Abstract
Plant tissue culture can readily be applied to the field of medicinal plants and secondary metabolites production. In this study, in vitro propagation protocol of Rumex cyprius Murb. was established. The highest shoot multiplication rate was observed from explants grown on MS medium supplemented with 1.5 mg/L BA (7.8 shoots per explant). For rooting, the maximum root induction rate was observed on MS medium supplemented with 1.5 mg/L IBA or 1.0 mg/L NAA. The longest roots were observed for plants grown on MS medium supplemented with 1.5 mg/L IBA. For callus induction, leaves from in vitro grown plants were cultured in various levels of 2,4-D or different combinations of BA and NAA. The highest fresh weight (5.86 g) of calli was observed at 1.5 and 0.5 mg/L BA and NAA, respectively. The second part of this work investigated the effects of abiotic factors (NaCl and Mannitol) and biotic factors (Yeast extract and Chitosan) on secondary metabolites accumulation of in vitro grown R. cyprius. Phenolic compounds were extracted, and the individual phenolic compounds and antioxidant activity of the extracts were determined of in vitro grown R. cyprius. The total phenol content and antioxidant activity increased significantly by the pervious factors. Reversed high-pressure liquid chromatography (RP-HPLC) was used to measure the effect of these factors on the contents of individual phenolic compound. New phenolic compounds (Gallic acid and Chlorogenic acid) not found in R. cyprius plants grown on control media were produced in R. cyprius plants grown on media supplemented with elicitors. Results indicate that using elicitors in plant tissue culture could be used to produce/enhance secondary metabolites accumulation in R. cyprius.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rumex cyprius Murb. is an annual wild plant species belonging to the family Polygonaceae. The genus Rumex has more than 200 species. It is distributed in the Mediterranean woodlands and shrub lands, Semi-steppe shrub lands, shrub-steppes, deserts and extreme deserts. In Jordan, R. cyprus is found along the roadsides, and in non-cultivated dry and/or salty soil.
The medicinal importance of this genus is a reflection of its chemical composition since plants contain many bioactive substances such as flavonoids (vitexin, isovitexin, orientin and isorientin) (Saleh et al. 1993). Rumex is also rich in anthraquinones, particularly in roots (emodin and chrysophanol). It contains carotenoids, vitamins (especially vitamin C), proteins, lipids and organic acids (Al Zghoul 2012). Also, it is a good source of minerals such as K, Na, Ca, Mg, Fe, Mn and Cu (Mostafa et al. 2011).
Traditional cultivation of many medicinal plants including R. cyprus is difficult because of low germination rate and/or specific ecological requirements. Also, their could be simply a lack of knowledge about the specific requirements for pollination, seed germination and growth requirements (Canter et al. 2005). Van Assche et al. (2002) studied germination requirements of various Rumex species. Results indicated that all studied Rumex species showed similar dormancy mechanisms; only two species out of nine were able to germinate after 6 months of storage, which indicates that some Rumex species have short-lived seeds and/or their germination is regulated by specific environmental conditions. Another study found that high proportion of Rumex acetosella L. seeds (46–99%) is dormant. Germination of dormant Rumex acetosella L. seeds was induced (up to 17%) by drying and then moistening of seeds (Hintikka 1990). In vitro propagation solved these problems along others. It is possible now to multiply these plants in the laboratory from little explants (Tripathi and Tripathi 2003; Al Khateeb et al. 2012). This will allow access to significant quantities within desired time and with less effort. In addition, we reduce the problem of the geographical boundaries where it was possible to germinate such plants at any time of the year in the laboratory regardless of geographical location. Plant tissue culture also helps to preserve the desired genotypes from genetic changes or extinction (Sujatha and Kumari 2008).
Secondary metabolites are a group of natural synthesized compounds with multi-chemical, functional and biological properties that help plants to survive under sever conditions. These metabolites are widely used in modern medicine either by direct isolation from plants or synthetic modification of a lead compound of natural origin (Vinale et al. 2008). Phenolic compounds are the most widely distributed type of plant secondary metabolites (Singh et al. 1998). These compounds have many significant bioactivities, such as scavenging free radicals, chelating metals, regulating enzyme activity, and modulating cell proliferation (Wang et al. 2011).
Oxidative stress results from free radicals and reactive oxygen species (ROS) that are formed under normal physiological conditions (Chanda and Dave 2009). Several studies reported that secondary metabolites can scavenge free radicals and may reduce such diseases (Gouthamchandra et al. 2010). Antioxidants are groups of secondary metabolites including peptides, amino acids, alkaloids, phenolic and metal ions that prevent or delay the oxidation of substrate against oxidative damage of human body (Dawidowicz et al. 2006).
