Abstract
Chromium (Cr) is considered to be one of the major environmental hazards and poses a threat to both plant and animal health. Selenium (Se), however, has been recognized as an essential micronutrient in plants. To understand the role of Se(VI) in oxidative stress management and regulation of antioxidative defence mechanism against heavy metal stress, the seedlings of Brassica juncea L. were raised in Petri plates containing nutrient media supplemented with only with Se(VI) and Cr(VI), or their combination. It was observed that of Cr(VI) causes an increase in reactive oxygen species (ROS) in the seedlings leading to oxidative stress. Histological studies using confocal and visible microscopy confirmed the biochemical results. Supplementation of up to 4 µM of Se(VI) to media containing 300 µM of Cr(VI) reduced the contents of ROS and increased enzymatic and non-enzymatic antioxidants in the seedlings. At a concentration of 6 µM, however, Se(VI) was toxic. The results suggested that at appropriate concentrations, the exogenous application of Se(VI) enabled the B. juncea seedlings to counteract the effects of Cr(VI), thereby increasing the resistance of plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The complex and unpredictable nature of environment along with harmful anthropogenic activities are creating adverse environmental conditions for living organisms (Mittler and Blumwald 2010). Such exposure of plants and animals to various abiotic and biotic stresses hampers their normal growth and development. Plants, in particular, are exposed to an array of environmental stresses like drought, salinity, extreme temperatures, and heavy metal contamination of soil and water. Out of these, heavy metals have proven to be perilous to plants and other forms of life. The contamination of food chain with heavy metals usually occurs when heavy metal rich soils or water are used for the production of crops. Upon entering the plant system, these metals either undergo redox reactions and lead to the formation of reactive oxygen species (ROS) by Haber–Weiss and Fenton reactions, or may become toxic by blocking the antioxidative defence system, increasing the rate of lipid degradation or hindering electron transport chain, thereby causing severe damage to membranes and biomolecules (Schützendübel and Polle 2002; Gill 2014). Chromium (Cr) is one such non-essential heavy metal, which even at low doses shows severe phytotoxic effects. Environmental contamination of Cr, caused due to various industrial activities, has become a matter of serious concern (Chanda and Parmar 2003; Schiavon et al. 2008). Several studies have proved that Cr can inhibit physiological and biochemical processes that negatively affect structure and function of enzymes, proteins, membranes and genome (Singh et al. 2013). Studies have also revealed the involvement of Cr in generation of superoxide radicals, hydrogen peroxide (H2O2) and reactive carbonyl groups, thereby causing oxidative damage to the plants (Shanker et al. 2004; Panda 2007; Gangwar and Singh 2011).
Scientific research pertaining to selenium (Se) as a potent stress ameliorator has gained momentum since few years. Se, which has a chemical similarity with sulphur (S), is easily taken up by plants through S transporters and it has an ability to replace S from biomolecules (Pilon-Smits and Quinn 2010). The beneficial roles of Se related to growth and development, including the ability to ameliorate oxidative damage caused by biotic and abiotic stresses, have been demonstrated in various studies. The members of the family Brassicaceae have been reported to take up more S from growth medium than members of families like Poaceae or Leguminosae (Marschner 1995). Therefore, it can be assumed that uptake and assimilation of Se, if supplied exogenously, will occur to a significant level as compared to the species of other families. Hence, in the present study, Brassica juncea seedlings were used as an experimental material to understand the role of Se(VI) in reducing the damage to the cells, and fortifying the defence system of plants against Cr(VI) stress. The interactive effect of Se(VI) and Cr(VI) on the working of Asada–Halliwell pathway that involves both antioxidants and antioxidative enzymes. The oxidative burst caused by Cr(VI) application was studied by estimating the contents of H2O2 and superoxide anions while damage was assessed by the content of malondialdehyde (MDA).
Materials and methods
Raising of plant material
The study was conducted on the seedlings raised from certified B. juncea (cv. RLC 1) seeds obtained from Punjab Agricultural University, Ludhiana, India. The surface sterilized seeds were soaked in distilled water for 2 h and then made to germinate in autoclaved Petri dishes lined with Whatman No. 1 filter paper. The seeds were supplemented with 2, 4 and 6 µM Na2SeO4 and 300 µM K2CrO4, in different binary combinations. These solutions were prepared in half strength Hoagland’s medium. The set of seedlings raised only with Hoagland’s nutrient medium was considered as control. The Petri dishes were kept in seed germinator and the seedlings were provided with 25 ± 2 °C temperature with 16 h of photoperiod. After 15 days, the seedlings were harvested and analysed for cellular damage, enzymatic and non-enzymatic antioxidative defence systems.
Cellular damage
The oxidative damage was estimated spectrophotometrically by estimating lipid peroxidation, superoxide anion production and H2O2 content.
The extent of lipid peroxidation was determined by estimating the content of MDA by the method of Heath and Packer (1968). Fresh seedling samples were homogenised in 0.1% trichloroacetic acid and centrifuged at 13,000×g for 20 min at 4 °C. To the supernatant, 0.5% thiobarbituric acid and 20% trichloroacetic acid were added and the mixture was heated at 95 °C for 30 min in water bath. To stop the reaction, the mixture was cooled quickly by placing the test tubes in an ice bath. The absorbance of the reaction mixture was recorded at 532 and 600 nm and the extinction coefficient used for the calculation of MDA content was 155/mM/cm.
The content of superoxide anions was estimated by Wu et al. (2010). Seedling extract was made in 50 mM potassium phosphate buffer (pH 7.8) and supernatant was used after centrifugation at 13,000×g at 4 °C. The reaction was carried out by adding 0.5 ml potassium phosphate buffer and 0.1 ml of 10 mM hydroxylamine hydrochloride to 0.5 ml of extract followed by incubation for 30 min at 25 °C. To the above reaction mixture, equal volumes of 58 mM 3-aminobenzenesulphonic acid and 7 mM 1-naphthylamine were added and incubated for another 20 min. The absorbance of the reaction mixture was recorded at 530 nm and the content of superoxide anions was calculated using standard curve obtained from NaNO2 as a standard.
