naphthoxy acetic acid
- SCoT:
-
Start codon targeted polymorphism
- SH:
-
Schenk and Hilderbrandt medium (1972)
- NN:
-
Nitsch and Nitsch medium (1969)
- B5:
-
Gamborg’s medium (Gamborg et al. 1968)
- WPM:
-
Woody Plant Medium (Lloyd and McCown 1980)
References
Abdullah AA, Yeoman MM, Grace J (1987) Micropropagation of mature Calabrian pine (Pinus brutia Ten.) from fascicular buds. Tree Physiol 3:123–136
Abeles FB, Morgan OW, Sahveit ME (1992) In: Ethylene in plant biology, 2nd edn. Academic Press, San Diego
Agarwal T, Gupta AK, Patel AK, Shekhawat NS (2015) Micropropagation and validation of genetic homogeneity of Alhagi maurorum using SCoT, ISSR and RAPD markers. Plant Cell Tiss Organ Cult 120:313–323
Aitken-Christie J, Jones C (1987) Towards automation: radiata pine shoot hedges in vitro. Plant Cell Tissue Org Cult 8:185–196
Arditti J, Ernst R (1993) Micropropagation of orchids. Wiley, New York
Arya HC, Shekhawat NS (1986) Clonal multiplication of tree species in Thar Desert through tissue culture. For Ecol Manag 16:201–208
Arya S, Sharma S, Kaur R, Arya ID (1999) Micropropagation of Dendrocalamus asper by shoot proliferation using seeds. Plant Cell Rep 18:879–882
Arya ID, Arya S, Kalia S, Kalia RK, Sharma SK (2005) Seasonal variation in the in vitro response of nodal explants of Dalbergia sissoo Roxb. Ann For 13:258–261
Aslam M, Singh R, Negi PS, Bhakuni DS, Das SC (2006) Enhanced in vitro regeneration from cotyledonary node explants of Tecomella undulata (Smith) Seem. Proc Nat Acad Sci India Sec B: Biol Sci 76:281–285
Bhansali RR (1993) Bud culture for shoot multiplication and plantlet formation of Tecomella undulata (Rohida) a wood tree of arid zone. Trop Sci 33:1–8
Bhatt ID, Dhar U (2005) Factors controlling micropropagation of Myrica esculenta buch.–Ham. ex D. Don: a high value wild edible of Kumaun Himalaya. Afr J Biotech 3:534–540
Bisht P, Pant M, Kant A (2010) In vitro propagation of Gigantochloa atroviolaceae Widjaja through nodal explants. J Am Sci 6:1019–1026
Carimi F, Zottini M, Formentin E, Terzi M, Lo Schiavo F (2003) Cytokinins: new apoptotic inducers in plants. Planta 216:413–421
Chhajer S, Kalia RK (2016) Seasonal variation in the in vitro responses of mature nodes of Tecomella undulata (Sm.) Seem. Ind For 142(9):827–832
Collard BCY, Mackill DJ (2009) Start codon targeted (SCoT) polymorphism: a simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol Biol Rep 27:86–93
Constabel F (1984) Callus culture: induction and maintenance. In: Vasil IK (ed) Cell culture and somatic cell genetics of plants, vol 1. Academic Press, New York
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:11–15
Funada R, Kubo T, Tabuchi M, Sugiyama T, Fushitani M (2001) Seasonal variations in endogenous indole-3-acetic acid and abscisic acid in the cambial region of Pinus densiflora Sieb. et Zucc. stems in relation to earlywood/latewood transition and cessation of tracheid production. Holzforschung 55:128–134
Gamborg OL, Miller RA, Ojima L (1968) Nutrient requirement of suspension culture of Soybean. Exp Cell Res 50:151–158
Gaspar T, Kevers C, Penel C, Greppin H, Reid DM, Thorpe T (1996) Plant hormones and plant growth regulators in plant tissue culture. In vitro Cell Dev Biol Plant 32:272–289
Kalia S, Kalia RK, Sharma SK (2004) Evaluation of clonal variability in shoot coppicing ability and in vitro responses of Dalbergia sissoo Roxb. Silvae Genet 53:212–220
Kalia RK, Arya S, Kalia S, Arya ID (2007) Plantlet regeneration from fascicular buds on seedling explants of Pinus roxburghii. Biol Plant 51:653–659
Kalia RK, Rai MK, Kalia S, Singh R, Dhawan AK (2011) Microsatellite markers: an overview of the recent progress in plants. Euphytica 177:309–334
Kalia RK, Rai MK, Sharma R, Bhatt RK (2014) Understanding Tecomella undulata: an endangered pharmaceutically important timber species of hot arid regions. Genet Res Crop Evol 61:1397. doi:10.1007/s10722-014-0140-3