Production of secondary metabolites in medicinal plants is limited as compared to the primary metabolites. Secondary metabolites present in low quantities; it is less than 1% of the total carbon content (Bourgaud et al. 2001; Al Zghoul 2012). Elicitation is usually one of the most successful ways to increase levels of secondary metabolites in medicinal plants (DiCosmo and Misawa 1985). It consists of applying chemical or physical stresses to plant cultures which will trigger the production of secondary metabolites that are normally not produced or produced in low quantities (Bourgaud et al. 2001). This could be achieved by applying biotic elicitors including chitosan, yeast extract and various protein extracts or abiotic factors, such as temperature, UV light, heavy metal, salts and pH (Dornenburg and Knorr 1995).
The aim of this study was to establish an in vitro propagation protocol for R. cyprius Murb. and to study the effect of different biotic and abiotic stresses on antioxidant activity and phenolic compounds of in vitro grown R. cyprius Murb.
Materials and methods
Plant materials
Wild plants of R. cyprius Murb. were collected from the Dead Sea area (31°41′44.83″N, 35°35′1.17″E) in Jordan during the period of October, 2013 (Fig. 1a). Fruits of R. cyprius Murb. were allowed to dry in the laboratory, and then seeds were collected (Fig. 1b).
In vitro propagation of R. cyprius L. a Wild grown R. cyprius plant near Dead Sea area/Jordan. b flowers and seeds of R. cyprius. c Germinated seedling after 10 days of inoculation on MS medium. d Microshoots after 5 weeks of inoculation on MS medium supplemented with 1.5 mg/L BA. e Explant used for shoot proliferation. f Rooting stage. g Explant used for callus induction. h Callus grown on MS medium supplemented with 1.5 mg/L BA and 0.5 mg/L NAA. Scale bar in a = 4 cm, b = 1 cm, c = 2 cm, d = 1.5 cm, e = 1 cm, g = 0.5 cm, h = 2 cm
Seed germination
In vitro cultures of R. cyprius Murb. were established from seeds. Seeds were washed with tap water and few drops of Tween-20 then with 70% alcohol for 30 s followed by washing with 30% sodium hypochlorite for 15 min. Finally, seeds were rinsed with sterile distilled water for three times (each time for 5 min). Sterilized seeds were inoculated into test tubes containing half-strength Murashige and Skoog (1962) medium (MS). The pH of the medium was adjusted to 5.8. Six gram per liter of agar was added to solidify the media. Sucrose (0.6%) and the essential vitamins were also added. Media were autoclaved at 121 °C and 1.15 kg/cm2 pressure for 20 min. Cultures were incubated in the growth chamber at 24 ± 2 °C and under 16/8 h (light/dark) photoperiod provided by cool-white fluorescent tubes.
In vitro shoot proliferation
Shoot segments (about 2 cm) of in vitro grown seedlings (2 weeks old) (Fig. 1e) were used as starting materials. Shoot tips were excised and cultured on MS medium supplemented with BA, TDZ or Kin at 0.0, 0.5, 1.0, 1.5, 2.0 and 3.0 mg/L, 3% sucrose and 0.6% agar, unless otherwise mentioned. The medium pH was adjusted to 5.8 before autoclaving. The cultures were maintained at 24 ± 2 °C under a 16/8 h (light/dark) photoperiod provided by cool-white fluorescent tubes for 6 weeks.
Data were taken after 6 weeks for number of leaves, number of shoots, callus formation and root formation.
In vitro root formation
Shoot segments (about 2 cm) were used as explants for the rooting experiment. Two rooting agents [1-napthaleneacetic acid (NAA) or indole-3-butyric acid (IBA)] at concentrations of 0.0, 0.5, 1.0, 2.0 mg/L were supplemented to MS medium to test their root induction ability. A number of roots and callus induction percentage were assessed after 6 weeks of culture.
Callus induction
Leaf segments (about 1 cm2) (Fig. 1g) from in vitro grown R. cyprius were used as starting material for callus induction. Different levels of BA, NAA, or 2,4-D were added to the basal medium either as single or in various combinations. Cultures were incubated at 24 °C and 16 h photoperiod for 4 weeks. Data were collected for callus induction rate % [(number of explants produced callus/total number of explants cultured) × 100%], callus texture, and fresh weight. To determine callus growth kinetics, callus was subcultured on medium supplemented with 1.5 BA + 0.5 NAA mg/L. Callus fresh and dry weight was measured at 3-day intervals for 30 days of cultivation.