To estimate the content of H2O2 in the seedling samples of B. juncea, the method of Velikova et al. (2000) was followed. For the preparation of plant extract, 100 mg of fresh seedlings were homogenised in 1.5 ml of 0.1% trichloroacetic acid and centrifuged at 12,000×g for 15 min. The reaction was started by adding 10 mM potassium phosphate buffer (400 µl) at pH 7 and 1 M potassium iodide (800 µl) to the supernatant (400 µl) and the absorbance was recorded at 390 nm. The content was calculated by a linear regression equation obtained from standard curve using H2O2 at different concentrations.
Extract preparation and estimation of enzymatic antioxidants
The enzyme extracts for the estimation of catalase (CAT), ascorbate peroxidase (APOX), guaiacol peroxidase (POD), glutathione peroxidase (GPOX), dehydroascorbate peroxidase (DHAR) and glutathione reductase (GR) were prepared in 50 mM phosphate buffer. One gram of fresh seedling samples were homogenised in 3 ml of phosphate buffer and the extracts were centrifuged at 13,000×g for 20 min. For estimation of superoxide dismutase (SOD), the seedling extracts were prepared in sodium carbonate buffer (50 mM; pH 10.2), while for monodehydroascorbate reductase (MDHAR), 50 mM of Tris–HCl buffer (pH 7.4) was used. The extracts were prepared in ice cold conditions with 0–4 °C temperatures.
The method given by Kono (1978) was used to estimate the activity of SOD. The reaction mixture contained 50 mM sodium carbonate buffer, 24 µM nitroblue tetrazolium, 0.1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM hydroxylamine hydrochloride (pH 6) and 0.03% triton X-100 with 2 min of incubation and the addition of plant extract. The absorbance was noted at 540 nm for 1 min.
CAT activity was estimated by following the protocol of Aebi (1984). The reaction mixture contained plant extract, potassium phosphate buffer (50 mM; pH 7) and 15 mM H2O2. The decline on absorbance was noted for 1 min at 240 nm and activity was calculated using an extinction coefficient of 39.4/mM/cm.
APOX activity was estimated using the method of Nakano and Asada (1981). The reaction mixture consisted of 50 mM potassium phosphate buffer (pH 7), 0.5 mM ascorbate, 1 mM H2O2 and plant extract. The decrease in absorbance was noted at 290 nm for 1 min and extinction coefficient of 2.8/mM/cm was used to calculate the enzyme activity.
The activity of POD was estimated by following the method of Putter (1974). The reaction mixture consisted of 50 mM potassium phosphate buffer (pH 7), 20.1 mM guaiacol solution, 12.3 mM H2O2 and plant extract. The increase in the absorbance was observed at 436 nm for 1 min and the activity of the enzyme was estimated using the extinction coefficient of 26.6/mM/cm.
GPOX was estimated according to the method given by Flohe and Gunzler (1984). The reaction mixture was prepared by adding 50 mM potassium phosphate buffer (pH 7), 0.5 mM EDTA, 1 mM reduced glutathione (GSH), 1 mM sodium azide, 0.15 mM reduced nicotinamide adenine dinucleotide phosphate (NADPH), 0.15 mM H2O2 and the plant extract. Decrease in absorbance was observed for 1 min at 340 nm and enzyme activity was calculated using extinction coefficient of 6.22/mM/cm.
The method given by Dalton et al. (1986) was used to determine the activity of DHAR. Estimation was done by mixing 50 mM potassium phosphate buffer (pH 7), 0.2 mM dehydroascorbate, 0.1 mM EDTA, 1.5 mM GSH and the plant extract. Change of absorbance was observed for 1 min at 265 nm, and the value of extinction coefficient used was 14/mM/cm.
MDHAR was estimated using the protocol given by Hossain et al. (1984). The reaction was carried out by adding 50 mM Tris–HCl (pH 7.6), 0.1 mM EDTA, 0.25% triton X-100, 3 mM ascorbate, 0.3 mM reduced nicotinamide adenine dinucleotide (NADH), 0.14 units of ascorbic acid oxidase to the plant extract. The decrease in absorbance was noted at 340 nm for 1 min and the calculation of enzyme activity was done using 6.2/mM/cm extinction coefficient.
GR activity was estimated using the protocol of Carlberg and Mannervik (1975). The reaction mixture consisted of 50 mM potassium phosphate buffer, 1 mM glutathione disulphide (GSSG), 1 mM EDTA, 0.1 mM NADPH and the seedling extract. Decrease in absorbance of the reaction mixture was noted for 1 min was noted at 340 nm and a value of 6.22/mM/cm was used as extinction coefficient for calculation of the activity of the enzyme.
Estimation of non-enzymatic antioxidants
GSH content was estimated by following the method of Sedlak and Lindsay (1968). One gram of fresh seedling samples were homogenised in Tris buffer (0.2 M, pH 8.2) under ice cold conditions and centrifuged at 13,000×g for 20 min at 4 °C. The content was estimated using 100 µl of supernatant, to which 0.2 M Tris buffer, 0.01 M 5,5′-dithiobis-(2-nitrobenzoic acid) and absolute methanol were added and incubated at room temperature for 15 min. The reaction mixture was centrifuged at 3000×g for 15 min and the absorbance of the supernatant was recorded at 412 nm.