Khalafalla MM, Daffalla HM (2008) In vitro micropropagation and micrografting of gum arabic tree [Acacia senegal (l.) wild]. Int J Sustain Crop Prod 3:19–27
Kozai T, Koyama Y, Watanabe I (1988) Multiplication of potato plantlets in vitro with sugar free medium under high photosynthetic photon flux. Acta Hort 230:121–127
Kumar S, Kumar P, Agrawal DK, Choudhary AK (2011) Rehabilitation of lignite mine backfill with indigenous desert tree, Tecomella undulata in Indian arid zone. J Trop For 27:17–28
Kumari S, Singh N (2012) Multiplication of desert teak Tecomella undulata under in vitro conditions. J Trop Med Plant 13:137–143
Kumari S, Singh N (2014) Micropropagation of Tecomella undulata (Sm.) Seem and genetic fidelity testing of in vitro raised plants. As Pac J Mol Biol Biotechnol 22:191–198
Lakshmanan V, Venkataramareddy SR, Neelwarne B (2007) Molecular analysis of genetic stability in long-term micropropagated shoots of banana using RAPD and ISSR markers. Electron J Biotechnol 10:106–113
Lloyd G, McCown BH (1980) Commercially feasible micropropagation of mountain laurel (Kalmia latifolia) by use of shoot tip culture. Int Plant Prop Soc Comb Proc 30:421–427
Malik SK, Chaudhury R, Kalia RK (2005) In vitro multiplication and conservation of Garcinia indica: a medicinal tropical tree. Sci Hortic 106:539–553
Marks TR, Simpson SE (1994) Factors affecting shoot development in apically dominant Acer cultivars in vitro. J Hortic Sci 69:543–551
Mathur N, Singh J, Bohra S, Bohra A, Mehboob M, Vyas A (2010) Phytoremediation potential of some multipurpose tree species of Indian Thar Desert in oil contaminated soil. Adv Environ Biol 4:131–137
McCown BH, Sellmer JC (1987) General media and vessels suitable for woody plant cultures. In: Bonga JM, Durzan DJ (eds) Tissue culture in forestry-General principles and biotechnology, vol 2. Martinus Nijhoff Publication, Dordrecht, pp 4–6
Mohammed GH, Vidaver WE (1988) Root production and plantlet regeneration in tissue cultured conifers. Plant Cell Tissue Org Cult 14:137–160
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assay with tobacco tissue culture. Physiol Plant 15:473–497
Nandwani D, Mathur N, Ramawat KG (1995) In vitro shoot multiplication from cotyledonary node explants of Tecomella undulata. Gartenbauwisseschaft 60:65–68
Nandwani D, Sharma R, Ramawat KG (1996) High frequency regeneration in callus culture of tree Tecomella undulata. Gartenbauwisseschaft 61:147–150
Negi D, Saxena S (2010) Ascertaining clonal fidelity of tissue culture raised plants of Bambusa balcooa Roxb. using inter simple sequence repeat markers. New For 40:1–8
Nitsch JP, Nitsch C (1969) Haploid plants from pollen grains. Science 163:85–87
Parasharami VA, Poonawala IS, Nadgauda RS (2003) Bud break and plantlet regeneration in vitro from mature trees of Pinus roxburghii Sarg. Curr Sci 84:203–208
Patel AK, Phulwaria M, Rai MK, Gupta AK, Shekhawat S, Shekhawat NS (2014) In vitro propagation and ex vitro rooting of Caralluma edulis (Edgew.) Benth. & Hook. f.: an endemic and endangered edible plant species of the Thar Desert. Sci Hortic 165:175–180
Randhawa GS, Mukhopadhyay A (1986) Floriculture in India. Allied Publishers, Mumbai
Rani V, Raina SN (2000) Genetic fidelity of organized meristem-derived micropropagated plants: a critical reappraisal. In Vitro Cell Dev Biol Plant 36:319–330
Rathore TS, Singh RP, Shekhawat NS (1991) Clonal propagation of desert tree (Tecomella undulata) through tissue culture. Plant Sci 79:217–222
Rathore JS, Rai MK, Phulwaria M, Shekhawat NS (2014) A liquid culture system for improved micropropagation of mature Acacia nilotica (L.) Del. ssp. indica and ex vitro rooting. Proc Natl Acad Sci India Sect B Biol Sci 84:193–200