Stress treatments
Microshoots (about 2 cm) of R. cyprius were cultured on MS medium containing 1.0 mg/L BA and supplemented with different concentrations of NaCl (0, 25, 50, 75 or 100 mM), Mannitol (0, 100, 200, 300 or 400 mM) as abiotic stresses or Yeast extract (100, 200, or 400 mg/L) or Chitosan (50, 100, or 200 mg/L) as biotic stresses. After 4 weeks, cultures were harvested and freeze dried to be used for phenolics analysis.
Phenolics analysis
Extraction
One gram of each freeze dried shoot sample was grounded and then subjected to extraction with 25 mL of methanol at 25 °C for 1 h in a shaking water bath. After that, the extracts were centrifuged at 3500 rpm for 10 min, and the supernatants were recovered and stored at −18 °C until used for the phenolics assays.
Determination of phenol content
Folion-Ciocalteu spectrophotometeric method was used to measure the phenol content for each extract according to Alu’datt et al. (2010). Gallic acid was used as the standard. Extract was mixed with distilled water and Folin-Ciocalteu at room temperature, and then sodium carbonate (Na2CO3) was added to neutralize the reaction. Phenol content in each extract was measured after 1 h at wavelength of 725 nm using spectrophotometer (UV 1800). The phenol content in each extract was expressed as milligram of gallic acid equivalents per gram of dry matter (mg of GAE/g).
Analysis of individual phenolic compounds using reversed phase high-pressure liquid chromatography (RP-HPLC)
Methanolic extracts from in vitro grown plants were evaporated under a stream of nitrogen and stored at −18 °C for HPLC analysis. The extract was subjected to RP-HPLC analysis using Agilent 1100 series HPLC system (Agilent Technologies, Waldbrom, Germany) liquid chromatography. For chromatographic separation, 100 µL sample was injected into C18 reversed phase column (5 μm, 250 × 4.6 mm, Varian, US). Elution was carried out at a flow rate of 0.75 mL/min with the following two buffer gradients system: solvent A, 0.2% triflouroacetic acid (TFA) in water (v/v) and solvent B, 100% methanol, with a linear gradient starting at 5% methanol within 60 min; initial conditions were then re-established over 10 min. Standards of gallic acid, vanillic acid, chlorogenic acid, caffeic acid, syringic acid, epicatechin, sinapic acid, rutin, hesperidin, rosmarinic, quercetin, and luteolin were used to quantify the contents of individual phenolics in extracts based on peak areas.
Determination of antioxidant activity
Antioxidant activity (AA %) of methanolic extracts was determined according to the method described by Alu’datt et al. (2010). β-Carotene was mixed with linoleic acid and Tween-20. The emulsion of β-carotene/linoleic acid was mixed with the sample solution and incubated in a water bath at 50 °C for 60 min. The antioxidant activity was evaluated by measuring the color absorbance at 470 nm using spectrophotometer after 60 min. Control of methanol instead of sample and water was used as blank. The antioxidant activity was expressed using the following equation:
where AA% is the antioxidant activity, DRC is the degradation rate of control, DRC = ln(A/B)/60, DRS is the degradation rate in the presence of the sample, DRS = in(A/B)/60. A is an initial absorbance at time 0 min, and B is the absorbance after 60 min.
Statistical analysis
All experiments were repeated at least three times. Each treatment consisted of at least eight petri dishes or flasks, each containing two explants in completely randomized design. Means, standard deviation, standard error and ANOVA were calculated using SPSS. Statistical significance was accepted at P ≤ 0.05.
Results
Seed germination and shoot proliferation
Surface sterilized R. cyprius seeds were inoculated aseptically in culture tubes containing germination medium (½ MS). Twenty percent of the inoculated seeds started to germinate after 5–10 days of inoculation and the remaining were germinated within 2–3 weeks (Fig. 1c).
To test the effect of type and level of cytokinin on shoot proliferation, different levels of BA, Kin, and TDZ were used. Uniform germinated seedlings (Fig. 1e) were inoculated on MS medium either free of growth regulators (control) or supplemented with different levels (0.5, 1.0, 1.5, and 2.0 mg/L) of three cytokinins (BA, Kin and TDZ).
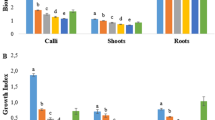
All the three growth regulators induced shoot proliferation of R. cyprius (Figs. 1d, 2). Seedlings cultured on medium supplemented with 1.5 mg/L BA showed the highest number of shoots (7 shoots per explant on average) compared to only one shoot in control.