The method of Roe and Kuether (1943) was used to determine the content of ascorbic acid. The seedling extract was prepared in 50 mM Tris buffer at pH 10. To estimate ascorbic acid, the reaction mixture comprising of plant extract, 50% trichloroacetic acid, distilled water and 100 mg activated charcoal was taken in test tubes. The mixture was filtered with Whatman filter paper No. 1 and 2,4-dinitrophenyl hydrazine (DNPH) reagent [2 g DNPH, 250 mg thiourea, 30 mg copper sulphate pentahydrate (CuSO4·5H2O) in 100 ml 9 N sulphuric acid (H2SO4)] was added and incubated at 37 °C for 3 h. The reaction mixture was then placed in ice bath and 65% cold H2SO4 was added. A second incubation for 30 min was given at room temperature and the absorbance was noted at 520 nm.
The content of tocopherol was determined by the method of Martinek (1964). Seedling extracts prepared in 50 mM Tris buffer (pH 10) were mixed with distilled water and 0.12% ethanolic ferric chloride hexahydrate (FeCl3·6H2O). The precipitates were obtained by vigorous shaking, and 0.5 ml of xylene was added followed by vortexing for 30 s. The reaction mixture was then centrifuged at 3000×g for 10 min. Two layers were formed and to the upper layer of xylene, 12% of 2,4,6-tripyridyl-S-triazine prepared in n-propanol was added. The absorbance of this layer was recorded at 600 nm.
Histochemical studies
Histochemical analysis was carried out on the roots of 15 days old B. juncea seedlings. The response of GSH, H2O2, membrane damage and nuclear damage to Cr(VI) and Se(VI), both alone and in binary combinations, were studied using confocal laser scanning microscope (Nikon A1R) with Plan Apo 20X objective lens, while MDA and cell viability were studied using light microscope (Magnus MLXi model).
The MDA was tagged by following the protocol of Wang and Yang (2005). The 15 days old roots of B. juncea were kept in Schiff’s reagent for 20 min and washed with 0.5% potassium metabisulphite prepared in 0.05 M HCl. The samples mounted on glass slides were observed under visible compound microscope.
The protocol of Ortega-Villasante et al. (2007) was followed to detect H2O2 in the roots of B. juncea. Tagging of H2O2 was done with 25 µM of 2′,7′-dichloroflourescien diacetate (DCF-DA) and staining the roots for 30 min. Staining was followed by washing of root samples thrice by distilled water and mounting on the glass slides. The observations were made at the excitation wavelength of 488 nm and emission wavelength of 530 nm.
The method given by Gutierréz-Alcala et al. (2000) was followed to test the membrane damage. The 15 days old root samples were treated with 50 µM of propidium iodide for 15 min and then washed with distilled water and mounted on glass slides. The excitation and emission wavelengths of 535 and 617 nm, respectively, were used to observe the samples under confocal laser scanning microscope.
The nuclear damage in the roots of B. juncea was assessed by the protocol proposed by Callard et al. (1996). The dye, 4,6-diamino-2-phenylindole (DAPI) was prepared by dissolving 0.1 mg in 100 ml phosphate buffer saline. The roots of B. juncea were kept for 30 min in the dye under dark conditions and then washed with phosphate buffer saline. The samples were mounted and viewed with excitation and emission wavelengths of 358 and 461 nm, respectively, under confocal laser scanning microscope.
The viability of cells was studied by the method given by Romero-Puertas et al. (2004). The dead cells in the roots of B. juncea were localized by 0.25% of aqueous solution of Evan’s Blue. The roots were kept in the dye for 10 min and washed many times with distilled water. The slides were observed under visible compound microscope.
GSH detection in the roots of B. juncea was done by the method of Fricker and Meyer (2001). The roots were dipped in monochlorobimane (MCB) dye at the concentration of 25 µM for 15–20 min. The dye also contained 5 mM sodium azide which prevented the sequestration of MCB-GSH conjugate to the vacuoles by diminishing the adenosine tri-phosphate (ATP) from the cells. The fluorescent adduct was viewed at an excitation wavelength of 351–364 nm and emission wavelength of 477 nm.
Statistical analysis
The results were analysed by applying one-way analysis of variance (ANOVA). Tukey’s honestly significant difference (HSD) was used to determine significance of differences among the mean values. The interaction between Cr(VI) and Se(VI) was analysed using linear multiple regression analysis (Sokal and Rholf 1981; Bailey 1995). The model used for multiple regression for binary combination was:
where Y was the parameter under study, X 1 and X 2 corresponded to Cr(VI) and Se(VI) in binary combinations, b 1 and b 2 were the partial regression coefficients due to the effects of X 1 and X 2, respectively, β 1 and β 2 signified the β-regression coefficients due to X 1 and X 2. The statistical analysis was done using self-coded programs in Microsoft Excel.
Results
Cellular damage
The stress caused by Cr(VI) resulted in loss of membrane integrity and increased the production of ROS, which led to oxidative stress. The effect of Cr(VI) and Se(VI) on cellular damage and its recovery was studied using spectroscopic and microscopic methods.
Spectroscopic studies
The MDA content of the seedlings represents the level of peroxidation occurring in membrane lipids. Cr(VI) stress caused a significant increase (34.4%) in MDA accumulation as compared to the untreated B. juncea seedlings. The application of Se(VI) in binary combination with Cr(VI) reduced MDA accumulation, and the maximum effect was observed at 4 µM concentration of Se(VI) which caused a reduction of 22.2% in MDA content as compared to Cr(VI) treated seedlings (Table 1). The multiple regression analysis showed positive value of β-regression coefficient for Cr(VI) which signified its role in enhancing the MDA accumulation. The β-regression coefficient for Se(VI) was, however, negative which implied reduction in accumulation of MDA due to Se(VI) application (Table 1).