Robinson R, Kumari B, Beniwal VS (2005) In vitro shoot multiplication of Tecomella undulata (Sm.) Seem: an endangered tree species. Indian J Plant Physiol 10:372–376
Sanyal I, Singh AK, Kaushik M, Amla DV (2005) Agrobacterium mediated transformation of chickpea (Cicer arietinum L.) with Bacillus thuringiensis cry1Ac gene for resistance against pod borer insect Helicoverpa armigera. Plant Sci 168:1135–1146
Schenk RU, Hildebrandt AC (1972) Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot 50:199–204
Singh R, Goyal SC (2008) Inflorescence nodal segment: a novel explant for rapid micropropagation of Chlorophytum borivilianum sant. et Fernand. Phytomorphology 58:187–193
Singh R, Rathore M, Mishra GP, Kumar M, Singh R, Ahmed Z (2009) Adventitious shoot regeneration and Agrobacterium tumefaciens mediated transformation in Rohida (Tecomella undulata). Indian For 135:751–764
Singh SR, Dalal S, Singh R, Dhawan AK, Kalia RK (2012a) Micropropagation of Dendrocalamus asper Schult. & Schult. F. Backer ex K. Heyne): an exotic edible bamboo. J Plant Biochem. doi:10.1007/s13562-011-0095-9
Singh SR, Dalal S, Singh R, Dhawan AK, Kalia RK (2012b) Seasonal influences on in vitro bud break in Dendrocalamus hamiltonii Arn. ex Munro nodal explants and effect of culture microenvironment on large scale shoot multiplication and plantlet regeneration. Indian J Plant Physiol 17:9–21
Singh SR, Singh R, Dalal S, Dhawan AK, Kalia RK (2013a) Evaluation of genetic fidelity of in vitro raised plants of Dendrocalamus asper (Schult. & Schult. F.) Backer ex K. Heyne using DNA-based markers. Acta Physiol Plant 35:419–430
Singh SR, Dalal S, Singh R, Dhawan AK, Kalia RK (2013b) Ascertaining clonal fidelity of micropropagated plants of Dendrocalamus hamiltonii Nees et Arn. ex Munro using molecular markers. In Vitro Cell Dev Biol Plant. doi:10.1007/s11627-013-9520-1
Sokolov RS, Atanassova BY, Iakimova ET (2014) Physiological response of in vitro cultured Magnolia sp. to nutrient medium composition. J Hortic Res 22:49–61
Swamy KM, Sudipta KM, Balasubramanya S, Anuradha M (2010) Effect of different carbon sources on in vitro morphogenetic response of patchouli (Pogostemon cablin Benth.). J Phytol 8:11–17
Tewari VP (2007) Comparing the model forms estimating generalised diameter-height relationships in Tecomella undulata plantations in hot arid region of India. J For Res 18:255–260
Tripathi JPM, Jaimini SN (2002) Floral and reproductive biology of Rohida (Tecomella undulata (Sm.) Seem.). Ind J For 25:341–343
Tyagi H, Tomar UK (2013) Factors affecting in vitro shoot proliferation and rooting of mature Tecomella undulata (Sm.) Seem tree. Res Plant Sci 1:38–44
Varshney A, Anis M (2012) Improvement of shoot morphogenesis in vitro and assessment of changes of the activity of antioxidant enzymes during acclimation of micropropagated plants of Desert Teak. Acta Physiol Plant 34:859–867
Venkatachalam L, Sreedhar RV, Bhagyalakshmi N (2007) Micropropagation in banana using high levels of cytokinins does not involve any genetic changes as revealed by RAPD and ISSR markers. Plant Growth Regul 51:193–205
Yokawa K, Kagenishi T, Baluska F (2013) Root photomorphogenesis in laboratory-maintained Arabidopsis seedlings. Trends Plant Sci 18:117–119
Zaerr JB, Mapes MO (1982) Action of growth regulators. In: Bonga JM, Durzan DJ (eds) Tissue culture in forestry. Martinus Nijhoff, Dordrecht, pp 231–255
Acknowledgement
The research Grant was provided vide Project No. BT/PR6558/PBD/16/998/2012 by DBT, Government of India, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Araniti.
Rights and permissions
About this article
Cite this article
Chhajer, S., Kalia, R.K. Seasonal and micro-environmental factors controlling clonal propagation of mature trees of marwar teak [Tecomella undulata (Sm.) Seem]. Acta Physiol Plant 39, 60 (2017). https://doi.org/10.1007/s11738-017-2364-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-017-2364-2