Shoot proliferation and rooting of R. cyprius. a Effects of Kin, BA, and TDZ on number of shoots. b Effects of Kin, BA, and TDZ on number of leaves. c Effects of NAA and IBA on number of roots. d Effects of NAA and IBA on root length. Data represent mean values ± standard error of eight replicates. Means followed by the same letter are not significantly different at the P ≤ 0.05 according to Tukey’s test
Similar to number of shoots, results showed that BA was the most effective type of cytokinin in inducing leaf formation with the maximum at 1.5 mg/L (22.8 leaves/microshoot) (Fig. 2b). Higher levels (2 mg/L) of the all used cytokinin types reduced the number of shoots and leaves significantly.
Root number and length
To test the effect of type and level of auxins on root formation, different levels of IBA and NAA were used. Roots of uniform germinated seedlings were trimmed 3 mm below their basal node and inoculated on MS medium either free of growth regulators (control) or supplemented with different levels (0.5, 1.0, 1.5, and 2.0 mg/L) of IBA or NAA. After 6 weeks, data were taken for number of developed roots and root length. The highest number of R. cyprius roots (16 roots/explant) was observed in MS medium supplemented with 1.5 mg/L IBA (Fig. 1f, 2c). High levels (1.5 and 2.0 mg/L) of NAA resulted in callus formation.
In general, root length of R. cyprius grown on MS medium supplemented with IBA was longer than that grown on MS medium supplemented with NAA. Results showed that the longest root was observed in the medium supplemented with 1.5 mg/L IBA (16.6 cm) followed by medium supplemented with 0.5 mg/L IBA (11.8 cm) (Fig. 2d).
Callus induction
Results showed that control media did not induce callus formation. Cultures grown on MS medium supplemented with various levels of 2,4-D (0.5, 1.0, 1.5 mg/L) were soft and yellow after 6 weeks (Fig. 1h; Table 1). BA and NAA combinations resulted in callus formation with 100% induction rate. The highest fresh weight (5.86 g) of calli was observed at 1.5 and 0.5 mg/L BA and NAA, respectively (Fig. 3a). Calli were produced after 6 weeks and they were friable, healthy, and green in all combinations. Growth curve of R. cyprius calli showed a sigmoidal-type pattern with three growth phases, the initial period of culturing after inoculation is referred to the lag phase, then the rate of cell division increases and reaches the maximum during the log phase and, finally, as nutrients in the growth medium gets consumed, and the number and size of cells remain constant, the cells enter the stationary phase (Fig. 3b). The fastest growth phase was observed in the period between day 15 and day 22.
Callus culture of R. cyprius. a R. cyprius callus fresh weight grown on MS medium supplemented with different growth regulators. b Growth curve of R. cyprius calli grown on MS medium supplemented with 1.5 mg/L BA + 0.5 mg/L NAA. I lag phase, II log phase, III stationary phase. Bars represent standard error
Effect of abiotic and biotic stresses on phenol level and antioxidant activity
Effect of abiotic stress
Rumex cyprius microshoots grown on MS media supplemented with different levels of NaCl (0, 25, 50,75 and 100 mM) or mannitol (100 mM) for 6 weeks were extracted with methanol and used to test the effect of such stresses on total phenol contents, individual phenolic acids, and antioxidant activity.
Phenol content
Phenol content increased significantly (P ≤ 0.05) using 50, 75 and 100 mM NaCl. The maximum level of phenol content (1.8 mg/g) was observed in microshoots grown on medium supplemented with 100 mM NaCl (Fig. 4a). Growing R. cyprius on media supplemented with 100 mM mannitol did not affect phenol content as compared to the control treatment.
a Effect of NaCl and Mannitol on total phenol content of R. cyprius microshoots grown on MS medium supplemented with 1.0 mg/L BA. b Effect of NaCl and Mannitol on the contents of phenolic compounds obtained using RP-HPLC technique for R. cyprius microshoots grown on MS medium supplemented with 1.0 mg/L BA. c Effect of NaCl and Mannitol on total antioxidant activity for R. cyprius microshoots grown on MS medium supplemented with 1.0 mg/L BA. Bars represent standard error. Means followed by the same letter are not significantly different at the P ≤ 0.05 according to Tukey’s test
RP-HPLC analysis for individual phenolic compounds
Twelve phenolic compounds were tested using RP-HPLC including gallic acid, vanillic acid, chlorogenic acid, caffeic acid, syringic acid, epicatechin, sinapic acid, rutin, hesperidin, rosmarinic, quercetin and luteolin. Results showed that vanillic acid is not detected in all treatments of R. cyprius samples.