The oxidative damage to the plants cells was also assessed by estimating the content of superoxide anions. Seedlings raised in 300 µM Cr(VI) showed a significant increase of 112.8% when compared to the control seedlings. Se(VI) supplementation helped in overcoming Cr(VI) stress by reducing the production of superoxide anions. Se(VI) at 2 µM in combination with Cr(VI), reduced the content of superoxide anions by 36.9%, to help the plants to combat stress (Table 1). Multiple linear regression analysis of the data also confirmed the protective effect of Se(VI) against Cr(VI). The β-regression coefficient for Cr(VI) was positive, while that for Se(VI) it was negative, thereby suggesting the stress ameliorative role of Se(VI) (Table 1).
H2O2, which is one of the most potent ROS, also got enhanced by significant levels (169.7%) due to the phytotoxic effects of Cr(VI). Addition of Se(VI) to the growth medium resulted in decrease of H2O2, thereby reducing the oxidative stress. As compared to Cr(VI) treated seedlings, the binary combination of 2 µM Se(VI) with Cr(VI) showed a significant reduction (31.5%) in the H2O2 content. The binary combination of the highest concentration of Se(VI) (6 µM) with Cr(VI), however, was not effective in reducing the H2O2 content, but enhanced its content to significant levels (Table 1). Multiple regression analysis revealed a significant correlation between the H2O2 content of the seedlings and the concentrations of Cr(VI) and Se(VI) treatments. The value of β-regression coefficient for Cr(VI) was positive which showed its harmful effects. The β-regression coefficient for Se(VI) was also positive, hence suggesting that Se(VI) is effective at lower concentrations but becomes harmful at higher concentrations (Table 1).
Microscopic studies
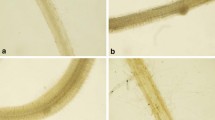
The results of spectroscopic studies on MDA were further confirmed by its localization in the roots of B. juncea seedlings by Schiff’s reagent. The Cr(VI) stressed roots showed a high intensity of pink colour in comparison to the roots of control seedlings. The binary combination of 4 µM Se(VI) and Cr(VI) showed reduced intensity of the dye due to lesser MDA content in comparison to Cr(VI) treated seedlings (Fig. 1).
The localization of H2O2 by DCF-DA produced green fluorescence when viewed under confocal laser scanning microscope. The intensity of fluorescence was higher in Cr(VI) treated roots. Se(VI) application at 2 µM concentration to stressed seedlings caused reduction in fluorescence intensity which further confirmed the spectroscopic estimation of H2O2 (Fig. 2).
The damage to the membrane was studied by propidium iodide. The dye has affinity for nucleotides, and hence binds them. But the membranes are impermeable to propidium iodide. Therefore, high intensity of red fluorescence confirms the damaged membranes. The Cr(VI) treated seedlings showed darkly stained cells of the roots, while addition of Se(VI) at 4 µM in combination with Cr(VI) resulted in lowering the red fluorescence which signified reduced membrane damage (Fig. 3). Similar effect was also observed for nuclear damage which was studied using DAPI. The blue fluorescence was maximum in roots treated with Cr(VI) while the roots from seedlings raised in solution containing 4 µM Se(VI) and 300 µM Cr(VI) showed relatively reduced intensity of blue fluorescence (Fig. 4). The damaging effect of Cr(VI) was also revealed by lesser viability of the cells in Cr(VI) stressed roots which showed highly stained blue colour with Evan’s Blue dye. The cell viability, however, was observed to improve under the influence of 4 µM Se(VI) which showed lightly stained cells, and thus proved protective effect of Se(VI) (Fig. 5).
Enzymatic antioxidants
The seedlings of B. juncea raised in 300 µM of Cr(VI) showed a significant increase (100.8%) in the activity of SOD as compared to the control seedlings. The binary combination of Se(VI) and Cr(VI), however, resulted in further increase in the activity of SOD, with maximum effect being observed when 4 µM Se(VI) was given along with 300 µM Cr(VI) (Table 2). Statistical analysis revealed significant effect of the treatments on the activity of SOD. The multiple regression analysis also showed positive values of β-regression coefficients for both Cr(VI) and Se(VI), which suggested that both the elements served to increase the enzyme activity (Table 2).
Cr(VI) application had similar effect on the activity of CAT in B. juncea seedlings. When compared to the untreated controls, an increase of 60.3% in the enzyme activity was observed in the seedlings grown in 300 µM Cr(VI). Se(VI) application in binary combination further enhanced the enzyme activity. Se(VI) at the concentration of 4 µM with 300 µM Cr(VI) showed maximum increase (25.5%) in the activity of CAT (Table 2). The statistical analysis by linear model of multiple regression showed significant correlation between treatments and CAT activity. The values for β-regression coefficients for both Cr(VI) and Se(VI) were positive which signified that both elements had positive influence on the activity of CAT (Table 2).
The activity of APOX, however, was decreased in response to Cr(VI) application in 15 days old B. juncea seedlings. A significant fall in its activity (41.9%) was observed in Cr(VI) treated seedlings as compared to the control seedlings. The application of Se(VI), both alone and in binary combinations, caused a significant improvement in the APOX activity. Supplementation of 4 µM Se(VI) with 300 µM Cr(VI) increased the enzyme activity by 61.8% as compared to the seedlings given only Cr(VI) treatment (Table 2). The correlation coefficient for the multiple linear regression between treatments and enzyme activity was significant. The harmful effect of Cr(VI) is also indicated by its negative β-regression coefficient value, while for Se(VI), the β-regression coefficient was positive which showed its stress protective ability (Table 2).
Similar effect was also observed for POD, which showed a considerable decline (22.9%) in its activity with Cr(VI) treatment. Se(VI) application aided in significantly improving the activity of POD, both alone and in combination with Cr(VI), which showed its stress alleviation property. Maximum improvement (18.6%) was observed at 4 µM Se(VI) in combination with Cr(VI) when compared to seedlings treated with only Cr(VI) (Table 2). Statistical analysis by multiple regression supported the observations. β-regression coefficient for Cr(VI) was negative that proved its deleterious effect on enzyme activity. Se(VI), on the other hand, showed positive value for β-regression coefficient that suggested its stress promoting property (Table 2).