Nine individual phenolic compounds were detected in the control sample of R. cyprius, namely caffeic acid, syringic acid, epicatechin, sinapic acid, rutin, hesperidin, rosmarinic, quercetin, and luteolin. The major phenolics in the control sample of R. cyprius as well as NaCl and Mannitol samples was epicatechin with 43, 40 and 56% from the total phenolic content, respectively (Fig. 4b). A decrease in epicatechin content was observed in R. cyprius samples grown on media containing NaCl. On the other hand, a significant increase in epicatechin level was observed in samples grown on media containing 100 mM mannitol (56% from total phenolic content) (Fig. 4b).
Growing R. cyprius on media supplemented with 100 mM NaCl significantly enhanced sinapic acid, rutin and rosmarinic accumulation. Similarly, a significant increase in syringic acid, rutin, epicatechin and luteolin level was observed in samples grown on media supplemented with 100 mM mannitol (Fig. 4b). Furthermore, new phenolic compounds (not found in plants grown on control media) were produced in plants grown on media supplemented with NaCl (chlorogenic acid) or Mannitol (gallic acid).
Antioxidant activity of R. cyprius
Results showed a significant (P < 0.05) increase in antioxidant activity as NaCl level increased in the growing medium. The maximum antioxidant activity (88.4%) was observed in microshoots grown on media supplemented with 75 mM NaCl (Fig. 4c). Similarly, growing R. cyprius microshoots on media supplemented with 100 mM mannitol significantly (P < 0.05) induced antioxidant activity (90.4%) compared to control plants.
Effect of biotic stress
In the present study, different levels of yeast extract (0, 100, 200, or 400 mg/L) or chitosan (0, 50, 100, or 200 mg/L) were used as biotic stress inducers. One gram of freeze dried tissues was subjected to methanolic extraction, and then the effect of these stresses on phenolics contents, individual phenolics, and antioxidant activities was evaluated.
Phenol contents
The phenol content was increased with the increase in yeast extract level (P < 0.05). The highest level of phenol content (1.7 mg/g) was observed in R. cyprius microshoots grown on media supplemented with 400 mg/L yeast extract (Fig. 5a). On the other hand, no significant effect of chitosan on phenol content of R. cyprius was observed (Fig. 5b).
RP-HPLC analysis for individual phenolic compounds
Growing R. cyprius on media supplemented with yeast extract resulted in new phenolic compounds (gallic acid and chlorogenic acid) not found in plants grown on control media (Fig. 6a). Similarly, growing R. cyprius on media supplemented with Chitosan resulted in production of gallic acid which was not found in plants grown on control media (Fig. 6b). Furthermore, yeast extract significantly enhanced other phenolic compounds accumulation in R. cyprius compared to control plants (caffeic acid, rutin, rosemeric acid and quercetin) (Fig. 6b).
Antioxidant activity of R. cyprius
Antioxidant activity increased significantly (P < 0.05) with the increase in yeast extract or Chitosan concentration compared to control plants. R. cyprius plants grown on media supplemented with high levels of yeast extract (200 and 400 mg/L) enhanced antioxidant activity and resulted in the maximum activity (93.4%). On the other hand, growing R. cyprius plants on media supplemented with either low or high level of Chitosan enhanced antioxidant activity significantly (95.1%) (Fig. 7a, b).
Discussion
The present study was undertaken to standardize an in vitro propagation protocol for R. cyprius. High seed germination rate on ½ MS medium was observed, possibly because of lower salt concentration in the ½ MS compared to that in full MS. Zeng et al. (2012) showed that Paphiopedilum wardii seeds prefer low salt medium for germination. Similarly, ½ MS was used for germination of many species including Paphiopedilum delenatii (Nhut et al. 2005), Bobgunnia madagascarensis (Thokozani et al. 2011), Cichorium pumilum (Al Khateeb et al. 2012) and Tabebuia impetiginosa (Vieira et al. 2010).
Multiple shoots proliferation was observed from shoots grown on MS medium supplemented with different concentrations of BA, Kin, and TDZ. However, the best results were observed on medium supplemented with different levels of BA. This is in agreement with another study conducted on Rumex vesicarius in which researchers found that shoot multiplication was the best from explants cultured on MS medium containing BA, compared to those on medium containing Kin (Abo El-soud et al. 2011).