The activity of GPOX in B. juncea seedlings was also observed to decline with the application of Cr(VI) which caused a decrease of 41.9% in the enzyme activity. The Se(VI) supplementation in binary combination with Cr(VI) caused the enzyme activity to enhance. The most effective concentration of Se(VI) was 2 µM, which in combination with 300 µM Cr(VI) resulted in 65.3% increase in enzyme activity (Table 2). The negative value of β-regression coefficient for Cr(VI) also confirmed its inhibitory effect, while Se(VI) showed a positive value for β-regression coefficient (Table 2).
The activities of DHAR and MDHAR also decreased significantly in response to Cr(VI) application. As compared to the control seedlings, Cr(VI) caused decline of 35.2 and 26.4% in DHAR and MDHAR activities, respectively. Se(VI) application, however, aided in reducing the oxidative stress caused by Cr(VI). The activities of both the enzymes showed a considerable increase when Se(VI) was supplemented along with Cr(VI). In binary combination with 300 µM Cr(VI), the activity of DHAR showed an increase of 125.2% at 2 µM Se(VI), while the activity of MDHAR showed an enhancement of 34.8% at 4 µM Se(VI) (Table 2). The correlation coefficients obtained from linear model of multiple regression supported the above results. The β-regression coefficients for Cr(VI) for both DHAR and MDHAR were negative, which signified its inhibitory effects on the enzyme activity. Se(VI), in contrast, showed positive values for β-regression coefficients for both enzymes, thereby suggesting its ameliorative property (Table 2).
The activity of GR suffered a significant decrease (50.6%) in response to Cr(VI) application when compared to the control seedlings. Se(VI) treatment at 4 µM in combination with Cr(VI) caused an increase in GR activity by 82.6% as compared to Cr(VI) treated seedlings (Table 2). This protective effect of Se(VI) against Cr(VI) for GR activity was also supported by multiple regression analysis which showed negative β-regression coefficient for Cr(VI), while a positive coefficient for Se(VI) (Table 2).
Non-enzymatic antioxidants
The non-enzymatic antioxidants involved in oxidative stress management showed a decrease in their contents with the application of 300 µM Cr(VI). The content of GSH declined significantly (25.9%) in the seedlings of B. juncea, while Se(VI) in combination with Cr(VI) decreased the effect of Cr(VI) stress and helped in enhancing the GSH content. Maximum effect was observed at 4 µM Se(VI) that caused 76.3% increase in the content of GSH (Table 3). The correlation coefficient derived from multiple linear regression showed a significant correlation between the treatments and GSH content (Table 3). The results from spectroscopic analysis are also in conformity with the microscopic analysis of GSH. The dye, MCB showed less intensity of blue fluorescence in the roots of Cr(VI) stressed B. juncea seedlings which signified the reduced content of GSH. However, Se(VI) application enhanced the GSH production which further led to higher intensity of the blue colour in root samples (Fig. 6).
Ascorbic acid is an important reductant in Asada–Halliwell pathway, and hence plays a key role in ROS scavenging. The content of ascorbic acid was observed to fall significantly by 54.7% in response to Cr(VI) treatment. Application of Se(VI) resulted in overcoming the Cr(VI) stress by significantly enhancing the content of ascorbic acid. Maximum increase (108.3%) occurred when Se(VI) at 6 µM was supplemented with 300 µM Cr(VI) (Table 3). The multiple regression analysis of the data further supported the results. The phytotoxic effect of Cr(VI) was revealed by the negative value of its β-regression coefficient. The protective effect of Se(VI) was verified by positive value of β-regression coefficient for Se(VI) (Table 3).
The content of tocopherol was also reduced significantly (21.2%) in 15 days old seedlings of B. juncea with 300 µM Cr(VI). Se(VI) application reduced the toxic effect of Cr(VI) and aided in enhancing the tocopherol content by 51.7% at a concentration of 4 µM (Table 3). The results were supported by multiple regression analysis that showed negative value of β-regression coefficient for Cr(VI) while positive value for Se(VI) (Table 3).
Discussion
Exposure of plants to heavy metals leads to enhanced production of ROS which further causes oxidative stress in plants. In the present study, Cr(VI) application resulted in enhanced accumulation of MDA, superoxide anions and H2O2. The participation of Cr in Fenton type reactions has been reported previously by Shi and Dalal (1989). In addition, Cr is known to have the ability to obstruct electron transport by binding to heme group of cytochrome which causes redox changes in Cu/Fe carriers (Dixit et al. 2002). These can lead to increased ROS which is responsible for oxidative damage. High levels of MDA accumulation also suggests that Cr(VI) stress led to enhanced leakiness in the membranes, damaged the membrane proteins, channels and enzymes (Garg and Manchanda 2009). Histological studies conducted on the roots of B. juncea also supported the observations on spectroscopic studies and confirmed the increase of MDA and H2O2 due to Cr(VI). Further, the increased membrane and nuclear damage along with loss in cell viability caused by oxidative stress by these damaging compounds, also strengthened the spectroscopic analysis of cellular damage by Cr(VI). The results of the present study are in conformity with the previous studies conducted on Vigna radiata, Ocimum tenuiflorum, Zea mays and Catharanthus roseus (Shanker et al. 2004; Rai et al. 2004, 2014; Zou et al. 2009; Maiti et al. 2012). Se(VI) application (up to 4 µM) to B. juncea, however, resulted in reduced MDA, superoxide anions and H2O2, and hence aided in minimising the effect of Cr(VI) stress. The concentration of 6 µM Se(VI), however, was observed to enhance the MDA, superoxide anions and H2O2 contents, whether alone or in combination with Cr(VI). The role of Se as an essential micronutrient and subsequently its biological activity is suggested to depend on the concentration used in plants. Three levels of biological activity were enumerated by Hamilton (2004) which included trace amounts for normal growth and development, moderate amounts for maintaining homeostatic functions and elevated concentrations leading to toxicity in plants. Microscopic studies also showed that Se(VI) reduced MDA, H2O2 contents and damage to the membranes and nucleus. This might be due to the potential of Se(VI) to stimulate the antioxidative defence system of the plants, thereby alleviating the oxidative stress. Reduced levels of MDA and H2O2 due to Se application have also been reported in Cd stressed Brassica napus plants (Filek et al. 2008). Similar effect of Se was also reported in Cd stressed Brassica oleracea which showed reduction in MDA with Se treatment (Pedrero et al. 2008). The plants of Phaseolus aureus subjected to As stress were also reported to show reduced contents of MDA and H2O2 in response to Se by Malik et al. (2012).
The enzymatic and non-enzymatic antioxidative defence systems in plants help in alleviating the oxidative stress. The application of Cr(VI), in the present study, led to enhanced activities of SOD and CAT in B. juncea seedlings. Superoxide anions undergo dismutation and get converted to H2O2 in a reaction catalysed by SOD. Further, CAT reduces H2O2 to H2O. Hence, both SOD and CAT form the first line of defence against the oxidative stress. The enhanced production of ROS might also enhance the tolerance mechanism in plants (Apel and Hirt 2004). Asada–Halliwell pathway, with the co-ordination of enzymatic and non-enzymatic antioxidants, also plays an instrumental role in the removal of H2O2. Cr(VI) caused a decrease in the activities of APOX, POD, GPOX, DHAR, MDHAR and GR. The contents of GSH, ascorbic acid and tocopherol were also observed to decrease with Cr(VI). The microscopic study of GSH also showed similar results. Such a decline of the defence system might be due to the susceptibility of plants to Cr(VI) stress that further reduced the ROS scavenging leading to oxidative damage (Tang et al. 2012). In addition, heavy metals can alter the active sites of the enzymes, thus rendering them nonfunctional (Sobolev and Begonia 2008). These results are in coherence with earlier studies carried out on several plant species. In a study conducted on Cr stressed Sorghum bicolor plants, a reduced content of GSH was reported by Shanker and Pathmanabhan (2004). Another study conducted by Panda (2007) on Oryza sativa showed an increase in SOD activity, while GPOX and GR activities declined in response to Cr(VI) application. Similar results were also reported in Cr(VI) stressed Triticum aestivum, which showed enhanced activity of SOD and reduced activities of CAT, APOX and GR (Subrahmanyam 2008). Cr(VI) stress in the plants of Pisum sativum also caused a reduction in the activities of CAT, GR and DHAR in roots as well as shoots. In the same study, reduction in the contents of ascorbic acid and GSH due to Cr(VI) was also reported (Gangwar et al. 2011).
Se(VI) application resulted in the fortification of the overall defence system of the B. juncea seedlings, hence it helped to reduce the effect of Cr(VI) stress. Both enzymatic and non-enzymatic antioxidants showed a significant increase on Se(VI) application to the Cr(VI) stressed seedlings. The enhanced activity of SOD in response to Se(VI) might be due to the potential of Se to alter its transcript levels (Djanaguiraman et al. 2010). The activity of CAT is also enhanced with Se(VI) addition which plays an imperative role in H2O2 scavenging. Scavenging of H2O2 also occurs via Asada–Halliwell pathway which uses both enzymes and antioxidants. APOX, however, is a primary enzyme of Asada–Halliwell pathway which uses ascorbate as a reductant (Asada 1994). The enzymes APOX and POD simultaneously work to scavenge H2O2 and convert it into H2O, with APOX having higher affinity for H2O2 (Apel and Hirt 2004). Se(VI) application to Cr(VI) stressed B. juncea seedlings, in the present work, caused enhanced activity of APOX. This might be also be the reason for reduced H2O2 content and increased content of ascorbic acid (Hasanuzzaman and Fujita 2011). The oxidised form of ascorbic acid requires DHAR, MDHAR and GSH for its regeneration. In the present study, Se(VI) application caused an increase in the activities of DHAR and MDHAR, as well as increased the content of GSH, which helped in maintaining the pool of ascorbic acid (Hasanuzzaman et al. 2012). Se(VI) also resulted in enhanced activity of GPOX which is a sulphur-containing enzyme. Se(VI) can get incorporated into this enzyme by replacing sulphur, and the selenoenzyme thus formed can scavenge H2O2 in the presence of GSH and cause reduction of organic and lipid hydroperoxides (Flohe 1982; Flohe and Gunzler 1984). GPOX also has the ability to repair lipid peroxidation in the membranes (Kühn and Borchert 2002) and is capable of managing oxidative damage caused by metal stress. The activity of GR in Cr(VI) stressed B. juncea seedlings was observed to increase in response to Se(VI). The enzyme GR is involved in the reduction of GSSG to GSH that increases the GSH/GSSG ratio (Chalapathi Rao and Reddy 2008). This high GSH/GSSG ratio further ensures the scavenging of H2O2 by Asada–Halliwell pathway, thereby strengthening the defence system of the plants (Pang and Wang 2010). Tocopherol content was also enhanced with Se(VI) supplementation to the Cr(VI) stressed plants. Tocopherols are important antioxidants that can scavenge singlet oxygen in chloroplasts, thereby enhancing the tolerance of plants to stress (Munné-Bosch and Alegre 2002; Munné-Bosch 2005). The observation is well supported by previous reports on many species. In a study by Filek et al. (2008), application of Se to Cd stressed B. napus plants was found to increase the activities of SOD, CAT and GPOX. Similarly, Se addition also enhanced the activities of SOD, CAT and peroxidase in S. bicolor plants subjected to heat stress, thereby reducing the effect of oxidative stress (Djanaguiraman et al. 2010). The Cd treated plants of B. oleracea, when treated with Se, showed increased contents of tocopherol (Pedrero et al. 2008). In another study on B. napus, Se aided in reducing the Cd stress by enhancing the activities of GPOX, MDHAR, DHAR and GR, as well as the contents of antioxidants (Hasanuzzaman et al. 2012). The plants of Lycopersicon esculentum subjected to salinity also showed increased activities of SOD, GPOX, DHAR, MDHAR and GR in response to Se. The contents of ascorbate and GSH also got enhanced on Se application (Diao et al. 2014).