Growth regulators (especially cytokinin) are one of the most important factors affecting shoot proliferation (Moncalean et al. 2003; Norton and Norton 1985). In this study, growing shoots on MS medium supplemented with TDZ and high concentration (2.0 mg/L) of BA and Kin resulted in compact dark callus formation at the base of the shoots. A similar result was also reported by Chengalrayan et al. (2005) as they found that high levels of TDZ more than 0.5 mg/L resulted in callus formation on shoot tips of Saccharum officinarum.
Roots induction at the base of in vitro grown shoots is essential and indispensable step to establish a successful micropropagation protocol. The most effective auxins for rooting of different species are IBA and NAA (Singh and Tiwari 2012; Simon and Petrasek 2011). Type and level of auxin for root induction are species specific. In this study, we found that IBA is more effective than NAA for number of roots formed and root length. Purkayastha et al. (2008) found that the highest root induction rate of in vitro grown shoots of Andrographis paniculata was using full MS medium supplemented with 1.5 mg/L IBA. In contrast, Balaraju et al. (2009) found that Swertia chirata were rooted most successfully using NAA-supplemented MS medium.
The best callus induction rate (100%) was achieved using MS medium supplemented with different ratios and combinations of BA and NAA which was found to be more effective for rapid production of green callus than 2,4-D. These findings are in agreement with the results obtained in Abelmoschus moschatus (Warghat et al. 2011) and in Cardiospermum halicacabum (Thomas and Maseena 2006). They found that good callus induction was found in MS medium supplemented with BA combined with NAA. Growth curve of R. cyprius callus was plotted based on fresh weights. Calli showed a sigmoid growth curve with lag, exponential, linear and stationary phases. These phases have been shown in other plant species including Linum usitatissimum (Cunha and Ferreira 1996), Eucommia ulmoides (Xing et al. 2001), and Typha latifolia (Estime et al. 2002). The lag phase, in which the cells of the explants prepare themselves for division, occurred after 9 days of inoculation. The exponential growth phase, which is a period in which the maximum rate of cell division is observed, occurred between the 9th and the 15th days after inoculation. In general, herbs showed faster exponential growth than woody plant species that need around 30–75 days (Abbade et al. 2010).
Elicitation is considered as the major method to increase secondary metabolites production in plants by enhancing compounds synthesis and gene expression of secondary metabolites. Results of this study showed that using different types of elicitors significantly increased total phenol contents, the level of predominant phenolic compounds, and antioxidant activity of in vitro grown R. cyprius.
No information is available in the literature until now about the effect of several elicitors on the levels of individual phenolic compounds in R. cyprius. Here, abiotic stresses increased gallic acid, syringic acid, epicatechin, sinapic acid, rutin, rosmarinic acid, and quercetin levels in R. cyprius. On the other hand, biotic stresses showed an increase in caffeic acid, rutin, rosmarinic acid, and quercetin in R. cyprius. Several studies reported that such phenolic compounds have numerous beneficial effects on human health including their antioxidant activities (Yang et al. 2008; Aytekin et al. 2011; Erkan et al. 2008; Pradeep et al. 2008).
Jaleel et al. (2006) studied factors that affect antioxidant activity of medicinal plants and the level of secondary metabolite production in Catharanthus roseus. They showed a significant enhancement of antioxidant activity under drought stress as compared to control samples. Similarly, salinity had a major role in the synthesis and accumulation of secondary metabolites. Wahid and Ghazanfar (2006) found that phenolic compounds, anthocyanins, and flavones contents in a group of medicinal plants were higher in salt stress conditions as compared to control samples. Chen et al. (2001) studied the effect of yeast elicitor on the growth and secondary metabolism of hairy root cultures of Salvia miltiorrhiza. They found that the production of both phenolic compounds and tanshinones was enhanced after treatment with yeast elicitors.
In conclusion, the present study describes for the first time a successful in vitro propagation protocol of R. cyprius. In addition, different elicitors were used to enhance the production of phenolics which possesses antioxidant properties. Results also showed that the phenolics content and antioxidant activity in R. cyprius grown on medium supplemented with elicitors were significantly higher than that of control (without elicitors). Phenol content increased by adding abiotic or biotic factors to the growing medium of R. cyprius. HPLC analysis of in vitro grown R. cyprius showed that the main individual phenolic compounds were caffeic acid, syringic acid, sinapic acid, rutin, hesperidin, rosmarinic, quercetin, luteolin, and epicatechin.
Author contribution statement
WAK Design of the experiment, in vitro establishment, data statistical analysis, results interpretation, drafting the article, final approval of the version to be published. MA helped in chemical analysis, results interpretation, critical revision of the article, final approval of the version to be published. HAZ in vitro propagation and chemical analysis, data collection and interpretation. RK Data collection, helped in chemical analysis and draft formatting. AE Plant collection and identification, helped in data interpretation and manuscript writing. JL Plant collection and identification, helped in data interpretation and manuscript writing.
Abbreviations
- BA:
-
6-Benzylaminopurine
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- TDZ:
-
Thidiazuron
- Kin:
-
Kinetin
- NAA:
-
1-Naphthaleneacetic acid
- IBA:
-
Indole-3-butyric acid
- MS:
-
Murashige and Skoog (1962) medium
References
Abbade L, Paiva P, Paiva R et al (2010) Growth curve and biochemical analysis of callus of IPE-BRANCO (Tabebuia roseo alba). Naturalia 33:45–56
Abo El-soud IH, Al-azab A, Koriesh EM et al (2011) Micropropagation of Rumex vesicarius L. through shoot tip culture. Catrina J 6:41–45
Al Khateeb W, Hussein E, Qouta L et al (2012) In vitro propagation and characterization of phenolic content along with antioxidant and antimicrobial activities of Cichorium pumilum Jacq. Plant Cell Tissue Organ Cult 110:103–110
Al Zghoul H (2012) Production of phytochemical compounds from in vitro grown Rumex cyprius L. and Artemisia herba alba L. M.Sc Thesis in Department of Biological Sciences, Yarmouk University, Jordan
Alu’datt M, Alli I, Ereifej K et al (2010) Optimisation, characterisation and quantification of phenolic compounds in olive cake. Food Chem 123:117–122
Aytekin A, Morimura S, Kida K (2011) Synthesis of chitosan caffeic acid derivatives and evaluation of their antioxidant activities. J Biosci Bioeng 111:212–216
Balaraju K, Agastian P, Ignacimuthu S (2009) Micropropagation of swertia chirata Buch.-Hams. ex Wall.: a critically endangered medicinal herb. Acta Physiol Plant 31:487–494
Bourgaud F, Gravot A, Milesi S et al (2001) Production of plant secondary metabolites: a historical perspective. Plant Sci 161:839–851
Canter PH, Thomas H, Ernst E (2005) Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotech 23:180–185
Chanda S, Dave R (2009) In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: an overview. Afr J Microbiol Res 3:981–996
Chen H, Chena F, Chiu F et al (2001) The effect of yeast elicitor on the growth and secondary metabolism of hairy root cultures of Salvia miltiorrhiza. Enzym Microb Technol 28:100–105
Chengalrayan K, Abouzid A, Gallo-Meacher M (2005) In vitro regeneration of plants from sugarcane seed-derived callus. Vitro Cell Dev Biol Plant 41:477–482
Cunha A, Ferreira M (1996) Somatic embryogenesis, organogenesis and callus growth kinetics of flax (Linum usitatissimum L.). Plant Cell Tissue Organ Cult 47:1–8
Dawidowicz A, Wianowska D, Baraniak B (2006) The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT Food Sci Technol 39:308–315
Dicosmo F, Misawa M (1985) Eliciting secondary metabolism in plant cell cultures. Trends Biotech 3:318–322
Dornenburg H, Knorr D (1995) Strategies for the improvement of secondary metabolite production in plant cell cultures. Enzym Microb Technol 17:674–684
Erkan N, Ayranci G, Ayranci E (2008) Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem 110:76–82
Estime L, O'Shea M, Borst M et al (2002) Suspension culture and plant regeneration of Typha latifolia. HortScience 37:406–408
Gouthamchandra K, Mahmood R, Manjunatha H (2010) Free radical scavenging, antioxidant enzymes and wound healing activities of leaves extracts from Clerodendrum infortunatum L. Environ Toxicol Pharmacol 30:11–18
Hintikka V (1990) Germination ecology and survival strategy of Rumex acetosella polygonaceae on drought exposed rock outcrops in south finland. Ann Bot Fenn 27:205–215
Jaleel C, Gopi R, Lakshmanan G (2006) Triadimefon induced changes in the antioxidant metabolism and ajmalicine production in Catharanthus roseus (L.) G. Don. Plant Sci 171:271–276