Conclusion
The results of the study suggest the importance of Se(VI) in the regulation of physiological responses of B. juncea against Cr(VI) stress. The application of Se(VI) at low concentrations significantly reduced the level of oxidative damage caused by Cr(VI) application. The defence system of B. juncea seedlings got suppressed under the effect of Cr(VI) that led to enhanced ROS production and membrane damage. The study confirms the potential of Se(VI) in counteracting the toxicity of Cr(VI) by making the defence system more effective. Hence, the use of Se(VI) at appropriate levels can become a tool to protect the crops against environmental stresses.
Author contribution statement
NH: Performed analysis on all samples, interpreted data and wrote manuscript. SKK: Participated in standardization of protocols for confocal and visible microscopy. Dr. AKT: Co-supervised the research work, helped in statistical analysis of data, its interpretation and manuscript evaluation. Dr. SA: Helped to evaluate and edit the manuscript. Dr. RB: Supervised development of research work, helped in data interpretation and manuscript evaluation.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Asada K (1994) Production and action of active oxygen in photosynthetic tissue. In: Foyer CH, Mullineaux PM (eds) Causes of photooxidative stress and amelioration of defense systems in plants. CRC, Boca Raton, pp 77–104
Bailey NTJ (1995) Statistical Methods in Biology. Cambridge University Press, Cambridge
Callard D, Axelos M, Mazzolini L (1996) Novel molecular markers for the late phases of the growth cycle of Arabidopsis thaliana cell suspension cultures are expressed during organ senescence. Plant Physiol 112:705–715
Carlberg I, Mannervik B (1975) Purification of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 250:5475–5480
Chalapathi Rao ASV, Reddy AR (2008) Glutathione reductase: a putative redox regulatory system in plant cells. In: Khan NA, Singh S, Umar S (eds) Sulfur assimilation and abiotic stresses in plants. Springer, Berlin, pp 111–147
Chanda SV, Parmar NG (2003) Effects of chromium on hypocotyl elongation, wall components, and peroxidase activity of Phaseolus vulgaris seedlings. New Zeal J Crop Hortic Sci 31:115–124
Dalton DA, Russell SA, Hanus FJ, Pascoe GA, Evans HJ (1986) Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc Natl Acad Sci USA 83:3811–3815
Diao M, Long M, Jianwei W, Jinxia C, Aifei F, Huiying L (2014) Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J Plant Growth Regul 33:671–682
Dixit V, Pandey V, Shyam R (2002) Chromium ions inactivate electron transport and enhance superoxide generation in vivo in pea (Pisum sativum L. cv: Azad) root mitochondria. Plant Cell Env 25:687–693
Djanaguiraman M, Prasad PVV, Seppanen M (2010) Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol Biochem 48:999–1007
Filek M, Keskinen R, Hartikainen H, Szarejko I, Janiak A, Miszalski Z, Golda A (2008) The protective role of selenium in rape seedlings subjected to cadmium stress. J Plant Physiol 165:833–844
Flohe L (1982) Role of GSH peroxidase in lipid in lipid peroxide metabolism. In: Yagi K (ed) Lipid peroxides in biology and medicine. Academic Press, New York, pp 1949–1959
Flohe L, Gunzler WA (1984) Assay of glutathione peroxidase. Methods Enzymol 105:114–121
Fricker MD, Meyer AJ (2001) Confocal imaging of metabolism in vivo: pitfalls and possibilities. J Exp Bot 52:631–640
Gangwar S, Singh VP (2011) Indole acetic acid differently changes growth and nitrogen metabolism in Pisum sativum L. seedlings under chromium (VI) phytotoxicity: implication of oxidative stress. Sci Hortic 129:321–328
Gangwar S, Singh VP, Srivastava PK, Maurya JN (2011) Modification of chromium (VI) phytotoxicity by exogenous gibberellic acid application in Pisum sativum (L.) seedlings. Acta Physiol Plant 33(4):1385–1397
Garg N, Manchanda G (2009) ROS generation in plants: boon or bane? Plant Biosyst 143:81–96
Gill M (2014) Heavy metal stress in plants: a review. Int J Adv Res 2(6):1043–1055
Gutierréz-Alcala G, Gotor C, Meyer AJ, Fricker M, Vega JM, Romero LC (2000) Glutathione biosynthesis in Arabidopsis trichome cells. Proc Natl Acad Sci USA 97:11108–11113
Hamilton SJ (2004) Review of selenium toxicity in the aquatic food chain. Sci Total Environ 326:1–31
Hasanuzzaman M, Fujita M (2011) Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol Trace Elem Res 143:1758–1776
Hasanuzzaman M, Hossain MA, Fujita M (2012) Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating the antioxidative defence and methylglyoxal detoxification systems. Biol Trace Elem Res 149(2):248–261
Heath RL, Packer L (1968) Photooxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25:385–395
Kono Y (1978) Generation of superoxide radical during autooxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186:189–195
Kühn H, Borchert A (2002) Regulation of enzymatic lipid peroxidation: the interplay of peroxidizing and peroxide reducing enzymes. Free Rad Biol Med 33:154–172
Maiti S, Ghosh N, Mandal C, Das K, Dey N, Adak MK (2012) Responses of maize plant to chromium stress with reference to antioxidation activity. Braz J Plant Physiol 24(3):203–212
Malik JA, Goel S, Kaur N, Sharma S, Singh I, Nayyar H (2012) Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ Exper Bot 77:242–248