Moncalean P, Rodriguez A, Fernandez B (2003) Effect of different benzyladenine time pulses on the endogenous levels of cytokinins, indole-3-acetic acid and abscisic acid in micropropagated explants of Actinidia deliciosa. Plant Physiol Bioch 41:149–155
Mostafa H, Elbakry A, Alam E (2011) Evaluation of antibacterial and antioxidant activities of different plant parts of Rumex vesicariusl. Int J Pharm Pharm Sci 3:109–118
Nhut D, Trang T, Vu N et al (2005) A wounding method and liquid culture in Paphiopedilum delenatii propagation. Propag Ornam Plants 5:156–161
Norton M, Norton C (1985) In vitro propagation of Ericaceae: a comparison of the activity of the cytokinins N6-benzyladenine and N6-isopentenyladenine in shoot proliferation. Sci Hortic 27:335–340
Pradeep K, Park S, Ko K (2008) Hesperidin a flavanoglycone protects against -irradiation induced hepatocellular damage and oxidative stress in Sprague Dawley rats. Eur J Pharmacol 587:273–280
Purkayastha J, Sugla T, Paul A et al (2008) Rapid in vitro multiplication and plant regeneration from nodal explants of Andrographis paniculata: a valuable medicinal plant. Vitro Cell Dev Biol Plant 44:442–447
Saleh N, El-Hadidi M, Arafa R (1993) Flavonoids and anthraquinones of some Egyptian Rumexspecies (Polygonaceae). Biochem Syst Ecol 21:301–303
Simon S, Petrasek J (2011) Why plants need more than one type of auxin. Plant Sci 180:454–460
Singh J, Tiwari K (2012) In vitro plant regeneration from decapitated embryonic axes of Clitoria ternatea L. An important medicinal plant. Ind Crops Prod 35:224–229
Singh S, Lewis N, Towers G (1998) Nitrogen recycling during phenylpropanoid metabolism in sweet potato tubers. J Plant Physiol 153:316–323
Sujatha G, Kumari B (2008) Micropropagation, encapsulation and growth of Artemisia vulgaris node explants for germplasm preservation. S Afr J Bot 74:93–100
Thokozani B, Zulu D, Sileshi G (2011) Seed germination and in vitro regeneration of the African medicinal and pesticidal plant, Bobgunnia madagascariensis. Afr. J. Biotechnol 10:5959–5966
Thomas T, Maseena E (2006) Callus induction and plant regeneration in Cardiospermum halicacabum Linn. an important medicinal plant. Sci Hortic 108(3):332–336
Tripathi L, Tripathi J (2003) Role of biotechnology in medicinal plants. Trop J Pharm Res 2:243–253
Van Assche J, Van Nerum D, Darius P (2002) The comparative germination ecology of nine Rumex species. Plant Ecol 159:131–142
Vieira C, Silva E, Alvarenga A et al (2010) Stress-associated factors increase after desiccation of germinated seeds of Tabebuia impetiginosa Mart. Plant Growth Regul 62:257–263
Vinale F, Sivasithamparam K, Ghisalberti E (2008) A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol Mol Plant Pathol 72:80–86
Wahid A, Ghazanfar A (2006) Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J Plant Physiol 163:723–730
Wang H, Hu Z, Wang Y (2011) Phenolic compounds and the antioxidant activities in litchi pericarp: difference among cultivars. Sci Hortic 129:784–789
Warghat A, Rampur N, Wagh P (2011) In vitro Callus induction of Abelmoschus moschatus L. by using different hormone concentration. Int J Pharm Sci Rev Res 10:82–84
Xing X, Ono A, Miyanaga K (2001) A kinetic model for growth of callus derived from Eucommia ulmoides aiming at mass production of a factor enhancing collagen synthesis of animal cells. Math Comput Simul 56:463–474
Yang J, Guo J, Yuan J (2008) In vitro antioxidant properties of rutin. Food Sci Technol 41:1060–1066
Zeng S, Wu K, Silva T (2012) Asymbiotic seed germination, seedling development and reintroduction of Paphiopedilum wardii Sumerh., an endangered terrestrial orchid. Sci Hortic 138:198–209
Acknowledgements
The authors would like to thank Adel Rababah for his technical assistance. This study was supported by the deanship of research at Yarmouk University/Jordan as M.Sc. thesis project for Haifa Al Zghoul. Funding was provided by Yarmouk University (Grant No. 12/2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Q. Wang.
Rights and permissions
About this article
Cite this article
Al Khateeb, W., Alu’datt, M., Al Zghoul, H. et al. Enhancement of phenolic compounds production in in vitro grown Rumex cyprius Murb.. Acta Physiol Plant 39, 14 (2017). https://doi.org/10.1007/s11738-016-2312-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2312-6