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, San Diego
Martinek RG (1964) Method for the determination of vitamin E (total tocopherols) in serum. Clin Chem 10:1078–1086
Mittler R, Blumwald E (2010) Genetic engineering for modern agriculture: challenges and perspectives. Annu Rev Plant Biol 61:443–462
Munné-Bosch S (2005) The role of α-tocopherol in plant stress tolerance. J Plant Physiol 162:743–748
Munné-Bosch S, Alegre L (2002) The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci 21:31–57
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Ortega-Villasante C, Hernández LE, Rellán-Alvarez R, Del Campo FF, Carpena-Ruiz RO (2007) Rapid alteration of cellular redox homeostasis upon exposure to cadmium and mercury in alfalfa seedlings. New Phytol 176:96–107
Panda SK (2007) Chromium mediated oxidative stress and ultrastructural changes in root cells of developing rice seedlings. J Plant Physiol 164:1419–1428
Pang CH, Wang BS (2010) Role of ascorbate peroxidase and glutathione reductase in ascorbate–glutathione cycle and stress tolerance in plants. In: Anjum NA, Chan MT, Umar S (eds) Ascorbate–glutathione pathway and stress tolerance in plants. Springer, Dordrecht, pp 91–112
Pedrero Z, Madrid Y, Hartikainen H, Camara C (2008) Protective effect of selenium in broccoli (Brassica oleracea) plants subjected to cadmium exposure. J Agric Food Chem 56:266–271
Pilon-Smits EAH, Quinn CF (2010) Selenium metabolism in plants. In: Hell R, Mendel RR (eds) Cell biology of metals and nutrients, plant cell monographs. Springer, Berlin, pp 225–241
Putter J (1974) Peroxidase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie, Weinhan, pp 685–690
Rai V, Vajpayee P, Singh SN, Mehrotra S (2004) Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorum L. Plant Sci 167:1159–1169
Rai V, Tandon PK, Khatoon S (2014) Effect of chromium on antioxidant potential of Catharanthus roseus varieties and production of their anticancer alkaloids: vincristine and vinblastine. Biomed Res Int 2014:934182
Roe JH, Kuether CA (1943) The determination of ascorbic acid in whole blood and urine through the 2,4-dinitrophenylhydrazine derivative of dehydroascorbic acid. J Biol Chem 147:399–407
Romero-Puertas MC, Rodríguez-Serrano M, Corpas FJ, Gómez M, del Río LA, Sandalio LM (2004) Cadmiuminduced subcellular accumulation of O ·−2 and H2O2 in pea leaves. Plant Cell Environ 27:1122–1134
Schiavon M, Pilon-Smits E, Wirtz M, Hell R, Malagoli M (2008) Interactions between chromium and sulfur metabolism in Brassica juncea. J Environ Qual 37:1536–1545
Schützendübel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53(372):1351–1365
Sedlak J, Lindsay RH (1968) Estimation of total, protein bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25(1):192–205
Shanker AK, Pathmanabhan G (2004) Speciation dependant antioxidative response in roots and leaves of Sorghum (Sorghum bicolor (L.) Moench cv. CO 27) under Cr(III) and Cr(VI) stress. Plant Soil 265:141–151
Shanker AK, Djanaguiraman M, Sudhagar R, Chandrashekar CN, Pathmanabhan G (2004) Differential antioxidative response of ascorbate glutathione pathway enzymes and metabolites in chromium speciation stress in green gram roots. Plant Sci 166:1035–1043
Shi X, Dalal NS (1989) Chromium (V) and hydroxyl radical formation during the glutathione reductase-catalyzed reduction of chromium (VI). Biochem Biophys Res 163:627–634
Singh HP, Mahajan P, Kaur S, Batish DR, Kohli RK (2013) Chromium toxicity and tolerance in plants. Environ Chem Lett 11:229–254
Sobolev D, Begonia MFT (2008) Effects of heavy metal contamination upon soil microbes: lead-induced changes in general and denitrifying microbial communities as evidenced by molecular markers. Int J Environ Res Public Health 5(5):450–456
Sokal RR, Rholf FJ (1981) Biometry: the principles and practice of statistics in biological research. WH Freeman and Co., San Francisco
Subrahmanyam D (2008) Effects of chromium toxicity on leaf photosynthetic characteristics and oxidative changes in wheat (Triticum aestivum L.). Photosynthetica 46:339–345
Tang J, Xu J, Wu Y, Li Y, Tang Q (2012) Effects of high concentration of chromium stress on physiological and biochemical characters and accumulation of chromium in tea plant (Camellia sinensis L.). African. J Biotechnol 11(9):2248–2255
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Wang YS, Yang ZM (2005) Nitric oxide reduces aluminium toxicity by preventing oxidative stress in roots of Cassia tora L. Plant Cell Physiol 46:1915–1923
Wu GL, Cui J, Tao L, Yang H (2010) Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa). Ecotoxicology 19:124–132
Zou J, Yu K, Zhang Z, Jiang W, Liu D (2009) Antioxidant response system and chlorophyll fluorescence in chromium (VI)-treated Zea mays L. seedlings. Acta Biol Crac Ser Bot 51:23–33
Acknowledgements
The study was supported by financial assistance from DRS-SAP (II) of University Grants Commission and DST-FIST programme of Government of India, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by NA Anjum.
Rights and permissions
About this article
Cite this article
Handa, N., Kohli, S.K., Thukral, A.K. et al. Role of Se(VI) in counteracting oxidative damage in Brassica juncea L. under Cr(VI) stress. Acta Physiol Plant 39, 51 (2017). https://doi.org/10.1007/s11738-017-2352-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-017-2352-6